Surface-modified spherical activated carbon for high carbon loading and its desalting performance in flow-electrode capacitive deionization†
Hong-ran Park‡
ab,
Jiyeon Choi‡a,
Seungcheol Yanga,
Sung Jo Kwaka,
Sung-il Jeonc,
Moon Hee Han*b and
Dong Kook Kim*c
aKorea Institute of Energy Research, Jeju Global Research Center, 200 Haemajihean-ro, 695-971, Republic of Korea
bChungnam National University, Graduate School of Green Energy Technology, 99 Daehak-ro, Yuseong-gu, Daejeon 305-764, Republic of Korea. E-mail: mhhan@cnu.ac.kr; Fax: +82 42 821 8839; Tel: +82 42 821 8601
cKorea Institute of Energy Research, 152 Gajeong-ro, Yuseong-gu, Daejeon 305-343, Republic of Korea. E-mail: dokkim@kier.re.kr; Fax: +82 42 860 3133; Tel: +82 42 860 3152
First published on 29th June 2016
Abstract
We have synthesized a new type of activated carbon (AC) containing ion-selective functional groups, trimethylammonium (AC-N) for anodes and sulfonate (AC-S) for cathodes, for high carbon loading of flow-electrodes. The AC-N and AC-S were partially covered with a 50 nm-thick polymer layer and their surfaces became more hydrophilic than that of bare AC. In the case of bare AC, the maximum carbon concentration in the flow electrodes was 10%, while in the case of the surface-modified AC (AC-N and AC-S), it increased to a maximum of 35% and decreased the viscosity due to the electrostatic repulsion. Moreover, with the increase in carbon concentration, the salt removal efficiencies were improved from 8.2% to 27.7%. This increase in efficiency was attributed to the formation of percolating networks, which occurred because of high carbon loading. The resulting improvement in electronic conductivity at higher loading led to a higher current, and thus an improved salt removal efficiency. Therefore, we expect that the surface-modified AC electrode can be used as a dispersant for hydrophobic AC particles in aqueous solution, as well as in flow electrodes to improve desalting performance in FCDI systems.
Introduction
Capacitive deionization (CDI) is an electrochemical method for removing ions dissolved in aqueous solutions.1 Recently, due to its advantages as an energy-efficient, cost-effective, and eco-friendly process, it has emerged as a promising desalination technique for water with a low or moderate salt concentration.2,3 CDI involves of a pair of porous carbon electrodes and salt water as an influent.4,5 In particular, CDI with an ion-exchange membrane (IEM) has been named membrane CDI (MCDI). The process of (M)CDI is based on electrical double layers (EDLs) formed on interfaces between carbon electrodes, such as porous activated carbons, and an electrolyte, such as NaCl solution.6 Salt ions are adsorbed and stored in the EDLs when a cell voltage in the range of 1–1.2 V is applied between the two carbon electrodes. It is also known that MCDI has much better salt removal efficiency than CDI because IEMs play a role in separating the counter-ions and the co-ions.6 Research on (M)CDI can be classified into the areas of concern of theoretical understanding,5–7 materials,8–11 and process optimization.7,12However, in conventional (M)CDI, which is based on static electrodes, salt removal has only been allowed to proceed for a finite time until the porous electrodes are fully charged. In order to regenerate the porous electrodes fully, until they are completely ion-adsorbed, the desalting process should be stopped because charging and discharging cannot be achieved in the same cell at the same time. This means that it is a discontinuous process, which is a major drawback because these operations require complicated handling of effluent (desalted water) and influent (salt water) at different times in the same cell.13 Finally, architectures of (M)CDI cells have been developed to overcome this limitation, resulting in a static electrode system.
