Synthesis and characterization of multifunctional superparamagnetic iron oxide nanoparticles (SPION)/C60 nanocomposites assembled by fullerene–amine click chemistry†
Manuel Cano*abd,
Rebeca Núñez-Lozanoab,
Yves Dumontc,
Chantal Larpent*d and
Guillermo de la Cueva-Méndez*ab
aBIONAND, Andalusian Centre for Nanomedicine and Biotechnology (Junta de Andalucía, Universidad de Málaga), Severo Ochoa 35, 29590 Campanillas, Málaga, Spain. E-mail: mcano@bionand.es; gdelacueva@bionand.es; Fax: +34 952367610; Tel: +34 952367611
bInstitute of Biomedical Research in Málaga, IBIMA, Avda. Jorge Luis Borges 15, Bloque 3, Planta 3a, 29010 Málaga, Spain
cGroupe d'Etudes de la Matière Condensée (GEMaC), UMR 8635, Université de Versailles St Quentin en Yvelines and CNRS, Université Paris-Saclay, 45 Avenue des Etats-Unis, 78035 Versailles, France
dInstitut Lavoisier de Versailles, UMR CNRS 8180, Université de Versailles-Saint-Quentin-en-Yvelines, Université Paris-Saclay, 45 Avenue des Etats-Unis, 78035 Versailles Cedex, France. E-mail: chantal.larpent@uvsq.fr; Fax: +33 139 254 452; Tel: +33 139 254 413
First published on 14th July 2016
Abstract
The fabrication of multifunctional nanostructures constitutes an interesting area of research. Here we used a simple fullerene–amine coupling chemistry to produce a photofunctional and antioxidant magnetic material composed of multiple C60 molecules covalently bound to core monodispersed superparamagnetic iron oxide nanoparticles (SPIONs). For this, core SPIONs were synthesized by thermal decomposition, using iron(III) acetylacetonate as the iron precursor, and the resulting hydrophobic SPIONs were coated with a thin amino-functionalized silica layer. C60 molecules were covalently linked to the core SPIONs by a simple fullerene–amine coupling reaction, a method promoting the assembly of the pursued nanocomposite. Fourier transform infrared spectroscopy, thermogravimetric analysis, and transmission electron microscopy were used all along the fabrication process to demonstrate the efficient assembly of the SPION/C60 nanocomposite. We confirm that the latter brings together the disparate physico-chemical properties of its components. Using vibrating sample magnetometry we demonstrate that the core SPION confers a superparamagnetic character to the nanocomposite. We also demonstrate that fullerenes confer photosensitizing properties to the latter, as illustrated by the photo-oxidation of 1,5-dihydroxynaphthalene. Interestingly, the hydrophobic SPION/C60 nanocomposite was easily solubilized in water using polyvinylpyrrolidone, offering the possibility of evaluating its properties in physiological conditions and paving the way to further biomedical applications. Therefore, we also demonstrate that water-soluble SPION/C60 has a good T2 relaxivity, conferred by its core superparamagnetic component, and could potentially be used as a contrast agent in magnetic resonance imaging. Furthermore, we show that the hydrophilic nanocomposite has a remarkable antioxidant capacity which is provided by its fullerene component. In summary, our work provides a facile method to produce composite nanostructures that bring together the attractive properties of monodispersed SPIONs and those of fullerene C60 molecules, which can be exploited in a broad range of applications, such as photodynamic therapy, photocatalytic oxidation of organic pollutants and radical scavenging, to name only a few.
1. Introduction
Fabrication of nanocomposites constitutes an attractive field of research in nanotechnology because this type of nanostructures have unique chemical and physical properties conferred by their individual nanoparticle (NP) constituents.1–4 Thus, the modular interconnection of NPs with different attributes enables ‘a-la-carte’ fabrication of complex nanostructures that combine disparate properties and confer to the whole novel functionalities with interesting potential applications. Another advantage of this type of assemblies is that they can be used to increase the local concentration of some of their constituents through a ‘crowding’ effect (and therefore also to enhance the intensity of the properties conferred to the nanocomposites by each individual component). This is, for instance, the case when multiple copies of superparamagnetic iron oxide NPs (SPIONs) are covalently linked to the surface of a larger silica NP core to form raspberry-like nanocomposites.1,5 The advantage of crowding SPIONs on larger silica cores is reflected by the magnetic force that can be applied to the resulting nanostructure, as a larger size (built up from smaller subunits) is generally preferable for a stronger magnetic response.6C60 constitutes an attractive component for the fabrication of nanocomposites. This fullerene is a closed-cage macromolecule made exclusively from carbon atoms and displaying an extended π-conjugated system, which confer unique physical–chemical properties.7,8 For instance, upon light excitation in the presence of free oxygen, this nanosized molecule acts as photosensitizer and produces reactive oxygen species (ROS) which can be exploited in photodynamic therapy (PDT)9,10 or in the photocatalytic oxidation of organic pollutants (PCO).11,12 C60 molecules also have high affinity for electrons, making them excellent radical scavengers with multiple applications, such as polymer stabilization or cellular protection.13,14
Combining the physical–chemical properties of C60 with those of other NPs may be interesting for certain applications. For instance, being able to modulate and/or monitor the localization of these nanosized carbon-based molecules could be useful for PDT, PCO and radical scavenging. Additionally, increasing the local concentration of C60 molecules at their desired sites of action could help to improve catalytic reactions or therapeutic outcomes. Also, magnetic separation allows a very useful approach for removing and recycling these photofunctional molecules in PCO by applying external magnetic field. All this may be achieved by linking multiple fullerenes to core NPs whose physical location can be both controlled and monitored. SPIONs are good candidates to achieve this: besides their biodegradability and biocompatibility, they display a superparamagnetic behavior (at particles diameters below 20–30 nm), thus providing a means to guide them to desired sites using a magnet and to monitor their location by magnetic resonance imaging (MRI) or magnetic particle imaging (MPI).15
Strategies to produce nanocomposites made of C60 and magnetic iron oxide NPs have been reported recently.11,16,17 However, these methods involve relatively complex synthesis steps, requiring chemical modification of C60 molecules, and that either produce large and poly-dispersed nanocomposites of complicate structures,11,17 or nanocomposites where the components are not linked to each other through covalent bonds.16 Thus, the development of methods enabling the facile and controlled production of small and well-defined monodisperse nanocomposites made of one core SPION linked to multiple pristine C60 molecules through covalent bonds, and displaying superparamagnetic, photochemical and antioxidant activities would be of great interest.
