 Open Access Article
Open Access ArticleTriple-decker complexes incorporating three distinct deck architectures†‡
Xiaofei
Sun
 a,
Akhil Kumar
Singh
a,
Ravi
Yadav
a,
Akhil Kumar
Singh
a,
Ravi
Yadav
 a,
Da
Jin
a,
Maria
Haimerl
b,
Manfred
Scheer
a,
Da
Jin
a,
Maria
Haimerl
b,
Manfred
Scheer
 b and
Peter W.
Roesky
b and
Peter W.
Roesky
 *a
*a
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, Karlsruhe, 76131, Germany. E-mail: roesky@kit.edu
bInstitute of Inorganic Chemistry, University of Regensburg, Regensburg 93040, Germany
First published on 17th December 2021
Abstract
The reactivity of the dilithioplumbole ([Li2(thf)2(μ,η5-LPb)], LPb = 1,4-bis-tert-butyl-dimethylsilyl-2,3-bis-phenyl-plumbolyl) towards the reactive pnictogen precursors P4, pentaphosphaferrocene, and pentaarsaferrocene ([Cp*Fe(η5-E5)] (Cp* = η5-C5Me5, E = P, As)) is reported. The reaction with P4 afforded a phospholyl lithium complex, via lead-phosphorus exchange, while the reactions with [Cp*Fe(η5-E5)] yielded the first examples of Pb–Fe–Li heterotrimetallic triple-decker polypnictogenides with three different deck motifs.
Since the milestone discovery of ferrocene in 19511 and bis(benzene)chromium in 1955,2 the chemistry of organometallic sandwich complexes has become an intensive subject of research, not only owing to their unique structural features but also due to their interesting physical and chemical properties.3 Most of the sandwich complexes contain carbocyclic ligands, among which, the cyclopentadienyl (Cp = η5-C5H5) anion and its derivatives have become the most frequently used ones.4 The possibility of expanding the dimension of sandwich complexes was achieved in 1972 by Salzer and Werner when they isolated the first triple-decker complex [Ni2Cp3]+.5 This was followed by a fast development of triple/multi-decker complexes comprising different ligand systems.6 However, such compounds with purely inorganic ring systems or heterocyclic ligands are still relatively unexplored compared to those with carbocylic ligand systems. The scarcity arises from the lack of suitable synthetic methods. The first successful stabilization of a cyclo-E5 (E = As, P) moiety as middle deck in triple-decker complexes was reported in 1982 ([(CpMo)2(μ,η4-As5)])7 and in 1986 ([(Cp*Cr)2(μ,η5-P5)], Cp* = η5-C5Me5),8 respectively. Due to the isolobal analogy between [E5]− (E = P, As) and Cp−, [cyclo-E5]− fragments have become potential ligands for inorganic sandwich complexes. The ferrocene analogues, pentaphosphaferrocene [Cp*Fe(η5-P5)]9 and pentaarsaferrocene [Cp*Fe(η5-As5)],10 were successfully synthesized and their reactivity has been investigated in the recent years.11 As the parent ferrocene usually features an iron-centred redox behaviour with no significant structural changes, in contrast, for [Cp*Fe(η5-E5)], due to the involvement of the [E5]− moiety in redox reactions, their reactivities show greater variety and redox reactions are often accompanied by structural modification such as hapticity change.12 Due to their redox non-innocent nature towards low-valent species, they can act as excellent building blocks for multimetallic complexes.12e,f,13 However, only a handful of triple-decker complexes bearing [Cp*FeE5] (E = P, As) fragments have been reported to date (Fig. 1A).12f,14
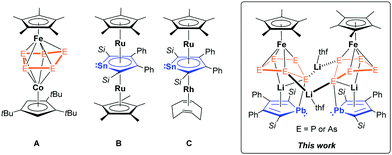 | ||
| Fig. 1 Selected examples of heterometallic triple-decker complexes with non-carbocyclic ligands (E = P or As, Si = SitBuMe2).12f,14a,b,d–f,15 | ||
Another possibility to generate heterometallic triple-decker complexes is to use heavy Cp analogues, which contain heavy group 14 metals in their π-skeleton.16 Those recently developed metalloaromatic species possess unique chemical and physical properties and therefore opened a new field for synthetic chemists.16c The group of Saito has accomplished the creation of novel Rh and Ru triple-decker complexes through salt metathesis reactions using the dianionic stannole ligand as middle deck (Fig. 1B and C).17 For the heaviest dianionic metallole, the plumbole,18 only anionic sandwich-type Ru and Rh complexes [Li(Cp*Ru)(η5-LPb)]19 (LPb = 1,4-bis-tert-butyl-dimethylsilyl-2,3-bis-phenyl-plumbolyl) and [Li(thf)4{(cod)Rh}-(η5-LPb)],20 and another inverted sandwich Rh complex [{(cod)Rh}2(μ,η5-LPb)]20 were reported. Related trimetallic triple-decker species with three distinct deck motifs have remained elusive. Generating such trimetallic triple-decker complexes with a plumbole deck is the aim of our work.