Flow-electrode capacitive deionization (FCDI) is a new and environmentally friendly technology for seawater desalination; it is based on MCDI. This system uses a suspension of flow electrodes composed of porous activated carbon and electrolyte (NaCl solution),14,15 and this is a representative architecture for new MCDI systems. The concept of a flow-electrode using carbon-based slurry was first reported by Kastening;16 this concept has recently been used for various applications such as electrochemical flow capacitors17 and semisolid lithium flow cells,18 as well as for FCDI.14,15 To the best of our knowledge, FCDI was the first application that allowed electrochemical desalination using EDLs within the range of seawater concentration; this system achieved encouraging results for salt removal efficiency, with an efficiency value of over 95% for an influent NaCl concentration of 3.5 mass%.14
As it does not require an in situ discharge step for regenerating the ion-saturated carbon electrodes, this process has the advantage of easy scale-up as well as being a continuous process. However, although FCDI has shown lots of potential as a powerful and alternative process for seawater desalination for a range of high salt concentrations, porous carbon materials should be developed for use as flow electrodes to allow the carbon load content to be as high as possible in order to improve the salt removal efficiency. Reduction in the viscosity of the flow electrode is desirable to decrease the amount of energy required for pumping.19
Porous activated carbon (AC) is a major component of flow-electrodes; it can easily agglomerate in an aqueous environment due to hydrophobic interactions. This property leads to clogging of the flow channel during the desalting process if AC is used as the flow electrode. The electrochemical performance is also dependent on the size,20 morphology,21,22 specific surface area,23 chemical surface charge24–26 and other physical properties of AC.27 In particular, high carbon mass loading in the flow-electrode enhances the ion removal efficiency because the connectivity between the carbon particles accelerates charge transportation and storage, and improves conductivity through the percolating networks.23,28,29 However, as the flow-electrode should flow along a narrow channel, the rheological properties are an important factor to ensure that there is no clogging. Thus, it is desirable that the flow electrodes have a low viscosity and a high carbon mass loading. A lot of research into flow-electrodes has been focused on the formation of structures with high carbon mass loading through various methods, such as oxidation and surface modification. For example, Hatzell et al.30 reported that granular AC with a heteroatom surface can be used as a flow electrode; its use caused the gravimetric capacitance to increase by up to 25%, resulting in an increase in the carbon loading. Boota et al.31 suggested that AC wrapped with highly conductive reduced graphene oxide sheets would improve the capacitance due to higher connectivity in the flow electrode.
Here, we hypothesize that a surface-modified AC suspension with ionic head-groups can be used as a flow electrode, one that will be well-dispersed in an aqueous electrolyte and that will lessen the intrinsic viscosity of the flow electrode. Besides this, ionic functional groups on the surface of the AC can help in the improvement of the ion adsorption efficiency to exclude co-ion expulsion.32 In this study, we prepared flow electrodes that were composed of spherical and porous activated carbon (AC) that was covered with ion-exchange polymers using the emulsion polymerization method. For the cathode and anode suspensions, ACs coated with anion- or cation-exchange polymers were denoted AC-N or AC-S because of the trimethylammonium (–N+(CH3)3) and sulfonate (–SO3−) groups, respectively (Fig. 1(a)). We characterized the surface-modified AC electrodes and confirmed the desalting effect through the presence of a high carbon mass loading in the FCDI system.
 | ||
| Fig. 1 Schematic representation of (a) surface modification of ACs and (b) The FCDI system using AC-S and AC-N as cathode and anode, respectively. | ||
Experimental
Materials and methods
Elemental analysis of the surface composition was performed via X-ray photoelectron spectroscopy (XPS, Kratos Axis Nova, UK) using AlKα radiation with a 20 mA current, 15 kV voltage (300 W), and a base pressure of 10−8 to 10−9 Torr in the sample chamber. The incident monochromated X-ray beam was focused on a 0.7 mm × 0.3 mm area of the surface. Nitrogen adsorption isotherms were measured at 77 K (ASAP 2010, USA).
The surface-modified AC particles were also characterized using a thermogravimetric analyzer (TGA; HI-Res TGA 2950, TA instrument, USA). TGA thermograms were recorded at a heating rate of 10 °C min−1 from 100 to 800 °C under an N2 atmosphere.