In this work, we describe a simple method to produce this type of nanocomposites using monodispersed 7 nm diameter core SPIONs coated with a thin amine-functionalized silica shell that can be easily linked to multiple pristine C60 molecules through fullerene–amine coupling click chemistry (Fig. 1A). We also demonstrate that the disparate physico-chemical properties of its components, iron oxide NPs and fullerenes, are brought together into the resulting photofunctional magnetic nanocomposite.
2. Experimental
2.1. Materials
Reagents were obtained from commercial suppliers and used without further purification. Absolute ethanol, acetic acid, chloroform, dimethylformamide (DMF), hexane, toluene, tetraethyl orthosilicate (TEOS), and (3-aminopropyl)trimethoxysilane (APTES) were from Alfa-Aesar. Diphenyl ether (99%), 1,2-tetradecanediol (97%), oleic acid (90%), oleylamine (>90%), iron(III) acetylacetonate (Fe(acac)3), polyvinylpyrrolidone (PVP-40), α,α-azodiisobutyramidine dihydrochloride (AAPH), fluorescein (FL) and 1,5-dihydroxynaphthalene (DHN) were purchased from Aldrich. Fullerene C60 (purity 99.5%) was obtained from MTR Ltd.2.2. Instrumentation
Fourier-transform infrared (FTIR) spectra were acquired using a Nicolet FT-IR 560 Magna spectrometer. Thermogravimetric analyses (TGA) were performed by simultaneous TG-DTA measurements using a Perkin-Elmer STA-600 TGA analyzer. Samples were heated from 20 °C to 600 °C at a heating rate of 5 °C min−1 under O2 flow. Magnetic properties of the SPIONs were recorded in the solid state at room temperature using a vibrating sample magnetometer (VSM, Quantum Design Inc. PPMS-9 Tesla) with fields up to 1 T. In a typical measurement, 2–15 mg were analyzed per sample. X-ray diffraction (XRD) patterns were recorded on a Siemens D5000 diffractometer with Cu-Kα (λ = 1.5405 Å) radiation over a 2θ range from 10 to 70° using a step size of 0.018 and a counting time per step of 20 s. UV-visible spectra were recorded on a Perkin-Elmer lambda19 UV/VIS/NIR spectrometer. Steady state fluorescence emission spectra were recorded on a spectrofluorimeter FluoroMax-3 equipped with 150 W xenon lamp and a slit width of 5 nm. Samples for transmission electron microscopy (TEM) analysis were prepared by drying, under ambient conditions, a dispersion of the particles on copper grids coated with Formvar film. Particles were imaged using a FEI Tecnai G2 20 TWIN TEM (100 kV). Fe concentrations were determined on an Inductively Coupled Plasma High Resolution Mass Spectroscope (ICP-HRMS, Element XR, Thermo Fisher). T1 and T2 relaxivities were calculated under two different magnetic fields, 1.5 T (Bruker Minispec) and 9.4 T (BrukerBiospec) using concentrations of NPs between 1.0 and 0.05 mM of Fe in PBS at 37 °C. Phantom of T1 and T2 were determined using an inversion-recovery sequence and the Carr-Purcell-Meiboom-Gill (CPMG) sequence, respectively.2.3. Synthesis and further characterization of monodisperse SPION–OA
Monodisperse SPION–OA of approximately 7 nm were synthesized following the standard thermal decomposition procedure reported by Sun et al.18 Briefly, 0.71 g Fe(acac)3, 2.30 g 1,2-tetradecanediol, 1.60 g oleic acid (OA), and 1.96 g oleylamine were suspended in 20 mL of diphenyl ether by mechanical stirring under an argon flow. The mixture was slowly heated to 200 °C for 1 h and then heated to reflux (∼265 °C) for 1 h. The resulting black-colored suspension was cooled to room temperature and then ethanol (∼50 mL) was added to precipitate the particles, which were separated by centrifugation (8586 × g × 10 min). The resulting SPION–OA were dispersed in hexane (∼40 mL) and precipitated again with ethanol to remove any remaining surfactants in the solution. Finally, SPIONs–OA were re-dispersed in hexane (50 mL) to produce a black NPs suspension at a concentration of ∼4 mg mL−1.The XRD spectrum of SPION–OA is showed in Fig. S1 (see ESI†). The 2θ peaks in this spectrum coincide with those corresponding to inverse spinel magnetite, according to JCPDS database (JCPDS file 19629; Joint Committee on Powder Diffraction: Swarthmore, PA), and confirmed that our material was crystalline. TEM images at different magnifications of SPION–OA are shown in Fig. S2 (see ESI†).