In our first attempt to synthesize a lead-containing polyphosphide, the dilithioplumbole [Li2(thf)2(μ,η5-LPb)]18 was reacted with white phosphorus (P4) in toluene at room temperature for 16 h (Scheme 1). The colour of the solution turned from green to colourless concomitant with the precipitation of grey metallic powder. After filtration and concentration of the toluene solution, colourless crystals of the reaction product were isolated in 60% yield, which was identified as phospholyl lithium 1. Its structure was determined by NMR spectroscopy and X-ray crystallography. The molecular structure of 1 (Fig. 2) shows that the phosphorus atom has replaced the lead atom in the five-membered ring and one lithium atom is η5-coordinated at the phospholyl moiety. In addition, one THF molecule is coordinated to the Li cation. Interestingly, a series of differently substituted phospholyl lithium species have been prepared by the group of Xi directly from P4 and organodilithium reagents.21 The 31P{1H} NMR shift of 1 at 154.1 ppm is in good agreement with the signals reported for the other phospholyl lithium species containing silyl groups (141.3–165.2 ppm).21a
 | ||
| Fig. 2 Molecular structures of 1, 2 and 3. All hydrogen atoms and non-coordinating solvent molecules are omitted for clarity. For selected bond distances and angles, see ESI.‡ | ||
Since the attempted preparation of Pb-polyphosphides was unsuccessful using P4 as phosphorus source, we tested the reaction between [Li2(thf)2(μ,η5-LPb)]18 and [Cp*Fe(η5-P5)]9 (Scheme 2). Stirring the two starting materials at room temperature in benzene led to an immediate color change from green to dark blue and formation of the triple-decker complex [{Cp*Fe(η4-P5)}Li(η5-LPb)Li(thf)]2 (2) (Scheme 2). During the reaction, formally, the plumbole dianion is oxidized to the neutral heterocycle, while [Cp*Fe(η5-P5)] is reduced to {Cp*Fe(η5-P5)}2− and both fragments are combined in a novel triple-decker scaffold. Complex 2 was crystallized from benzene in 41% yield (Fig. 2). The molecular structure of 2 in the solid state consists of two identical triple-decker motifs, generated by rotation around the crystallographic C2 axis. These two units are interconnected through Li2 and Li2′. Each triple-decker motif is constituted by a {Cp*Fe(η4-P5)} unit, which is π-coordinated by a {(LPb)Li} fragment. The π-coordinated Li atoms are in closer spatial proximity to the plumbole moiety of 2 (Li–C: 2.261–2.337 Å, Li–Pb = 2.794 Å) than in the dilithioplumbole (Li–C 2.281–2.448 Å, Li–Pb 2.854–2.897 Å).18 Due to the two-electron reduction, the cyclo-P5 fragment loses its planarity and adopts an envelope-shaped conformation, which coordinates in an η4 fashion to the {Cp*Fe} fragment.12d,f,22 The resulting P4 (P1–P2–P3–P4) unit is nearly planar (torsion angle = 1.9°), P5 deviates from the plane and is coordinated to Pb with a distance of 2.8357(9) Å, which is slightly longer than the Pb–P bonds in the cage complexes [Pb6(PSiiPr3)6] and [Pb7(tBuP)7] (2.666–2.754 Å).23 After reduction and coordination toward Li and Pb, the P–P bond distances (2.149–2.197 Å) are only slightly longer than those in the starting material (average 2.120 Å), indicating partial double bond character of these bonds.24 The plumbole maintained planarity with the sum of the internal angles being 537.5°. In contrast to the dilithioplumbole with no C–C bond alternation in the ring,18 the C–C bond lengths in 2 alter remarkably (C1–C2 = 1.355(4), C2–C3 = 1.515(4) and C3–C4 = 1.364(4) Å), rendering the heterocycle non-aromatic but the expected 1,3-diene character. Similar geometry was found in the base-stabilized plumbacyclopentadienylidenes.25