The viscosity of each suspension electrode was measured with a cone/plate rheometer (DV-III Ultra Rheometer, Brookfield, USA) with a spindle of CPE-51 and CPE-41 at a shear rate ranging from 50 to 950 s−1. For these tests, suspended electrodes were prepared by mixing AC, AC-N, and AC-S with an electrolyte solution of NaCl (2.5%). Each of these samples had a concentration ranging from 5–35%.
As can be seen in Fig. 1(b), the FCDI unit cell was mainly made of three parts: a pair of endplates (polyvinylchloride), a pair of graphite current collectors (104 mm × 56 mm × 12 mm), and a pair of ion exchange membranes (Neosepta AMX and Neosepta CMX, Astom Corp., Japan). The current collector had an intagliated flow channel whose width and depth were 2 mm and 2 mm, respectively; the collector is serpentine in shape. A flow channel was connected from the inlet to the outlet; the length per column was 30 mm and the total number of columns was 23. The contact area between the ion exchange membranes and the flow electrode was 12.7 cm2. The area ratio over the total membrane was 0.22. The flow channel was a gasket (silicone rubber, 0.3 mm), and a spacer (polyester, 0.3 mm) was used for stacking between the ion exchange membranes.
| Concentration (%) | Carbon content (g) | NaCl (g) | Deionized water (g) |
|---|---|---|---|
| 10 | 20 | ||
| 20 | 40 | ||
| 25 | 50 | 5 | 200 |
| 30 | 60 | ||
| 35 | 70 |
To determine the desalting performance, a constant cell voltage of 1.2 V was maintained using a potentiostat (Iviumstat Standard, Ivium Technologies, the Netherlands). The salt concentration in the effluent was calculated from the effluent electrical conductivity, which was measured every 10 s using a conductivity meter (S47, Mettler-Toledo, Switzerland). The experiment was conducted after confirming the stabilization of effluent concentration through the conductivity change.
The salt removal efficiency (η) was calculated using the equation below
 | (1) |
The current efficiency (λ) was calculated according to:
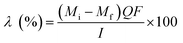 | (2) |
Results and discussion
Morphology and porosity characterizations
Fig. S1† shows the surface morphologies and thicknesses of the polymer layers before and after polymerization on the AC surfaces. While bare AC particles seemed significantly rough (Fig. S1a and d†), surface-modified AC particles, AC-N and AC-S, changed and had smooth surfaces. Moreover, as can be seen in the TEM images (Fig. S1e and f†), polymer layers with anion/cation-exchange functional groups were formed and can be observed to have an approximate thickness of 50 nm on the AC surfaces.To compare the AC and surface-modified ACs, and to determine differences, adsorption isotherms of N2 on the carbon particles were used to quantify the changes in the pore structure and to determine the specific surface area. The results are shown in Fig. S2; †it can be seen that these results correspond to a type 1 isotherm.33 The hysteresis loops were flat in the intermediate and final sections; it is evident that a microporous structure dominates in the AC structure and this microporous structure includes AC-N and AC-S particles. The pore properties of AC, AC-N, and AC-S are summarized in Table 2.
| Sample | Specific surface area (m2 g−1) | Average pore diameter (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|
| AC | 3020 | 2.49 | 1.63 |
| AC-N | 1064 | 2.52 | 0.54 |
| AC-S | 1638 | 2.44 | 0.85 |
The AC particles showed a specific surface area of 3020 m2 g−1, while the AC-N and AC-S samples had surface area values of 1064 and 1638 m2 g−1, respectively. Although both AC-N (2.01 nm) and AC-S (2.06 nm) maintained constant average pore diameters, in contrast to AC (2.10 nm), the pore volumes of AC-N (0.54 cm3 g−1) and AC-S (0.85 cm3 g−1) decreased steeply from that of AC (1.63 cm3 g−1). The decrease in the specific surface area and pore volume can be attributed to the ion-exchange polymer coverage on the AC surfaces. When both ionic monomers and AC particles were mixed in the aqueous electrolyte, monomers formed micelles around the AC surfaces and were then polymerized by the thermal initiator. This is a kind of emulsion polymerization. In effect, the polymer seemed to partially cover micro- and meso-pores, which resulted in decreased specific surface area and pore volume by about half, compared with bare AC.