2.4. Synthesis of SPION–TEOS
SPION–TEOS were prepared using the silane ligand exchange reaction described in De Palma et al.19 Briefly, 10 mL of the hydrophobic SPION–OA preparation were diluted in 200 mL hexane. 300 μL of TEOS and 3 μL of acetic acid were added and the mixture was stirred using an orbital shaker at room temperature for 72 h. The surface modification leads to the precipitation of the resulting SPION–TEOS NPs, which produces a dark brown precipitate that was isolated with the aid of a magnet. To remove all excess of TEOS, the NPs were washed with 20 mL ethanol and centrifuged at 8586 × g for 5 min at least three times. The isolated particles were dried under vacuum at 50 °C before storage.2.5. Synthesis of SPION–TEOS/APTES
SPION–TEOS/APTES were prepared according the procedure described by Li et al.20 Briefly, 40 mg of dried SPION–TEOS were suspended in 20 mL of toluene in a 50 mL flask. Then, 22 μL of APTES were added to the mixture. Air was evacuated from the flask using argon flow for 20 min, and the flask was then immersed in an oil bath at 90 °C and the mixture stirred for 16 h. After this, the solution was cooled and exposed to air, and the resulting SPION–TEOS/APTES were pelleted by centrifugation (8586 × g x 10 min) and re-suspended in 20 mL of toluene in an ultrasonic bath. The NPs were pelleted again by centrifugation and re-suspended in 20 mL of acetone under sonication. Finally, the NPs were centrifuged a third time and dried under vacuum for 24 h at room temperature.2.6. Synthesis of the SPION/C60 nanocomposite
The functionalization of SPION–TEOS/APTES with fullerene-C60 was performed following a procedure adapted from Lu et al.24 Briefly, 100 mg of SPION–TEOS/APTES were added to a suspension of C60 (50 mg) in 20 mL of DMF, and the mixture was kept under magnetic stirring. Air was evacuated using an argon flow for 20 min, and stirring was continued for 48 h at room temperature. To facilitate a two phase separation of the resulting nanocomposites, toluene (∼50 mL) and water (∼25 mL) were added and the mixture was vigorously shaked. The aqueous phase was discarded and the SPION/C60 nanocomposites were isolated from the organic phase by magnetic attraction. The solvent was carefully removed and the nanocomposites were washed 3 times with toluene to remove any free C60, which could be monitored measuring the absorption peak of the latter molecule at 335 nm. Finally, the resulting particles were washed with acetone and vacuum dried at room temperature for 48 h.2.7. Photo-oxidation of DHN
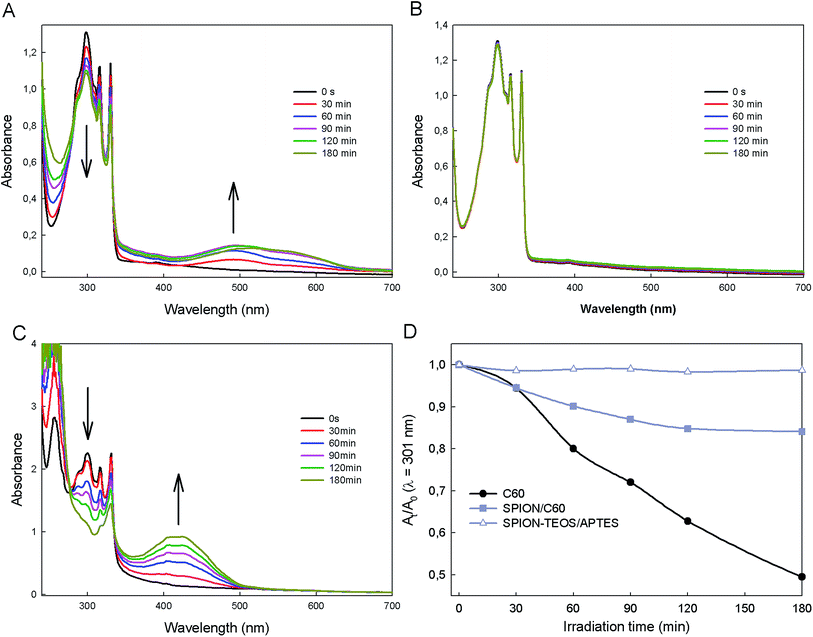
Enough SPION/C60 nanocomposite as to provide 20 mg of C60 content were re-suspended in 25 mL of a 2.0 × 10−4 M solution of DHN in ethanol in a round bottom flask. O2 was bubbled through the solution for 10 min before irradiating it using two 50 W white-light lamps. Irradiation was carried out with the round bottom flask submerged into a 0.72 M NaNO2 solution in water, which is transparent to light at wavelength higher than 385 nm. UV-vis absorption spectra were recorded for three hours at 30 minutes intervals (Fig. 4). For this a sample was taken and SPION/C60 nanocomposites were removed using a magnet before measuring UV-visible spectra of the supernatant. DHN consumption was revealed by a decrease of the UV absorption peak at 301 nm. Alternatively, the conversion of DHN into juglone could be monitored observing the appearance of a band above 400 nm in the UV-visible spectrum.27 Identical experiments using samples bearing the nanocomposite precursors (i.e. free C60 alone, SPION–TEOS/APTES alone) or no particle at all (i.e. only DHN) were carried out in parallel to perform a comparative analysis.2.8. Solubilization of SPION/C60 in water using PVP
Solubilization of SPION/C60 in water was facilitated using PVP following a procedure similar to that described in Yamakoshi et al.26 Briefly, SPION/C60 (0.8 mg) dispersed in toluene (1.0 mL) were added dropwise to 2.0 mL of chloroform containing 100 mg of PVP-40, under magnetic stirring. After this, the solvent was thoroughly evaporated and the remaining residue was re-dissolved in 2.0 mL of water under sonication for 15 min. Solubilized SPION/C60 was separated from excess of PVP by magnetic attraction and re-dispersed in the same volume of pure water. The resulting aqueous solution of SPION/C60 remains stable for more than a week at room temperature.2.9. Determination of antioxidant capacity by ORAC assay