The 1H NMR spectrum of 2 in C6D6 reveals four sharp singlet signals corresponding to the SitBuMe2 group and the Cp* moiety. In the 13C{1H} NMR spectrum, signals assignable to the plumbole ring Cα atom (212.5 ppm) is only marginally shifted compared to that of the starting material (213.5 ppm), whereas the signal for the Cβ atom (172.8 ppm) is observed in a much lower field compared to the dilithioplumbole (159.4 ppm). The two different Li atoms observed in the solid state (sandwich type and bridging type) do not scramble in solution because two different signals are observed in the 7Li{1H} NMR spectrum at 1.2 and −3.9 ppm. The former signal is assignable to the bridging Li atom and the latter one to the π-coordinated Li nucleus, corroborated by DFT computations.18 At room temperature, the 31P{1H} NMR of 2 shows two broad signals at 42.4 ppm and 18.4 ppm and a doublet of doublets at −14.3 ppm. When low temperature VT NMR studies in toluene-d8 were carried out (see Fig. S17, ESI‡), an apparent AA′MM′X spin system, typical for envelope-shaped P5 systems, could be observed.12e,13a The 207Pb NMR spectrum exhibits one resonance at 2587 ppm, bearing good similarity to the dilithioplumbole (2573 ppm).18
Given the aforementioned reactivity with [Cp*Fe(η5-P5)], we turned to its heavier arsenic analogue [Cp*Fe(η5-As5)].10 In general, the reactivity of the latter one is significantly less explored, and reduction often resulted in mixtures of different arsenic-rich species.26–28 As an example, the reduction of [Cp*Fe(η5-P5)] with KH afforded [K(dme)2K(dme)][(Cp*Fe)2(μ,η4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4-P10)] only,12d while the reaction of [Cp*Fe(η5-As5)] with KH resulted in a mixture of different polyarsenic Asn (n = 4, 10, 14, 18) scaffolds stabilized by {Cp*Fe} fragments.26 Herein, we were interested whether the reduction of the heavier arsenic congener [Cp*Fe(η5-As5)] with the dilithioplumbole will proceed in a similar way as that of its lighter phosphorus derivative or will rather generate other polyarsenides. The reaction between [Li2(thf)2(μ,η5-LPb)]18 and [Cp*Fe(η5-As5)]10 was carried out in a similar manner as with its phosphorus analogues. As a result, brown crystals of the reaction product [{Cp*Fe(η4-As5)}Li(η5-LPb)Li(thf)]2 (3) (Scheme 2), suitable for X-ray structural analysis were obtained in 48% yield. Analogous to the reduction of [Cp*Fe(η5-P5)], the two-electron reduction of the cyclo-As5 unit was observed (Fig. 2). Although, 3 forms a similar dimeric triple-decker structure as 2 in the solid state, there are some slight differences, as the two triple-decker subunits are symmetry-independent. The lithium atoms, which are part of the triple-decker structure (Li1 and Li2) are η4-coordinated by the cyclo-As5 units with interatomic distances ranging from 2.630(6) to 3.001(7) Å, whereas in compound 2, the corresponding lithium atoms are η3-coordinated by the cyclo-P5 unit with interatomic P–Li distances ranging from 2.484(6) to 2.794(6) Å. In these units, As5 and As10 are bent out of the As4 planes with an angle of 54.4° and 55.4°, respectively. The envelope-shaped As5 moiety and the As–As bond distances (2.376–2.442 Å) are similar to that observed in the f-element polyarsenide complexes.13a,22 The Pb–As distances of 2.9171(5) Å (Pb1–As5) and 2.9132(4) Å (Pb2–As10) are longer than those in the cage complex [PbAsSiiPr3]6 (2.794–2.837 Å),29 indicating a weaker interaction between Pb and As. It is noteworthy to mention that molecular compounds with Pb–As bonds are scare and only known in some cage complexes.29,30
4-P10)] only,12d while the reaction of [Cp*Fe(η5-As5)] with KH resulted in a mixture of different polyarsenic Asn (n = 4, 10, 14, 18) scaffolds stabilized by {Cp*Fe} fragments.26 Herein, we were interested whether the reduction of the heavier arsenic congener [Cp*Fe(η5-As5)] with the dilithioplumbole will proceed in a similar way as that of its lighter phosphorus derivative or will rather generate other polyarsenides. The reaction between [Li2(thf)2(μ,η5-LPb)]18 and [Cp*Fe(η5-As5)]10 was carried out in a similar manner as with its phosphorus analogues. As a result, brown crystals of the reaction product [{Cp*Fe(η4-As5)}Li(η5-LPb)Li(thf)]2 (3) (Scheme 2), suitable for X-ray structural analysis were obtained in 48% yield. Analogous to the reduction of [Cp*Fe(η5-P5)], the two-electron reduction of the cyclo-As5 unit was observed (Fig. 2). Although, 3 forms a similar dimeric triple-decker structure as 2 in the solid state, there are some slight differences, as the two triple-decker subunits are symmetry-independent. The lithium atoms, which are part of the triple-decker structure (Li1 and Li2) are η4-coordinated by the cyclo-As5 units with interatomic distances ranging from 2.630(6) to 3.001(7) Å, whereas in compound 2, the corresponding lithium atoms are η3-coordinated by the cyclo-P5 unit with interatomic P–Li distances ranging from 2.484(6) to 2.794(6) Å. In these units, As5 and As10 are bent out of the As4 planes with an angle of 54.4° and 55.4°, respectively. The envelope-shaped As5 moiety and the As–As bond distances (2.376–2.442 Å) are similar to that observed in the f-element polyarsenide complexes.13a,22 The Pb–As distances of 2.9171(5) Å (Pb1–As5) and 2.9132(4) Å (Pb2–As10) are longer than those in the cage complex [PbAsSiiPr3]6 (2.794–2.837 Å),29 indicating a weaker interaction between Pb and As. It is noteworthy to mention that molecular compounds with Pb–As bonds are scare and only known in some cage complexes.29,30
To gain deeper insight into the electronic structure of complexes 2 and 3, we have performed density functional theory (DFT) calculations (for details, see ESI‡). The geometries of complexes 2 and 3 were optimized in the gas phase without any constraints (see Fig. S24, ESI‡) and retained the original structural motifs. We have also examined the Kohn Sham orbitals to get more details of electronic structure of our novel triple-decker complexes (Fig. 3 and Fig. S25, S26, ESI‡). HOMO to HOMO−3 orbitals are mainly localized on iron and phosphorus centers having very small energy difference between them. While HOMO−4 is localized on plumbole ring with larger component on Pb with an energy difference of 0.45 eV, LUMOs are localized on the plumbole and P5 rings. Similarly, in complex 3 HOMO to HOMO−3 orbitals are mainly localized on iron and arsenic centers with an energy span of 0.54 eV while the LUMOs are localized on the plumbole and As5 rings.
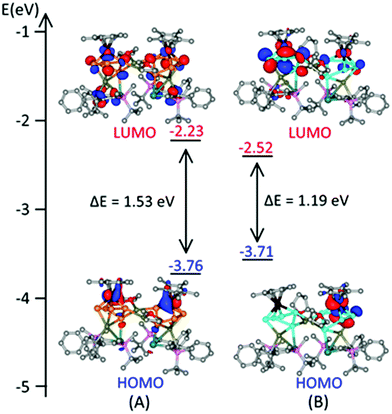 | ||
| Fig. 3 Energy diagrams and selected Kohn–Sham orbitals (side view) of UB97D/6-31G**/DEF2SVP optimized geometry of (A) complex 2 and (B) complex 3. | ||
We have also examined the topologies of Pb–P5 and Pb–As5 linkage within the framework by Bader theory.31 We were able to identify the bond critical point (BCP) for both Pb–P5 and Pb–As5 interactions in complex 2 and 3, respectively. The electron density [ρ(r)] values between the Pb–P5 and Pb–As5 pairs for complexes 2 and 3 are 0.041 and 0.038 a.u., which suggest electrostatic bonding with modest covalent contributions. The Laplacian of the electron density [∇2ρ(r)] was found to be in the range of 0.049–0.056.
In summary, we have shown a new and straightforward reaction pathway to generate unprecedented trinuclear triple-decker complexes by the reduction of pentaphosphaferrocene and pentaarsaferrocene with the dilithioplumbole. Both triple-decker complexes comprise an envelope-shaped E5 polypnictogen middle deck and a planar lead-containing heterocycle as the lower deck revealing the first Pb–Fe–Li heterotrimetallic triple-decker polypnictogenides. These results spotlight the use of the heaviest group 14 metallole dianion as a unique two-electron reducing agent to generate previously unknown structural motifs.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) with the project No. 470309834 (Ro2008/21-1 and Sche384/45-1). A. S. acknowledges funding from the DFG-funded transregional collaborative research center SFB/TRR 88 “Cooperative Effects in Homo and Heterometallic Complexes (3MET)”.
Conflicts of interest
There are no conflicts of interest.References
- (a) T. J. Kealy and P. L. Pauson, Nature, 1951, 168, 1039–1040 CrossRef CAS; (b) E. O. Fischer and W. Pfab, Z. Naturforsch., B: J. Chem. Sci., 1952, 7, 377–379 CrossRef; (c) G. Wilkinson, M. Rosenblum, M. C. Whiting and R. B. Woodward, J. Am. Chem. Soc., 1952, 74, 2125–2126 CrossRef CAS.
- E. O. Fischer and W. Hafner, Z. Naturforsch., B: Anorg. Chem., Org. Chem., Biochem., Biophys., Biol., 1955, 10, 665–668 CrossRef.
- (a) A. N. Nesmeyanov and N. S. Kochetkova, Russ. Chem. Rev., 1974, 43, 710–715 CrossRef; (b) D. Bravo-Zhivotovskii, R. Dobrovetsky, D. Nemirovsky, V. Molev, M. Bendikov, G. Molev, M. Botoshansky and Y. Apeloig, Angew. Chem., Int. Ed., 2008, 47, 4343–4345 CrossRef CAS PubMed.
- (a) C. Janiak and H. Schumann, in Advances in Organometallic Chemistry, ed. F. G. A. Stone and R. West, Academic Press, 1991, vol. 33, pp. 291–393 Search PubMed; (b) H. Bauer, A. Glöckner, A. C. Tagne Kuate, S. Schäfer, Y. Sun, M. Freytag, M. Tamm, M. D. Walter and H. Sitzmann, Dalton Trans., 2014, 43, 15818–15828 RSC.
- (a) A. Salzer and H. Werner, Angew. Chem., Int. Ed. Engl., 1972, 11, 930–932 CrossRef CAS; (b) H. Werner and A. Salzer, Synth. React. Inorg. Met.-Org. Chem., 1972, 2, 239–248 CrossRef CAS.
- (a) H. Werner, Angew. Chem., Int. Ed. Engl., 1977, 16, 1–9 CrossRef; (b) W. Siebert, Russ. Chem. Rev., 1991, 60, 784–791 CrossRef; (c) G. E. Herberich, U. Englert, F. Marken and P. Hofmann, Organometallics, 1993, 12, 4039–4045 CrossRef CAS; (d) A. R. Kudinov, A. A. Fil’chikov, P. V. Petrovskii and M. I. Rybinskaya, Russ. Chem. Bull., 1999, 48, 1352–1355 CrossRef CAS; (e) V. Beck and D. O’Hare, J. Organomet. Chem., 2004, 689, 3920–3938 CrossRef CAS.
- A. L. Rheingold, M. J. Foley and P. J. Sullivan, J. Am. Chem. Soc., 1982, 104, 4727–4729 CrossRef CAS.
- O. J. Scherer, J. Schwalb, G. Wolmershäuser, W. Kaim and R. Gross, Angew. Chem., Int. Ed. Engl., 1986, 25, 363–364 CrossRef.
- O. J. Scherer and T. Brück, Angew. Chem., Int. Ed. Engl., 1987, 26, 59 Search PubMed.
- O. J. Scherer, C. Blath and G. Wolmershäuser, J. Organomet. Chem., 1990, 387, C21–C24 CrossRef CAS.
- E. Peresypkina, A. Virovets and M. Scheer, Coord. Chem. Rev., 2021, 446, 213995 CrossRef CAS.
- (a) R. F. Winter and W. E. Geiger, Organometallics, 1999, 18, 1827–1833 CrossRef CAS; (b) T. Li, J. Wiecko, N. A. Pushkarevsky, M. T. Gamer, R. Köppe, S. N. Konchenko, M. Scheer and P. W. Roesky, Angew. Chem., Int. Ed., 2011, 50, 9491–9495 CrossRef CAS PubMed; (c) T. Li, M. T. Gamer, M. Scheer, S. N. Konchenko and P. W. Roesky, Chem. Commun., 2013, 49, 2183–2185 RSC; (d) M. V. Butovskiy, G. Balázs, M. Bodensteiner, E. V. Peresypkina, A. V. Virovets, J. Sutter and M. Scheer, Angew. Chem., Int. Ed., 2013, 52, 2972–2976 CrossRef CAS PubMed; (e) R. Yadav, T. Simler, S. Reichl, B. Goswami, C. Schoo, R. Köppe, M. Scheer and P. W. Roesky, J. Am. Chem. Soc., 2020, 142, 1190–1195 CrossRef CAS PubMed; (f) R. Yadav, T. Simler, B. Goswami, C. Schoo, R. Köppe, S. Dey and P. Roesky, Angew. Chem., Int. Ed., 2020, 132, 9530–9534 CrossRef.
- (a) N. Reinfandt, N. Michenfelder, C. Schoo, R. Yadav, S. Reichl, S. N. Konchenko, A. N. Unterreiner, M. Scheer and P. W. Roesky, Chem. – Eur. J., 2021, 27, 7862–7871 CrossRef CAS PubMed; (b) R. Yadav, B. Goswami, T. Simler, C. Schoo, S. Reichl, M. Scheer and P. W. Roesky, Chem. Commun., 2020, 56, 10207–10210 RSC; (c) R. Yadav, M. Weber, A. K. Singh, L. Münzfeld, J. Gramüller, R. M. Gschwind, M. Scheer and P. W. Roesky, Chem. – Eur. J., 2021, 14128–14137 CrossRef CAS PubMed; (d) M. E. Moussa, M. Fleischmann, G. Balázs, A. V. Virovets, E. Peresypkina, P. A. Shelyganov, M. Seidl, S. Reichl and M. Scheer, Chem. – Eur. J., 2021, 27, 9742–9747 CrossRef CAS PubMed.
- (a) M. Piesch and M. Scheer, Organometallics, 2020, 39, 4247–4252 CrossRef CAS; (b) M. Detzel, G. Friedrich, O. J. Scherer and G. Wolmershäuser, Angew. Chem., Int. Ed. Engl., 1995, 34, 1321–1323 CrossRef CAS; (c) M. Detzel, T. Mohr, O. J. Scherer and G. Wolmershäuser, Angew. Chem., Int. Ed. Engl., 1994, 33, 1110–1112 CrossRef; (d) O. J. Scherer, S. Weigel and G. Wolmershäuser, Chem. – Eur. J., 1998, 4, 1910–1916 CrossRef CAS; (e) K. Mast, J. Meiers, O. J. Scherer and G. Wolmershäuser, Z. Anorg. Allg. Chem., 1999, 625, 70–74 CrossRef CAS; (f) M. Piesch, F. Dielmann, S. Reichl and M. Scheer, Chem. – Eur. J., 2020, 26, 1518–1524 CrossRef CAS PubMed.
- M. Detzel, T. Mohr, O. J. Scherer and G. Wolmershäuser, Angew. Chem., Int. Ed. Engl., 1994, 33, 1110–1112 CrossRef.
- (a) M. Saito and M. Yoshioka, Coord. Chem. Rev., 2005, 249, 765–780 CrossRef CAS; (b) M. Saito, Acc. Chem. Res., 2018, 51, 160–169 CrossRef CAS PubMed; (c) J. Wei, W.-X. Zhang and Z. Xi, Chem. Sci., 2018, 9, 560–568 RSC.
- (a) T. Kuwabara, J.-D. Guo, S. Nagase, T. Sasamori, N. Tokitoh and M. Saito, J. Am. Chem. Soc., 2014, 136, 13059–13064 CrossRef CAS PubMed; (b) M. Saito, N. Matsunaga, J. Hamada, S. Furukawa, M. Minoura, S. Wegner, J. Barthel and C. Janiak, Dalton Trans., 2018, 47, 8892–8896 RSC.
- M. Saito, M. Nakada, T. Kuwabara and M. Minoura, Chem. Commun., 2015, 51, 4674–4676 RSC.
- M. Nakada, T. Kuwabara, S. Furukawa, M. Hada, M. Minoura and M. Saito, Chem. Sci., 2017, 8, 3092–3097 RSC.
- M. Saito, M. Nakada, T. Kuwabara, R. Owada, S. Furukawa, R. Narayanan, M. Abe, M. Hada, K. Tanaka and Y. Yamamoto, Organometallics, 2019, 38, 3099–3103 CrossRef CAS.
- (a) L. Xu, Y. Chi, S. Du, W.-X. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2016, 55, 9187–9190 CrossRef CAS PubMed; (b) S. Du, W.-X. Zhang and Z. Xi, Organometallics, 2018, 37, 2018–2022 CrossRef CAS.
- C. Schoo, S. Bestgen, M. Schmidt, S. N. Konchenko, M. Scheer and P. W. Roesky, Chem. Commun., 2016, 52, 13217–13220 RSC.
- S. Almstätter, M. Eberl, G. Balázs, M. Bodensteiner and M. Scheer, Z. Anorg. Allg. Chem., 2012, 638, 1739–1745 CrossRef.
- P. Pyykkö and M. Atsumi, Chem. – Eur. J., 2009, 15, 12770–12779 CrossRef PubMed.
- M. Saito, T. Akiba, M. Kaneko, T. Kawamura, M. Abe, M. Hada and M. Minoura, Chem. – Eur. J., 2013, 19, 16946–16953 CrossRef CAS PubMed.
- M. Schmidt, D. Konieczny, E. V. Peresypkina, A. V. Virovets, G. Balázs, M. Bodensteiner, F. Riedlberger, H. Krauss and M. Scheer, Angew. Chem., Int. Ed., 2017, 56, 7307–7311 CrossRef CAS PubMed.
- T Li, J. Wiecko, N. A. Pushkarevsky, M. T. Gamer, R. Köppe, S. N. Konchenko, M. Scheer and P. W. Roesky, Angew. Chem., Int. Ed., 2011, 50, 9491–9495 CrossRef CAS PubMed.
- N. Arleth, M. T. Gamer, R. Köppe, S. N. Konchenko, M. Fleischmann and M. Scheer, Angew. Chem.. Int. Ed., 2016, 55, 1557–1560 CrossRef CAS PubMed.
- C. von Hänisch and D. Nikolova, Z. Anorg. Allg. Chem., 2004, 630, 345–346 CrossRef.
- (a) S. Traut and C. von Hänisch, Chem. Commun., 2010, 46, 1538–1540 RSC; (b) S. Traut and C. von Hänisch, Z. Anorg. Allg. Chem., 2011, 637, 1777–1783 CrossRef CAS.
- R. F. W. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
Footnotes |
| † Dedicated to Prof. Wolfgang Beck on the occasion of his 90th birthday. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2119835–2119837. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc06182h |
| This journal is © The Royal Society of Chemistry 2022 |