In addition, the thermal properties of AC, AC-N, and AC-S were measured by TGA (Fig. 2). Both AC-N and AC-S exhibited profiles of two-step weight loss. In AC-N, the thermal decomposition of the ammonium groups began at around 170 °C and was complete at approximately 270 °C.34 In this temperature region, the weight decreased by 10%. In AC-S, the weight loss at above 250 °C indicates the desulfonation of sulfonic acid groups, the decrease in weight had a value of 9%.35 The last weight loss region, occurring at 600–700 °C, is attributed to the decomposition of the AC particles.
Characterization of surface chemistry by FT-IR and XPS
FT-IR was used to confirm the surface modification of the AC particles: this process was used to determine whether trimethylammonium or sulfonate groups on AC-N and AC-S were covered on the AC surfaces or not. Fig. 3 shows the FT-IR spectra of (a) AC, (b) AC-N, and (c) AC-S. In AC-N, peaks corresponding to N–H bending and –C–N stretching were observed at 1563 and 1092 cm−1, respectively. The peak at 1647 cm−1 corresponds to the asymmetric bending vibration of the quaternary amine cation.36 Moreover, the symmetric vibration of the SO3− group was assigned to the peak at 1181 cm−1 and the peak at 1101 cm−1 was attributed to the bending vibration of the phenyl ring. Stretching of the aromatic ring was observed at 1625 cm−1. The peaks of weak intensity in the range of 2893–2846 cm−1 are due to the stretching vibration of the C–H bonds, irrespective of whether the sample is AC-N or AC-S.37XPS is a powerful method that can be used to confirm the presence of surface functional groups and to obtain the chemical composition. For further investigation, the surface chemistry of AC-N and AC-S was characterized by XPS, the results are displayed in Fig. 4 and the respective atomic concentrations are listed in Table 3. As can be seen in Fig. 4(A) and in Table 3, AC particles mostly consisted of carbon and oxygen, with atomic concentrations of 93.8 and 6.2%, respectively. However, sulfur (1.09%) and nitrogen (5.23%), as well as carbon and oxygen, in accordance with the sulfonate and trimethylammonium groups, were observed in the AC particles after surface modification. Chloride and sodium as counter-ions were detected as well. Fig. 4(B) shows the XPS spectra corresponding to AC-S. In particular, the C 1s peak (a) is deconvoluted into the C–C (285.0 eV) and C–N (283.3 eV) peaks; the N 1s peak (b) is divided into two peaks of N–R (401.8 eV) and R–NH2 (398.9 eV).37,38 Moreover, in the spectral analysis of AC-S (Fig. 4(C)), it was possible to deconvolute the carbon peak into two components corresponding to C–C (285.0 eV) and C–S (288.1 eV),31 though the intensity relating to the C–S bond was slightly weak. As these peaks are ascribed to the trimethylammonium groups (–N+(CH3)3) and sulfonate groups (–SO3−), the achievement of successful surface modification of the AC particles by emulsion polymerization was confirmed by the presence of sulfur and nitrogen.