The oxygen radical absorbance capacity (ORAC) assay28 was used to determine the antioxidant capacity of SPION/C60 nanocomposite. For this, all required solutions were made freshly in 75 mM phosphate buffer (PBS) at pH 7.4. Fluorescein sodium salt (FL) was prepared at 0.2 μM from a 4 mM aqueous stock (which was prepared fresh monthly and stored in the dark at 4 °C). A 30 mM solution of α,α-azodiisobutyramidine dihydrochloride (AAPH) was prepared in PBS and kept in an ice bath until added to the system. The stock solution of racemic 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (trolox) and ascorbic acid sodium salt (Vit C) were 20 mg L−1 and 22 mg L−1, respectively. The concentrations of SPION and C60 in the SPION/C60 stock solution were 500 mg L−1 and 18 mg L−1, respectively. Each assay was made in triplicate using polystyrene disposable cuvettes, and average values and standard deviations were calculated.In order to account for the background and any fluorescence loss during the experiments, PBS was substituted for AAPH and FL in control samples. Therefore, each sample was analyzed three times as follows:
1. Assay = 2.4 mL FL + 0.4 mL sample + 1.2 mL AAPH.
2. Control 1 (minus AAPH) = 2.4 mL FL + 0.4 mL sample + 1.2 mL PBS.
3. Control 2 (minus FL) = 2.4 mL PBS + 0.4 mL sample + 1.2 mL AAPH.
Cuvettes containing FL and sample under scrutiny were incubated at 37 °C in water bath, in the dark, for 15 min. Then, the ice-cold AAPH was added to the cuvette and the sample was excited at 485 nm to measure its fluorescence at 530 nm every 5 minute for 90 min. These analyses were carried out for trolox, SPION–TEOS/APTES, SPION/C60, AA (Vit C) and PBS (Fig. 5).
Values obtained in control 2 samples were subtracted from the corresponding time point for each assay and control 1 samples. The resulting assay value was divided by the resulting control 1 value for each time point to provide the relative fluorescein intensity, which was plotted against time (Fig. 5). The area under each resulting curve (AUC) was determined electronically. These areas were used to calculate Trolox Mass Equivalents (TME) according to the following equations (see inset in Fig. 5):
 | (1) |
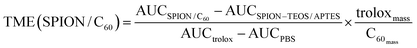 | (2) |
3. Results and discussion
3.1. Synthesis and characterization of the SPION/C60 nanocomposite
Monodisperse ∼7 nm SPIONs coated with oleic acid were synthetized in diphenyl ether by thermal decomposition of an iron(III) acetylacetonate precursor, as described by Sun et al.18 The resulting hydrophobic SPIONs were thin coated with silica through a ligand exchange/condensation reaction using tetraethyl orthosilicate (TEOS) as described by De Palma et al.19 The precipitation of the NPs as the reaction progressed was indicative of the successful silica coating that converts hydrophobic SPIONS–OA into hydrophilic SPIONS@SiO2 NPs. Then, a further silanization step was carried out using (3-aminopropyl)triethoxysilane (APTES) in toluene as reported by Li et al.,20 to coat the surface of these SPIONs with amine groups.The efficiency of all these steps was determined by Fourier transform infrared spectroscopy (FTIR, Fig. 1B). Analysis of the SPION–OA precursor revealed the expected strong band corresponding to Fe–O vibration in the magnetite core (585 cm−1). The appearance of the C–H stretching bands (2930 and 2854 cm−1) and carboxylate vibrations (1528 and 1406 cm−1) confirmed the adsorption of oleic acid onto the surface of our SPIONs, as previously reported for this type of particles.21 The analysis of the SPION–TEOS particles revealed an additional strong band at 1100 cm−1 which corresponds to Fe–O–Si bonds typically observed in silanized SPIONs,19 thus confirming the successful silanization of SPION–OA with TEOS. The latter process was further confirmed by the appearance of characteristic O–H vibrations (3425 and 1634 cm−1) in the spectra obtained for our SPION–TEOS precursors. Further silanization with APTES resulted in the appearance of a band at 1566 cm−1 that corresponds to the characteristic N–H bending of primary amines, confirming the successful synthesis of SPION–TEOS/APTES.