 | ||
| Fig. 4 XPS spectra of bare AC, AC-N, and AC-S samples. (A) Wide-scan of (a) AC, (b) AC-N and (c) AC-S. (B) (a) C 1s and (b) N 1s of AC-N, and (C) (a) C 1s and (b) S 2p of AC-S. | ||
| Sample | Atomic concentration% | |||||
|---|---|---|---|---|---|---|
| C 1s | O 1s | N 1s | Cl 2p | Na 1s | S 2p | |
| AC | 93.79 | 6.21 | — | — | — | — |
| AC-N | 92.69 | 1.56 | 5.23 | 0.53 | — | — |
| AC-S | 87.83 | 9.41 | — | — | 1.67 | 1.09 |
Rheological properties and deionization performance
The rheological properties are an important factor in the FCDI system, one that must be considered for suspension electrodes. Fig. 5 shows the viscosity of AC, AC-N, and AC-S as a function of shear rate according to the suspension concentrations. All of the samples were non-Newtonian fluids and exhibited shear-thinning behavior. The suspensions were analyzed with the Ostwald-de Waele power law model, using eqn (3):| η = kγn−1 | (3) |
 | ||
| Fig. 5 Rheograms of flow electrodes of (a) AC-N and (b) AC-S according to the carbon concentration in 2.5 wt% NaCl electrolyte. | ||
Table 4 summarizes these factors. All of the samples showed shear-thinning behavior. Despite their high carbon mass loading, the AC-S and AC-N electrodes at 30% demonstrated significantly lower viscosity compared to that of the AC electrode, which had a value of 12.5%. The flow electrode improved the flowability; this improvement resulted from a decrease in the hydrophobic interactions between AC particles as the outer layer of the AC became more hydrophilic, increasing the electrostatic repulsion. After the surface modification, isoelectric points against AC-S and AC-N were evaluated using the titration method (Fig. S3†). While bare AC showed an isoelectric point at pH 6, the isoelectric point of AC-S was shifted to a lower pH value of 2; that of AC-N moved toward a higher pH value of 9. This movement results from surface modification by cationic (ammonium group) and anionic (sulfonate group) monomers. Besides this, a significant decrease in the viscosity is indirect evidence that supports the presence of electrostatic repulsion due to these functional groups. In addition, it was thought that the viscosity difference between AC-N and AC-S was attributed to the extent of surface modification as recognized by specific surface area and pore volume (Table 2).
| Material | Carbon concentration (%) | Consistency index k | Shear thinning index n | R2-Value |
|---|---|---|---|---|
| AC | 12.5 | 373.13 | 0.422 | 0.986 |
| AC-N | 10 | 17.458 | 0.619 | 0.903 |
| 25 | 53.851 | 0.614 | 0.968 | |
| 30 | 106 | 0.541 | 0.945 | |
| AC-S | 10 | 25.596 | 0.577 | 0.970 |
| 25 | 130.15 | 0.468 | 0.995 | |
| 30 | 339.65 | 0.406 | 0.955 |
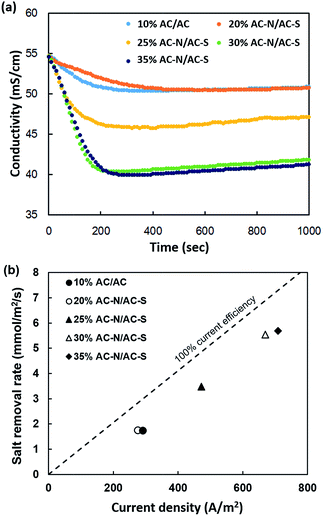
The salt removal efficiencies were calculated using eqn (1) in the FCDI system according to the concentration of the suspension electrode. The conductivity matches the ion concentrations of the NaCl solution in the effluent. That is, when external power is applied, ionic adsorption occurs around the flow electrode through the EDLs. Fig. 6(a) shows the results of changes in the conductivity as a function of time. The results are summarized in Table 5. In the case of the AC/AC flow electrode (10%), the maximum desalting efficiency and the current efficiency were 8.2 and 74.7%, respectively. However, in the case of the AC-N/AC-S flow electrode, the maximum desalting efficiency increased along with an increase in the carbon loading. At high carbon content, such as 35%, the desalting rate was 27.2%. The current of the AC-N/AC-S flow electrode was stable during the salt removal test. In order to calculate the current density, we used the effective membrane area, as this takes into account the membrane in contact with the flow electrode. Thus, the membrane area of the open flow channel was chosen because this is where ion adsorption occurs. According to the concentration of the AC-N/AC-S flow electrode, the values of current density (A m−2) increased to 275 (20%), 472 (25%), 669 (30%), and 709 (35%); their efficiencies were over 60% as shown in Fig. 6(b).