The successful coating and functionalization was further evidenced by thermogravimetric analyses (TGA) of all the NPs described above (Fig. 1C). TGA, which measures the percentage of weight loss induced in samples when they are heated above temperatures that induces decomposition of their organic constituents (components), enables to infer the amount of organic material coating each one of our NP precursor. The TGA curve for SPION–OA revealed a 14.34% weight loss when this NP precursor was heated above 200 °C. Total decomposition of the oleic acid layer occurred between 180 °C and 360 °C, approximately. The ligand exchange reaction performed on SPION–OA to produce SPION–TEOS displaces the oleic acid ligands and substitutes them by a organo-silica layer. Accordingly the TGA curve of SPION–TEOS shows a lower weight loss (8.1%), with a decomposition starting at approximately 200 °C. For SPION–TEOS/APTES NPs, made by adding a thin silane–amine layer on the SPION–TEOS core, the TGA curve reveals an increased weight loss upon heating (10.98%). From this, it can be inferred that 2.88% of the total mass of these NPs corresponds to the propyl-amine residues in the external (peripheral) layer, i.e. an overall content of amine groups of about 0.49 mmol g−1.
We also analyzed the superparamagnetic properties of SPION–OA, SPION–TEOS and SPION–TEOS/APTES using a Vibrating Sample Magnetometer (VSM). As expected for superparamagnetic materials, all these SPIONs lacked coercivity and remanence in magnetization (M) versus magnetic field strength (H) cycles at 300 K (Fig. 2A). Therefore, their magnetization curves cross the origin of coordinates in M–H plots (Fig. 2A). Further confirming the superparamagnetic nature of the NPs above, this analysis revealed that the saturation magnetization values (A m2 kg−1) of SPION–OA, SPION–TEOS and SPION–TEOS/APTES at 300 K were 59.1, 30.5 and 14.6, respectively. The progressive decrease of this value as silanization of the core SPION increased (first with TEOS and then with APTES) can be explained by the diamagnetic character of the silica shell. In spite of this the magnetization values of our SPION–TEOS/APTES is suitable for magnetic guiding and imaging.20,22
When a sample is composed by monodisperse non interacting magnetic NP, its magnetization curves can be adjusted to the Langevin function L(α) = nμ[coth(α) − 1/α],23 with α = μ0HμNP/kT the scaling variable. To determine whether our SPIONs preparations were monodisperse we analyzed the fitting of the data in Fig. 2A with the Langevin equation:
| M(H) ∼ nμL(α) = nμL(μ0HμNP/kT) | (3) |
Fig. 2B shows a plot of M/Mmax (reduced magnetization) vs. μ0H (applied magnetic field) calculated from data in Fig. 2A. Mmax is here magnetization at the maximum used field of 1 T. Good fitting of these curves with the Langevin function is obtained for a total magnetic moment (macrospin) of 6.89 ± 0.04 × 103 μB and 3.45 ± 0.01 × 103 μB in the case of SPION–OA and of SPION–TEOS, SPION–TEOS/APTES, respectively. These results demonstrate the monodispersity of our SPION–OA preparation and that this feature is retained all along the synthesis process to produce our core SPION–TEOS/APTES.
Next, we fabricated the SPION/C60 nanocomposite. For this, C60 molecules were grafted onto core SPION–TEOS/APTES using a simple fullerene–amine coupling reaction.24 The grafting of C60 was easily achieved by reacting an excess of pristine C60 molecules with the amino-functionalized NPs (SPION–TEOS/APTES) in DMF at room temperature. The fullerene functionalized NPs were obtained after magnetic separation and extensive washings with toluene to remove the un-reacted C60 molecules. The FTIR spectrum (Fig. 1B) of the resulting SPION/C60 nanocomposite shows peaks between 1500 and 1100 cm−1 that could be attributed to the C60 residues.25 TGA analysis further confirmed the attachment of C60 molecules to the surface of SPION–TEOS/APTES cores: the total weight loss is 15.49% for SPION/C60 nanocomposite weight when heated above 240 °C to be compared to 10.98% for the SPION–TEOS/APTES precursor (Fig. 1C). This result indicates that fullerene content in the nanocomposite accounts for 4.51% of its total weight which corresponds to about 0.06 mmol C60 per g. Interestingly, the SPION/C60 nanocomposite also maintained a superparamagnetic character, keeping a saturation magnetization value almost identical to that of its un-grafted precursor (13.52 A m2 kg−1) (Fig. 2A), and also remained monodispersed (Fig. 2B).