| Flow electrode composition (anode/cathode) | Concentration (%) | Maximum salt removal efficiency (%) | Current efficiency (%) |
|---|---|---|---|
| AC/AC | 10 | 8.4 | 74.7 |
| AC-N/AC-S | 20 | 8.2 | 68.7 |
| 25 | 16.7 | 76.7 | |
| 30 | 26.5 | 81.3 | |
| 35 | 27.2 | 85.6 |
Although the surface-modified flow electrodes (AC-N and AC-S) showed a lower salt removal efficiency than the bare AC flow electrode at the same concentration (data not shown), their efficiency can compensate through high carbon loading of over 20%. While the salt removal test proceeded in the FCDI unit cell for 1000 s, the conductivities decreased steeply during the initial 200 s and gradually increased for the remainder of the test. At the same time, all of the samples were observed to exhibit a decrease in current. The increase in the conductivity and the decrease in current are attributed to the saturation of the ion adsorption in the flow electrode; this occurred because, in this study, we used a two tank mode, as was referred to earlier, and limited the amount of the flow electrode to 200 mL per electrode. It was confirmed that the amount of carbon loading affects the salt removal efficiency and the current as well.
Unfortunately, bare AC could not be used as a flow electrode over the range of 12.5% due to the significantly high viscosity (Fig. 5). The flow electrodes, composed of bare AC particles, easily aggregate and clog the microchannel due to their high van der Waals interactions in aqueous electrolytes. For these reasons, the usage of bare AC particles might not be advantageous in the case of flow electrodes. However, it can be seen that AC-N and AC-S flow electrodes, with a high carbon concentration of 35%, operated smoothly and showed a much higher desalting efficiency (27%) than did the other electrodes.
Salt adsorption depends on the specific surface area of AC, with increased adsorption resulting from a larger available surface area. Thus, modification of the AC surface may be detrimental to salt adsorption. However, the partial modification of AC by our polymer coating meant that some conductive AC remained exposed. This partial coating negated the detrimental effects of poor conductivity and low specific surface area. We found that a percolating network was readily formed among the surface-modified AC particles at high carbon loading, and concluded that the salt removal efficiency increased, despite the low specific surface area of the surface-modified AC.
Surface-modified AC particles, AC-N and AC-S, can be used as electrode materials irrespective of cathode and anode, respectively. Specifically, the flow electrode (500 mL) composed of AC-N particles (35%) in a 2.5% NaCl solution was used for salt removal tests in 1-tank mode (Fig. S4†). The salt removal efficiency was 20.5% and the current efficiency was 85.0% (Table S1†). In 1-tank mode, the mixing of the flow-anode and the flow-cathode can lead to desorption of Na+ and Cl− ions adsorbed on the AC-N particles by electrical neutralization.15 However, despite having the same carbon content (AC-N, 35%), the desalting efficiency was slightly low, compared to 2-tank mode (AC-N/AC-S, 35%). It might be explained by relatively low polarizability and high resistance due to the ammonium groups of the AC-N particles in the cathode side.
Consequently, as carbon loading is a key factor in improving the desalting efficiency in the flow electrode, surface-modified AC particles are favourable for reducing the viscosity at high loading. The use of such particles should result in an increase in the desalting efficiency. Subsequently, this result means that AC-N or AC-S can be used as dispersants. In addition, current efficiencies increased as the carbon loading concentration increased from 20 to 35%. Further investigation is ongoing to understand whether the ion exchange polymer on the outer layer affects the capacitance and resistance during the FCDI process.
Conclusions
To achieve high carbon mass loading in a flow electrode, we successfully performed surface modification of AC particles, which were covered with a polymer with ion-selective functional groups using the emulsion polymerization method. Due to its ammonium and sulfonate groups, which induced electrostatic repulsion, the ion exchange polymer that covered the AC acted as a dispersant. In particular, these AC-S and AC-N samples decreased the viscosity in the suspension with a high loading of carbon. Subsequently, high loading of carbon content enabled an improvement in the salt removal efficiency in the FCDI system. Therefore, we expect that this material will have high potential to homogeneously disperse AC particles without aggregation in an aqueous environment. We are proceeding with further investigation regarding seawater desalination using FCDI unit cells with flow electrodes of high carbon mass loading mixed with AC as a salt adsorbent and surface-modified AC as a dispersant.Acknowledgements
This work was conducted under the framework of the Research and Development Program of the Korea Institute of Energy Research (KIER) (B6-2483).Notes and references
- S. Porada, R. Zhao, A. van der Wal, V. Presser and P. M. Biesheuvel, Prog. Mater. Sci., 2013, 58, 1388–1442 CrossRef CAS.
- M. A. Anderson, A. L. Cudero and J. Palma, Electrochim. Acta, 2010, 55, 3845–3856 CrossRef CAS.
- Y. Oren, Desalination, 2008, 228, 10–29 CrossRef CAS.
- J.-B. Lee, K.-K. Park, H.-M. Eum and C.-W. Lee, Desalination, 2006, 196, 125–134 CrossRef CAS.
- P. M. Biesheuvel and A. van der Wal, J. Membr. Sci., 2010, 346, 256–262 CrossRef CAS.
- P. M. Biesheuvel, R. Zhao, S. Porada and A. van der Wal, J. Colloid Interface Sci., 2011, 360, 237–248 CrossRef PubMed.
- R. Zhao, O. Satpradit, H. H. M. Rijnaarts, P. M. Biesheuvel and A. van der Wal, Water Res., 2013, 47, 1941–1952 CrossRef CAS PubMed.
- H. Li and L. Zou, Desalination, 2011, 275, 62–66 CrossRef CAS.
- Y.-J. Kim and J.-H. Choi, Water Res., 2012, 46, 6033–6039 CrossRef CAS PubMed.
- X. Gao, A. Omosebi, J. Landon and K. Liu, Energy Environ. Sci., 2015, 8, 897–909 CAS.
- J. Lee, S. Kim and C. Yoon, Energy Environ. Sci., 2014, 7, 3683–3689 CAS.
- R. Zhao, P. M. Biesheuvel and A. van der Wal, Energy Environ. Sci., 2012, 5, 9520–9527 CAS.
- M. E. Suss, S. Porada, X. Sun, P. M. Biesheuvel, J. Yoon and V. Presser, Energy Environ. Sci., 2015, 8, 2296–2319 CAS.
- S.-I. Jeon, H.-R. Park, J.-G. Yeo, S. Yang, C. H. Cho, M. H. Han and D. K. Kim, Energy Environ. Sci., 2013, 6, 1471–1475 CAS.
- S.-I. Jeon, J.-G. Yeo, S. Yang, J. Choi and D. K. Kim, J. Mater. Chem. A, 2014, 2, 6378–6383 CAS.
- B. Kastening, T. Boinowitz and M. Heins, J. Appl. Electrochem., 1997, 27, 147–152 CrossRef CAS.
- V. Presser, C. R. Dennison, J. Campos, K. W. Knehr, E. C. Kumbur and Y. Gogotsi, Adv. Energy Mater., 2012, 2, 895–902 CrossRef CAS.
- M. Duduta, B. Ho, V. C. Wood, P. Limthongkul, V. E. Brunini, W. C. Carter and Y.-M. Chiang, Adv. Energy Mater., 2011, 1, 511–516 CrossRef CAS.