The size of the starting NPs and the final nanocomposites were studied by transmission electronic microscopy (TEM, Fig. 3). The starting oleic acid coated iron oxide NPs (SPION–OA) were remarkably homogeneous in size and shape, with an average diameter of 7 nm (Fig. 3A). As expected from the synthetic procedure that only adds a thin silica coat, no significant morphological changes were observed in the silanized SPIONs (Fig. 3B). Indeed, considering the Si/Fe content in the NPs, one can estimate that the thickness of the peripheral silica layer is about 0.4 nm in amine functionalized SPION–TEOS/APTES. Fullerenes and nanocomposites bearing pristine C60 molecules have a strong tendency to form large aggregates through strong hydrophobic interactions.17 Accordingly, we observed large nanocomposite clusters of 7–8 nm individual particles in our SPION/C60 preparations (Fig. 3C). This observation is indicative of an effective surface coverage of the particles by C60 molecules which makes them strongly hydrophobic, in good agreement with the C60 content which confirms a complete coverage of the surface with fullerene entities with about 30–35 C60 molecules grafted per NP.
 | ||
| Fig. 3 TEM images of (A) SPION–OA, (B) SPION–TEOS/APTES, (C) SPION/C60 in the absence or (D) the presence of PVP. | ||
Interestingly the C60 coated nanocomposites could be dispersed in aqueous solutions by using polyvinylpyrrolidone (PVP-40), a well-known and innocuous solubilizing agent for fullerenes.26 Analysis of these preparations by TEM confirmed the effective disaggregation of large aggregates into single nanocomposite units (Fig. 3D).
3.2. Analysis of the combined properties of the SPION/C60 nanocomposite
Next, we analyzed the extent to which the physico-chemical properties of SPION and C60 were imported into the nanocomposite, starting from those conferred to the nanocomposite by the fullerene moieties. We first studied the ability of SPION/C60 as photosensitizer for photo-oxidation of 1,5-dihydroxynaphthalene (DHN) into juglone.27 Progress of the reaction was followed by UV-vis absorption (Fig. 4), which enabled us to monitor both the amount of DHN oxidized (peak at 301 nm) and the amount of juglone produced (peak at 450 nm). The kinetics of this reaction were compared to those of parallel control reactions where the individual components of the nanocomposite (SPION–TEOS/APTES or pristine C60 molecules), were used as sensitizers, instead. As expected, SPION–TEOS/APTES showed no photo-oxidative behavior (Fig. 4B). However, linkage of C60 molecules to the surface of this core NP produced a nanocomposite that displayed a clear photo-oxidative character (Fig. 4A). Normalized comparison of efficiency with which free- and bound-C60 molecules transformed DHN into juglone revealed that each fullerene moiety attached to the SPION–TEOS/APTES NP core retained approximately 33% of the photo-oxidative character of its unbound precursor (Fig. 4C). This observed decrease of the photosensitizing ability of the individual grafted C60 molecules may be due to the covalent linkage with amine groups since, owing to the close vicinity of amine functions on the surface, one C60 can react with 2 or 3 amines.24 However, the average number of C60 molecules attached to each SPION–TEOS/APTES core in our nanocomposite preparations is 33, which can be deduced from the size and density of the magnetite core and their analysis by TGA. Consequently, although individual C60 molecules in the nanocomposite have a reduced photo-oxidative capacity compared to free precursors each nanocomposite has a photosensitizing activity that is roughly 10 times higher than that of pristine C60 molecules.Next, we measured whether the grafted C60 molecules conferred an antioxidant character to the SPION/C60 nanocomposite. For this we used the standard oxygen radical absorbance capacity (ORAC) assay, which monitors the loss of fluorescence intensity of a fluorescent probe (fluorescein, FL) in the presence of oxygen radicals, generated by thermal activation of a ROS initiator (AAPH).28 When oxygen-radical scavengers are added to the reaction, the fluorescent intensity of the probe persists until the scavenger is consumed. The assay readout compares the radical scavenging ability of test compounds to that of the standard radical scavenger trolox, a water-soluble vitamin E derivative.28 We monitored changes in fluorescein intensity over time upon induction of oxygen radicals in the presence of the SPION/C60 nanocomposite, and compared them to those observed in control reactions using either precursor SPION–TEOS/APTES core NPs, vitamin C, trolox, or PBS, as scavengers (Fig. 5). Our results showed that the SPION/C60 nanocomposite displayed an oxygen-radical scavenging capacity that was similar to that of vitamin C, a well characterized strong antioxidant. Calculation of the trolox mass equivalent (TME) for SPION/C60 and vitamin C (i.e. their relative antioxidant capacity compared to the same mass of trolox) revealed that the antioxidant activity of each C60 molecule bound to SPION–TEOS/APTES was only slightly lower than that of vitamin C (inset in Fig. 5). Thus, following the argument above regarding SPION![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C60 ratios in our nanocomposites, each C60 decorated NP concentrates an antioxidant capacity that is 23 fold higher than that of single vitamin C moieties.
C60 ratios in our nanocomposites, each C60 decorated NP concentrates an antioxidant capacity that is 23 fold higher than that of single vitamin C moieties.
 | ||
| Fig. 5 ORAC assay. Relative fluorescein intensity versus time and inset comparison of the trolox mass equivalent for SPION/C60 and vitamin C. | ||
Finally, we examined the extent to which the superparamagnetic properties of core SPIONs were also retained in the nanocomposite. For this we analyzed the longitudinal (T1) and transversal (T2) relaxation times of SPION/C60 nanocomposite solubilized in water with PVP, at low (1.5 T) and high (9.4 T) magnetic fields. This showed that the average transverse relaxivity values of the nanocomposite were 272 and 176 mM−1 s−1, at 1.5 T and 9.4 T, respectively (Table 1), suggesting that, due to their SPION core, the nanocomposite could be a good T2 (negative) contrast agent,22,29 which was further confirmed in an MRI-phantom experiment at 9.4 T (Fig. 6A). The magnetic character was also evident macroscopically (Fig. 6B).
| Field | Longitudinal relaxation | Transversal relaxation | r2/r1 | ||
|---|---|---|---|---|---|
| T1a (ms) | r1 [mM−1 s−1] | T2a (ms) | r2 [mM−1 s−1] | ||
| a The longitudinal (T1) and transverse (T2) relaxation times were measured at 0.5 mM Fe (as measured by ICP-AES). | |||||
| 1.5 T | 248.8 ± 4.2 | 5.87 ± 0.07 | 6.18 ± 0.02 | 272.74 ± 3.6 | 46.4 |
| 9.4 T | 1012 ± 23 | 1.35 ± 0.02 | 11.4 ± 0.05 | 176.60 ± 1.2 | 130.8 |
4. Conclusions
Our work here demonstrates the feasibility of using fullerene–amine coupling chemistry for the facile and effective construction of a SPION/C60 nanocomposite that brings together disparate physico-chemical properties characteristic of its individual components into a more complex hybrid nanostructure. Our method uses monodisperse core SPION (which is an important requisite for the fabrication of sensitive and reliable magnetic nanodevices) that can be easily produced on a large scale and at low cost. It also uses pristine C60 molecules, which can be covalently linked to core SPIONs through a very simple click chemical reaction, in the absence of any other additive. This method produces monodisperse nanocomposites of small size that bear a single SPION core and multiple C60 molecules covalently linked to the latter. This nanocomposite inherits superparamagnetic properties, conferred by its SPION-core constituent, and links them to other disparate properties, such as photochemical reactivity and antioxidant capacity, that are conferred to the final product by the fullerene moieties. Moreover, the nanocomposite is amenable to further modification, as the fabrication method used to build it leaves large numbers of free amine groups on the surface of core-SPIONs, which could be used for subsequent functionalization of this composite, and therefore the expansion of its multifunctional character and potential applications. These assemblies may have value in fields as diverse as photodynamic therapy, photocatalytic oxidation, or radical scavenging, in particular for applications where being able to determine and/or monitor the localization of the nanocomposite, or concentrating and disposing it afterwards, would be important.Acknowledgements
Research in the GCM lab is supported by the Consejería de Igualdad, Salud y Políticas Sociales (PI-0044-2014) and the Consejería de Economía, Innovación, Ciencia y Empleo (Proyecto de Investigación de Excelencia BIO 3120), from the Junta de Andalucía, as well as by the Instituto de Salud Carlos III (FIS-2013, PI13/02753, co-financed with FEDER funds); and the European Commission (FP7 Marie Sklodowska-Curie Fellowship 2013 –ref. 623906-). MC was also supported by a Postdoctoral fellowship in Nanomedicine (ref. 0399) from Consejería de Salud from Junta de Andalucía, Spain for his stay at the Institut Lavoisier de Versailles (2010–2012) and at BIONAND (2013–2014). Research in the CL and YD labs was supported by the CNRS and the University of Versailles. We acknowledge Ile de France “C'Nano – IdF” network for VSM measurements (NOVATECS program no. IF-08-1453/R).References
- S. I. Stoeva, F. Huo, J.-S. Lee and C. A. Mirkin, J. Am. Chem. Soc., 2005, 127, 15362–15363 CrossRef CAS PubMed.
- C. Mi, J. Zhang, H. Gao, X. Wu, M. Wang, Y. Wu, Y. Di, Z. Xu, C. Mao and S. Xu, Nanoscale, 2010, 2, 1141–1148 RSC.
- S. Behrens, Nanoscale, 2011, 3, 877–892 RSC.
- Z. Ji, X. Shen, X. Yue, H. Zhou, J. Yang, Y. Wang, L. Ma and K. Chen, J. Colloid Interface Sci., 2015, 459, 79–85 CrossRef CAS PubMed.
- M. Cano and G. de la Cueva-Méndez, Chem. Commun., 2015, 51, 3620–3622 RSC.
- A. B. Dávila-Ibánez, M. A. López-Quintela, J. Rivas and V. Salgueirino, J. Phys. Chem. C, 2010, 114, 7743–7750 Search PubMed.
- A. Dellinger, Z. Zhou, J. Connor, A. B. Madhankumar, S. Pamujula, C. M. Sayes and C. L. Kepley, Nanomedicine, 2013, 8, 1191–1208 CrossRef CAS PubMed.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marks and M. C. Hersam, Chem. Soc. Rev., 2013, 42, 2824–2860 RSC.
- Y. Yamakoshi, N. Umezawa, A. Ryu, K. Arakane, N. Miyata, Y. Goda, T. Masumizu and T. J. Nagano, J. Am. Chem. Soc., 2003, 125, 12803–12809 CrossRef CAS PubMed.
- P. Mroz, G. P. Tegos, H. Gali, T. Wharton, T. Sarna and M. R. Hamblin, Photochem. Photobiol. Sci., 2007, 6, 1139–1149 CAS.
- Y. Choi, Y. Ye, Y. Mackeyev, M. Cho, S. Lee, L. J. Wilson, J. Lee, P. J. Alvarez, W. Choi and J. Lee, Carbon, 2014, 69, 92–100 CrossRef CAS.
- J. Lee, S. Hong, Y. Mackeyev, C. Lee, E. Chung, L. J. Wilson, J. H. Kim and P. J. Alvarez, Environ. Sci. Technol., 2011, 45, 10598–10604 CrossRef CAS PubMed.
- P. J. Krusic, E. Wasserman, P. N. Keizer, J. R. Morton and K. F. Preston, Science, 1991, 254, 1183–1185 CAS.
- J. Tong, M. C. Zimmerman, S. Li, X. Yi, R. Luxenhofer, R. Jordan and A. V. Kabanov, Biomaterials, 2011, 32, 3654–3665 CrossRef CAS PubMed.
- R. Hufschmid, H. Arami, R. M. Ferguson, M. Gonzales, E. Teeman, L. N. Brush, N. D. Browning and K. M. Krishnan, Nanoscale, 2015, 7, 11142–11154 RSC.
- L. Yan, M. Wang, N. P. Raju, A. Epstein, L. S. Tan, A. Urbas, L. Y. Chiang and B. Hu, J. Am. Chem. Soc., 2012, 134, 3549–3554 CrossRef CAS PubMed.
- J. Shi, X. Yu, L. Wang, Y. Liu, J. Gao, J. Zhang, R. Ma, R. Liu and Z. Zhang, Biomaterials, 2013, 34, 9666–9677 CrossRef CAS PubMed.
- S. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. Li, J. Am. Chem. Soc., 2004, 126, 273–279 CrossRef CAS PubMed.
- R. De Palma, S. Peeters, M. J. Van Bael, H. Van den Rul, K. Bonroy, W. Laureyn, J. Mullens, G. Borghs and G. Maes, Chem. Mater., 2007, 19, 1821–1831 CrossRef CAS.
- Q. Li, L. Zhang, L. Bai, Z. Zhang, J. Zhu, N. Zhou, Z. Cheng and X. Zhu, Soft Matter, 2011, 7, 6958–6966 RSC.
- M. Cano, K. Sbargoud, E. Allard and C. Larpent, Green Chem., 2012, 14, 1786–1795 RSC.
- J. Kim, H. S. Kim, N. Lee, T. Kim, H. Kim, T. Yu, I. C. Song, W. K. Moon and T. Hyeon, Angew. Chem., Int. Ed., 2008, 47, 8438–8441 CrossRef CAS PubMed.
- A. E. Berkowitz, J. A. Lahut and C. E. Van Buren, IEEE Trans. Magn., 1980, 16, 184–190 CrossRef.
- Z. Lu, C. He and T. S. Chung, Polymer, 2001, 42, 5233–5237 CrossRef CAS.
- A. M. Vassallo, L. S. K. Pang, P. A. Cole-Clarke and M. A. Wilson, J. Am. Chem. Soc., 1991, 113, 7820–7821 CrossRef CAS.
- Y. N. Yamakoshi, T. Yagami, K. Fukuhara, S. Sueyoshi and N. Miyata, J. Chem. Soc., Chem. Commun., 1994, 517–518 RSC.
- S. Y. Takizawa, R. Aboshi and R. S. Murata, Photochem. Photobiol. Sci., 2011, 10, 895–903 CAS.
- R. M. Lucente-Schultz, V. C. Moore, A. D. Leonard, B. K. Price, D. V. Kosynkin, M. Lu, R. Partha, J. L. Conyers and J. M. Tour, J. Am. Chem. Soc., 2009, 131, 3934–3941 CrossRef CAS PubMed.
- H. B. Na, I. C. Song and T. Hyeon, Adv. Mater., 2009, 21, 2133–2148 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: XRD analysis (Fig. S1) and additional TEM images (Fig. S2) of SPION–OA. See DOI: 10.1039/c6ra14047e |
| This journal is © The Royal Society of Chemistry 2016 |