- G. J. Doornbusch, J. E. Dykstra, P. M. Biesheuvel and M. E. Suss, J. Mater. Chem. A, 2016, 4, 3642–3647 CAS.
- C. Portet, G. Yushin and Y. Gogotsi, J. Electrochem. Soc., 2008, 155, A531–A536 CrossRef CAS.
- M. Boota, K. B. Hatzell, M. Beidaghi, C. R. Dennison, E. C. Kumbur and Y. Gogotsi, J. Electrochem. Soc., 2014, 161, A1078–A1083 CrossRef CAS.
- J. W. Campos, M. Beidaghi, K. B. Hatzell, C. R. Dennison, B. Musci, V. Presser, E. C. Kumbur and Y. Gogotsi, Electrochim. Acta, 2013, 98, 123–130 CrossRef CAS.
- S. Porada, L. Borchardt, M. Oschatz, M. Bryjak, J. S. Atchison, K. J. Keesman, S. Kaskel, P. M. Biesheuvel and V. Presser, Energy Environ. Sci., 2013, 6, 3700–3712 CAS.
- X. Gao, S. Porada, A. Omosebi, K.-L. Liu, P. M. Biesheuvel and J. Landon, Water Res., 2016, 92, 275–282 CrossRef CAS PubMed.
- X. Gao, A. Omosebi, J. Landon and K.-L. Liu, Environ. Sci. Technol., 2015, 49, 10920–10926 CrossRef CAS PubMed.
- P. M. Biesheuvel, H. V. M. Hamelers and M. E. Suss, Colloids Interface Sci. Commun., 2015, 2, 1–5 CrossRef.
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- S. Porada, D. Weingarth, H. V. M. Hamelers, M. Bryjak, V. Presser and P. M. Biesheuvel, J. Mater. Chem. A, 2014, 2, 9313–9321 CAS.
- K. B. Hatzell, M. Boota, E. C. Kumbur and Y. Gogotsi, J. Electrochem. Soc., 2015, 162, A5007–A5012 CrossRef CAS.
- K. B. Hatzell, M. C. Hatzell, K. M. Cook, M. Boota, G. M. Housel, A. Mcbride, E. C. Kumbur and Y. Gogotsi, Environ. Sci. Technol., 2015, 49, 3040–3047 CrossRef CAS PubMed.
- M. Boota, K. B. Hatzell, M. Alhabeb, E. C. Kumbur and Y. Gogotsi, Carbon, 2015, 92, 142–149 CrossRef CAS.
- J. Yang, L. Zou and N. R. Choudhury, Electrochim. Acta, 2013, 91, 11–19 CrossRef CAS.
- D. J. Shaw, Introduction to colloid and surface chemistry, Butterworth Heinemann Ltd, oxford, 1992, 4th edn, ch. 5, pp. 121–123 Search PubMed.
- H. He, B. Adzima, M. Zhong, S. Averick, R. Koepsel, H. Murata, A. Russell, D. Luebke, A. Takahara, H. Nulwala and K. Matyjaszewski, Polym. Chem., 2014, 5, 2824–2835 RSC.
- L. Zhou, X. Lin, T. Huang and A. Yu, J. Mater. Chem. A, 2014, 2, 5117–5123 CAS.
- B. Yameen, M. Ali, M. Alvarez, R. Neumann, W. Ensinger, W. Knoll and O. A. Azzaroni, Polym. Chem., 2010, 1, 183–192 RSC.
- X. Han, L. Zhang and C. Li, RSC Adv., 2014, 4, 30536–30541 RSC.
- L. Meng, X. Zhang, Y. Tang, K. Su and J. Kong, Sci. Rep., 2015, 5, 7910 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM, TEM images, nitrogen adsorption isotherm and pore size distribution of AC materials. And point of zero charge (PZC) and profiles of NaCl conductivity changes and current during 1-tank mode. See DOI: 10.1039/c6ra02480g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |