Block copolymer-guided fabrication of shuttle-like polyaniline nanoflowers with radiating whiskers for application in supercapacitors
Hongyu Mi*a,
Jiapan Zhoua,
Zongbin Zhaob,
Chang Yub,
Xuzhen Wangb and
Jieshan Qiu*b
aXinjiang Laboratory of Advanced Functional Materials, School of Chemistry and Chemical Engineering, Xinjiang University, Urumqi 830046, China. E-mail: mmihongyu@163.com; Fax: +86 991 8582809
bCarbon Research Laboratory, School of Chemical Engineering, State Key Lab of Fine Chemicals, Dalian University of Technology, Dalian 116023, China. E-mail: jqiu@dlut.edu.cn; Fax: +86 411 84986072
First published on 24th November 2014
Abstract
Superfine shuttle-shaped polyaniline (PANI) nanoflowers with radiating whiskers at the edge of the flowers have been synthesized using a block copolymer-assisted reverse microemulsion system. The copolymer poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (P123) served as the surfactant stabilizing reverse micelles for regular assembly of the flower-like structures. The flowers formed had widths of ca. 40–150 nm and lengths of ca. 80–180 nm, far less than the typical sizes reported (over several microns). The correlation between some important factors (polymerization method, P123 and aniline concentrations, and reaction time) and the morphology was discussed, and a plausible mechanism for formation of the nanoflowers was put forward. More importantly, the unique architecture showed good electrochemical properties with a high reversible capacitance (622 F g−1 at 0.5 A g−1, and 484 F g−1 at 2 A g−1) and good durability (76% capacitance retention at 5 A g−1 after 1000 cycles) due to the nano size and V-type channels. These characteristics make it promising for supercapacitor applications.
1. Introduction
Owing to its promising prospects in the areas of supercapacitors, batteries, chemical sensors, corrosion protection and drug delivery, polyaniline (PANI), as one of the popular conductive polymers, has aroused lots of attention.1–3 Under increasingly serious resource and environmental problems, PANI, especially nanostructured PANI, is more often studied as a supercapacitor electrode material, and nanostructured PANI achieves electrochemical properties superior to its bulk counterparts owing to a nano-size effect that offers larger electrode–electrolyte interfaces, shorter ion/electron transport lengths, faster electrochemical responses and better strain buffering.4–6 Hence, much effort has focused on the fabrication and assembly of nano-sized PANI with a controllable doped structure and morphology. Thus far, diverse microstructures ranging from one-dimensional (1D) tubes/wires/fibers/rods/belts7–15 and two-dimensional (2D) plates/film/rings16–19 to three-dimensional (3D) flowers20–25 have been made. Of those, 1D PANI has been frequently synthesized using many approaches, such as a conventional chemical oxidation method, interfacial polymerization method, soft/hard templating method, microemulsion method and electrochemical method, and it showed enhanced electrochemical properties. For instance, Alshareef et al.8 assembled full-cell pseudocapacitors based on PANI nanotubes obtained by a reactive oxide templating method, which delivered a remarkable energy density of 84 W h kg−1 and a power density of 182 kW kg−1. Sk et al.26 synthesized heparin-controlled porous PANI nanofiber networks using a chemical oxidation method, which showed a capacitance of about 732.18 F g−1 at 1 mA and a long cycle life (72.28% retention after 1000 cycles). Mallouk and coworkers27 fabricated a template grown PANI, and it showed a capacitance of 700 F g−1 at a current density of 5 A g−1. More recently, Qin et al.28 achieved core–shell PANI/PPy using in situ chemical polymerization and further seed polymerization, which exhibited 346 F g−1 at a scan rate of 5 mV s−1. However, approaches to 3D PANI are the least reported, and mainly concentrate on chemical oxidation routes in the presence of various additives.20,23–25 Besides, studies concerning the electrochemical performance of 3D PANI are scarce, despite possibly achieving remarkable properties which significantly benefit from their multidimensional structure.20Poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (P123), an amphiphilic triblock copolymer, popularly acts as a template for guiding the formation of mesoporous silicates29,30 and other inorganic nanomaterials.31–34 There are to our knowledge few literature procedures involving block copolymer-assisted synthesis of 3D PANI. For instance, in the work of Guo et al.,16 a block copolymer F127 template in an aqueous system was used to fabricate leaf-structured PANI. Srivastava et al.35 synthesized a mesostructured PANI by a templating approach using mixed surfactants of anionic sodium dodecylsulfate and non-ionic block copolymer P123 in an aqueous system. However, there are no reports on the combination of a block copolymer-templated strategy with a reverse microemulsion polymerization method for fabricating 3D nanostructured PANI. The advantage of this approach is that it can achieve strict control over the PANI growth in a confined microenvironment derived from surfactant micelles within an oil phase, thus providing a simple and effective way to synthesize 3D PANI with a very small size. Besides, owing to the rich phase behavior of block copolymers,36 the block copolymer-guided method can provide an alternative route to synthesize 3D PANI superstructures in a stable water-in-oil (W/O) system, which may be applicable to the ordered assembly of other nanomaterials. Based on the above analysis, in this work, we present an original method to prepare flower-shaped PANI multidimensional superstructures using P123-based micelles as the template in a W/O system for the first time. Some important factors that influence the morphology and size of PANI are explored, and a possible mechanism for flower growth is proposed. Especially, the electrochemical performance of the PANI nanoflowers is investigated and compared with that of well-developed PANI nanofibers in order to verify the potential of PANI nanoflowers as an electrode material in supercapacitors.
2. Experimental
2.1 Materials
Poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (denoted as P123 or PEO20PPO70PEO20, MW = 5800) was purchased from Sigma Aldrich. Camphor sulfonic acid (CSA) was received from J&K Scientific Ltd. Aniline (AN) was purified by distillation before use. All other chemicals and solvents were purchased from commercial suppliers, which were of analytical grade and used as received.2.2 Synthesis of PANI nanoflowers
Two reverse microemulsion systems (RMS1 and RMS2) were firstly prepared. Generally, P123 (0.25 mmol) and CSA (20 mmol) were dissolved in deionized water (15 mL) with vigorous stirring for 30 min, followed by the addition of hexane (25 mL) and n-hexanol (4.5 mL). After stirring for 30 min, AN (5.5 mmol) was added and the mixture continued to be magnetically stirred for 2 h in an ice–water bath, forming RMS1. The concentrations of P123 and AN in RMS1 were about 0.006 M and 0.12 M, respectively. RMS2 was prepared under parallel conditions with APS (5 mmol) instead of AN. Next, RMS2 was slowly added to RMS1 under stirring in an ice–water bath, and the resultant solution was left undisturbed for 24 h at 8–10 °C. Finally, the dark green PANI was obtained by centrifuging–rinsing repeatedly and freeze-drying. For investigating the effect of some reaction factors on the PANI morphology, various PANI samples were prepared by varying the type of the surfactant and the concentrations of P123 and AN following the above procedure.2.3 Material characterization
Transmission electron microscopy (TEM) micrographs of the samples were obtained with a HITACHI-600 transmission electron microscope. Infrared (IR) spectra in the range 400–4000 cm−1 were recorded using a BRUKER-EQUINOX-55 infrared spectrometer using KBr pellets. X-ray diffraction (XRD) patterns were recorded on a BRUKER D8 Advance powder diffractometer with a Cu Kα target ranging from 5 to 80 °C. The surface area of the sample was examined by Brunauer–Emmett–Teller (BET) measurements on an automatic ASAP 2020 analyzer.2.4 Electrochemical measurement
The working electrode (∼0.5 cm2) composed of 80 wt% PANI, 10 wt% acetylene black and 10 wt% polytetrafluorethylene (the loading of PANI in the electrode was 2.4 mg cm−2), using carbon paper as the current collector, was immersed in 1 M H2SO4 electrolyte overnight and then taken for electrochemical tests on a CHI660C electrochemical workstation and a CT2001A Land Battery Testing System in a classical three-electrode mode at room temperature. The reference electrode was a saturated calomel electrode (SCE), and the counter electrode was a Pt electrode. Cyclic voltammetry (CV) and galvanostatic charge–discharge (CD) tests were performed in a sweep potential ranging from −0.2 V to 0.8 V. The specific capacitance (Cm) can be calculated from the CD curves using the equation: Cm = (IΔt/mΔV), where I is the discharge current (mA), Δt is the discharge or charge time (s), ΔV is the potential window and m is the weight of active material in the electrode (mg). The electrochemical impedance spectroscopy (EIS) test was carried out in a frequency range of 104 to 0.01 Hz.3. Results and discussion
3.1 Morphology
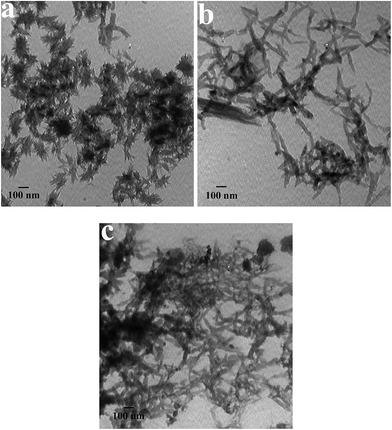
Typical TEM micrographs of PANI products made from reverse and conventional microemulsion polymerization methods are presented in Fig. 1. It was found that both the synthetic approach and the type of the surfactant noticeably affect the morphology of the products. As revealed in Fig. 1a, PANI synthesized by P123-assisted reverse microemulsion polymerization consists of small shuttle-shaped flower-like nanostructures with widths of ca. 40–150 nm and lengths of ca. 80–180 nm. Its shape and size are significantly different from previous results reported.20,21 For instance, the spherical flowers described by Alshareef et al.,8 Guo et al.20 and Wang et al.21 are around 3 μm, 4.5 μm and 10–20 μm in size, respectively. Further careful observation found that the edges of the flowers have some radiating whiskers, constructing rich V-type channels on the surfaces. When P123 is replaced by CTAB without changing the other conditions (Fig. 1b), well dispersed short nanofibers rather than nanoflowers appear in the product. Their diameters and lengths are estimated to be ca. 20–30 nm and ca. 100–200 nm, respectively, which are very small compared to the previous reports.11,26,37–39 With the conventional microemulsion method in the presence of P123 (Fig. 1c), the as-prepared PANI is still present as short nanofibers. Compared with those from the above case, although the fibers so fabricated have a smaller size (ca. 15–30 nm diameter and ca. 50–100 nm length), their dispersity is not very good. TEM results manifest that a micelle templating approach readily yields PANI with a small size, and the P123-stabilized reverse microemulsion route has the ability to generate special flower-structured PANI.Fig. 2 depicts TEM images of PANI obtained with P123 concentrations ranging from 0 M to 0.011 M. Apparently, the concentration of P123 has a significant effect on the morphology of the products. In the absence of P123 (Fig. 2a), PANI has irregular fiber-like structures with diameters and lengths of ca. 30–70 nm and ca. 80–250 nm, respectively. In this case, the PANI nanofibers are actually synthesized in an immiscible aqueous/organic bi-phasic system by interfacial polymerization. This result verifies again that the interfacial polymerization method is an effective and simple strategy to fabricate a fibrous morphology. At a low P123 concentration of 0.002 M (Fig. 2b), some flower-like aggregates appear, but a few fiber-like structures still exist. When the P123 concentration is increased to 0.006 M, the PANI is shuttle-like nanoflowers (Fig. 2c). At a higher concentration (0.011 M) (Fig. 2d), the flower shape still remains but its size is slightly decreased, possibly because excessive adsorption of P123 on the surfaces of the PANI nuclei limits their further growth.31 The above results indicate that, with an increasing P123 concentration from 0 to 0.011 M, the morphology of the PANI varies from nanofibers to nanoflowers, accompanied with a slight decrease of the size of the nanoflowers. Considering that for the removal of a surfactant with a high concentration from the system, it is very time-consuming to purify the product as much as possible, therefore, a P123 concentration of 0.006 M was regarded as the appropriate condition for the preparation of flower-shaped PANI.
Fig. 3 shows TEM images of PANI synthesized with AN concentrations ranging from 0.07 M to 0.17 M. At a low concentration of AN (0.07 M), some irregular particles close to a flower shape start to form, with detectable short whiskers on the edge of the flowers (Fig. 3a). As the AN concentration increases to 0.12 M (Fig. 1a and 2c), the product exhibits well-defined flower structures with pronounced whiskers. With an AN concentration of 0.17 M, one can see only short fibers instead of flowers, which may be caused by a high reaction rate that is not beneficial to the assembly of flowers. These fibers have very coarse surfaces and diameters of ca. 40–70 nm. The above morphology investigation well reveals that, with the increase of AN concentration, the as-obtained PANI is inclined to form fibrous structures. The possible reason for this is that a high monomer (AN) concentration leads to a high reaction rate, which may not be beneficial to the assembly of fibers into nanoflowers.
 | ||
| Fig. 3 TEM images of PANI prepared with different concentrations of AN. (a) [AN] = 0.07 M; (b) [AN] = 0.17 M. The concentration of P123 is 0.006 M. | ||
The images of PANI obtained at various polymerization stages are displayed in Fig. 4, which experimentally indicates an evolution process of PANI from 1D nanofibers to 3D nanoflowers. At the initial stage of the polymerization (about 10 min) (Fig. 4a), nanofibers with ca. 10–20 nm diameters are a dominating morphology of the PANI. After reaction for 30 min, most of the nanofibers have been converted to nanoflowers, leading to coexisting morphologies of a large majority of nanoflowers and a few nanofibers in the product (Fig. 4b). After the reaction time increases to 1 h, almost all of the PANI sample exhibits a nanoflower morphology but some of the nanoflowers are still in an incomplete growth state (Fig. 4c). After the reaction has proceeded for 5 h (Fig. 4d), the PANI shows a uniform flower shape. After reaction for 24 h, well-grown PANI nanoflowers with more abundant whiskers on the surfaces are successfully achieved.
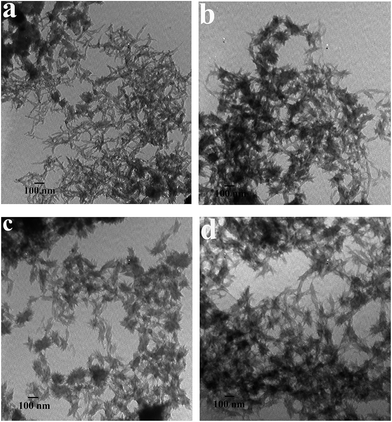 | ||
| Fig. 4 TEM images of PANI obtained at various reaction stages. (a) 10 min; (b) 30 min; (c) 1 h and (d) 5 h. The concentrations of P123 and AN are 0.006 M and 0.12 M, respectively. | ||
3.2 Formation mechanism
On the basis of the above results and previous studies on block copolymer-assisted organic/inorganic nanomaterials,20,31,33 the fabrication process and possible mechanism for the evolution from randomly oriented nanofibers to higher order nanoflowers are depicted in Fig. 5. Step 1 is the formation of a stable hexane/n-hexanol/water/P123/AN system (RMS1). From the molecular structure presented, it is clear that P123 is an amphiphilic molecule consisting of a hydrophilic (PEO) block and a hydrophobic (PPO) block. The introduction of P123 into deionized water easily leads to the generation of P123 micelles with a hydrated PEO corona and a PPO core.40,41 As a large amount of organic solvent is mixed with the P123 micelles, these micelles are dispersedly confined within the oil phase by the barrier role of the P123 surfactant located on the water/oil interfaces. Here, a confined aqueous microenvironment derived from the P123 micelles readily allows the nucleation, growth and further assembly of PANI.29 With the introduction of AN into this W/O system and further stirring for 2 h, RMS1 forms with AN adsorbed on the P123 chains through electrostatic attraction. Step 2 is the polymerization of AN. When the hexane/n-hexanol/water/P123/APS system (RMS2) is added dropwise into RMS1, AN is firstly polymerized at the oil–water interface and then grows towards the water phase. Initially, randomly oriented short fibers are formed, and then these fibers act as nucleation sites for the subsequent assembly of ordered nanoflowers through the intra-/inter-molecular interaction between the fibers.25 The above analysis illustrates that P123-stabilized reverse micelles can serve as the microreactor and template for constructing flower-based superstructures. Such 3D nanoscale architectures with open V-type channels on the surfaces can greatly boost the migration of electrolyte ions into the interior of the active materials through these channels. Therefore, one can predict that the as-formed PANI may possess favorable electrochemical characteristics. | ||
| Fig. 5 The conformation of P123, and the fabrication procedure and possible formation mechanism for the PANI nanoflowers. | ||
3.3 Chemical structure
The chemical structure of the resultant PANI nanoflowers was examined by IR spectroscopy, as shown in Fig. 6a. Some typical bands can be clearly seen at 1563, 1480, 1290, 1131, 1044, 784 and 581 cm−1. They correspond respectively to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of a quinoid ring, C
C stretching of a quinoid ring, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of benzenoid, C–N stretching of a secondary aromatic amine, N
C stretching of benzenoid, C–N stretching of a secondary aromatic amine, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching (Q represents a quinoid ring), S
N stretching (Q represents a quinoid ring), S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, C–H out-of-plane bending vibration, and S–O stretching, indicating the formation of CAS-doped PANI. Generally, PANI has several forms, i.e. leucoemeraldine base (fully reduced form), emeraldine base (half-oxidized form), and pernigraniline base (fully oxidized form). When the intensity ratio (R) of quinoid-to-benzenoid is 1, PANI is in a conductive emeralding salt state. In Fig. 6a, the R value of 0.91 verifies that the PANI obtained is close to the conducting emeraldine salt fashion and is identical to that in the previous report.35 The crystalline structure of the product was examined using its XRD pattern. Different from amorphous PANI with a relatively plane pattern, the nanoflowers show three pronounced diffraction peaks (Fig. 6b), such as (15.4°), (20.5°) and (25.5°). They represent the repeat unit of the PANI chain, the periodicity parallel and perpendicular to the polymer chain, respectively, indicating that PANI nanoflowers have a crystalline structure. Its crystalline degree is estimated to be about 0.48, based on the integral intensities of the radiation diffracted by the crystalline (Ic) and amorphous (Ia) phases: Wcr = Ic/(Ia + Ic).42 Generally, PANI with an ordered structure favors a rapid charge–discharge process by providing a high conductivity pathway,43 and thus the PANI nanoflowers obtained may show improved electrochemical performance. Finally, N2 adsorption–desorption isotherms and the corresponding pore size distribution of the PANI nanoflowers are presented in Fig. 6c. IV-type isotherms with a hysteresis loop indicate a porous characteristic of the PANI nanoflowers. The specific surface area of the PANI nanoflowers is calculated to be about 30 m2 g−1.
O stretching, C–H out-of-plane bending vibration, and S–O stretching, indicating the formation of CAS-doped PANI. Generally, PANI has several forms, i.e. leucoemeraldine base (fully reduced form), emeraldine base (half-oxidized form), and pernigraniline base (fully oxidized form). When the intensity ratio (R) of quinoid-to-benzenoid is 1, PANI is in a conductive emeralding salt state. In Fig. 6a, the R value of 0.91 verifies that the PANI obtained is close to the conducting emeraldine salt fashion and is identical to that in the previous report.35 The crystalline structure of the product was examined using its XRD pattern. Different from amorphous PANI with a relatively plane pattern, the nanoflowers show three pronounced diffraction peaks (Fig. 6b), such as (15.4°), (20.5°) and (25.5°). They represent the repeat unit of the PANI chain, the periodicity parallel and perpendicular to the polymer chain, respectively, indicating that PANI nanoflowers have a crystalline structure. Its crystalline degree is estimated to be about 0.48, based on the integral intensities of the radiation diffracted by the crystalline (Ic) and amorphous (Ia) phases: Wcr = Ic/(Ia + Ic).42 Generally, PANI with an ordered structure favors a rapid charge–discharge process by providing a high conductivity pathway,43 and thus the PANI nanoflowers obtained may show improved electrochemical performance. Finally, N2 adsorption–desorption isotherms and the corresponding pore size distribution of the PANI nanoflowers are presented in Fig. 6c. IV-type isotherms with a hysteresis loop indicate a porous characteristic of the PANI nanoflowers. The specific surface area of the PANI nanoflowers is calculated to be about 30 m2 g−1.
 | ||
| Fig. 6 (a) IR spectrum and (b) XRD pattern of the PANI nanoflowers. (c) N2 adsorption–desorption isotherms of the PANI nanoflowers. | ||
3.4 Electrochemical performance
As far as we know, it is well established that many 1D nano-structured PANI samples exhibit a good capacitive performance due to the nano-size effect and high doping level.8,9,11,13,26–28,39,44–46Among the various PANI nanofibers obtained in this study, the PANI nanofibers prepared using CTAB instead of P123 possess uniform morphology and very small size, which may deliver a good performance. Thus, to verify that the PANI nanoflowers obtained can also perform well electrochemically, PANI nanoflowers and nanofibers were subjected to cyclic voltammetry (CV) and galvanostatic charge–discharge (CD) tests in a classical three-electrode cell, and the results are compared and summarized in Fig. 7. Firstly, rate-dependent CV tests of the two electrodes were performed in 1 M H2SO4 solution over a range of 1 to 9 mV s−1. As shown in Fig. 7a and b, several couples of anodic and cathodic redox peaks can be observed in all of the CV curves, manifesting that their charge storage mode follows a Faradaic redox mechanism. The peak pairs in O1/R1 and O3/R3 are ascribed to the leucoemeraldine–polaronic emeraldine redox transition, while the O2/R2 and O4/R4 peak pairs come from the emeraldine–pernigraniline transformation.13,47 Notably, with increasing scan rate the cathodic and anodic peaks for the two electrodes slightly shift positively and negatively, respectively, which is mainly caused by the resistance of the electrode. In addition, the fact that the redox peak currents increase with the increase in scan rate confirms a fast current response of the PANI nanoflowers and nanofibers.
Galvanostatic CD curves of the PANI nanoflowers and nanofibers at current densities of 0.5, 1, 1.5 and 2 A g−1 were obtained to determine their specific capacitance (Cm) values, as shown in Fig. 7c and d. A near symmetric feature of the charge–discharge curves at a high current density suggests that the two electrodes have good electrochemical reversibility. The charge and discharge capacitances of the PANI nanoflowers were calculated to be 1010 and 622 F g−1 at a current density of 0.5 A g−1, respectively, which are slightly lower than those of the PANI nanofibers (1155 and 705 F g−1). Furthermore, the theoretical capacitance of the PANI nanoflowers was also calculated for comparison. According to the equation:26,48 Cmax = αF/dVM + CdlA, where α, F, dV, M, Cdl and A are the fraction of electron shared on each monomer unit (the maximum value of α is 1 for PANI), the Faraday constant (96485.3 C mol−1), the potential range (V), the molecular weight of aniline (91.1 g mol−1), the double layer capacitance (about 30 μF cm−2)48 and the specific surface area (m2 g−1), respectively. Based on a specific surface area of the PANI nanoflowers of 30 m2 g−1, their theoretical Cmax was calculated to be about 1068 F g−1. Therefore, the discharge capacitance of the PANI nanoflowers (622 F g−1 at 0.5 A g−1) can deliver about 58% of its theoretical value due to a low α value. Although the use of a very low current density can lead to a significantly high Cm, close to its theoretical value, this strategy is not advocated because a low current is unsuitable for high power applications in supercapacitors.26
The specific capacitance–current profiles of the two electrodes are shown in the inserts of Fig. 7c and d, respectively. With an increase of current density, their Cm values decrease, caused by internal resistance. In spite of this, the PANI nanoflowers still maintain charge and discharge capacitances of 510 and 484 F g−1, even at a rate of 2 A g−1. Although the Cm values of the PANI nanoflowers are somewhat lower than those of the PANI nanofibers (634 and 578 F g−1 at 2 A g−1), their capacitance retention is comparable (78% for nanoflowers and 82% for nanofibers). This confirms that both PANI electrodes have the ability to yield high capacitance at a high current density. On the other hand, the Cm values of the PANI nanoflowers are also superior to those from crosslinked PANI nanorods (297 F g−1 at 1 A g−1),13 PANI nanofibers (192 F g−1 at 0.1 A g−1),40 and PANI nanofibers (548 F g−1 at 0.18 A g−1).41 These data can well illustrate that PANI nanoflowers and nanofibers have a high specific capacitance and good rate performance. The high performance may be ascribed to more and shorter diffusion paths of ions and electrons, a highly accessible specific surface area and a good electrical conductivity brought about by the 1D or 3D nano-sized structure of the doped PANI.
The cycling properties of the PANI nanoflowers and nanofibers are measured by charge–discharge cycling at a current density of 5 A g−1. As shown in Fig. 7e, the PANI nanoflowers can retain about 76% of the initial capacitance at a high current density of 5 A g−1 after 1000 charge–discharge cycles, which is higher than that of the PANI nanofibers (69%), demonstrating that the PANI nanoflowers exhibit better electrochemical stability due to their multidimensional superstructures with nano size and V-type channels.
The Nyquist plots of the PANI nanoflowers and nanofibers are depicted in Fig. 7f. The two electrodes show similar impedance characteristics. At low frequency, the two straight lines are nearly parallel to the imaginary axis, confirming a good capacitive behavior. At high frequency, one can estimate from the enlarged plots in the inset of Fig. 7f that the charge-transfer resistance (Rct) values of the PANI nanoflowers and nanofibers are around 1.3 and 1.4 Ω based on the diameter of the semicircle, and that their internal resistance (Rs) values are about 1.9 and 1.5 Ω based on the high frequency intercept of the real axis. The small Rct and Rs values indicate that the two electrodes have low charge-transfer resistance and ion diffusion resistance during the electrochemical charge–discharge process, which has a contribution to their high-performance electrochemical behavior.
4. Conclusions
A P123-assisted reverse microemulsion method has been demonstrated to be effective for tailoring PANI architectures from 1D fibers to 3D flowers. The reverse P123 micelles play important roles in controlling the flower growth. The novelty of the present work mainly has two points. Firstly, through the exploration of the formation mechanism of the nanoflowers, one can clearly understand the formation of the shuttle-like PANI nanoflowers obtained from an original approach. This will provide some inspiration for designing other interesting multidimensional structures. Secondly, particular emphasis is placed on the capacitive properties of such PANI flowers for the first time. The results demonstrate that the electrochemical performance of the PANI nanoflowers is comparable to that of well-developed PANI nanofibers, exhibiting high specific capacitance, good rate capability and good cycling stability due to their nanoscale size, open V-shape channels and high doping level. Therefore, the PANI nanoflowers obtained may be a promising candidate for energy storage applications.Acknowledgements
This work is supported by the Scientific Research Program of the Higher Education Institution of XinJiang (no. XJEDU2012I05), the National Natural Science Foundation of China (no. 21363023, and no. U1203292), and the Open Project Program of Xinjiang Laboratory Advanced Functional Materials, China (XJDX0902-2012-05).References
- K. Lee, S. Cho, S. H. Park, A. J. Heeger, C. W. Lee and S. H. Lee, Nature, 2006, 441, 65–68 CrossRef CAS PubMed.
- C. Li, H. Bai and G. Shi, Chem. Soc. Rev., 2009, 38, 2397–2409 RSC.
- J. F. Mike, L. Shao, J. W. Jeon and J. L. Lutkenhaus, Macromolecules, 2014, 47, 79–88 CrossRef CAS.
- A. N. Aleshin, Adv. Mater., 2006, 18, 17–27 CrossRef CAS.
- G. P. Hao, F. Hippauf, M. Oschatz, F. M. Wisser, A. Leifert, W. Nickel, N. Mohamed-Noriega, Z. Zheng and S. kaskel, ACS Nano, 2014, 8, 7138–7146 CrossRef CAS PubMed.
- M. A. Bavio, G. Acosta and T. Kessler, J. Power Sources, 2014, 245, 475–481 CrossRef CAS PubMed.
- J. X. Huang, S. N. Virji, B. H. Weiller and R. B. Kaner, J. Am. Chem. Soc., 2003, 125, 314–315 CrossRef CAS PubMed.
- W. Chen, R. B. Rakhi and H. N. Alshareef, J. Mater. Chem. A, 2013, 1, 3315–3324 CAS.
- J. Mu, G. Ma, H. Peng, J. Li, K. Sun and Z. Lei, J. Power Sources, 2013, 242, 797–802 CrossRef CAS PubMed.
- Z. D. Zujovic, C. Laslau, G. A. Bowmaker, P. A. Kilmartin, A. L. Webber, S. P. Brown and J. Travas-Sejdic, Macromolecules, 2010, 43, 662–670 CrossRef CAS.
- K. Wang, J. Huang and Z. Wei, J. Phys. Chem. C, 2010, 114, 8062–8067 CAS.
- Y. Liao, V. Strong, W. Chian, X. Wang, X. G. Li and R. B. Kaner, Macromolecules, 2012, 45, 1570–1579 CrossRef CAS.
- X. Wang, J. Deng, X. Duan, D. Liu, J. Guo and P. Liu, J. Mater. Chem. A, 2014, 2, 12323–12329 CAS.
- P. Anilkumar and M. Jayakannan, Macromolecules, 2008, 41, 7706–7715 CrossRef CAS.
- G. R. Li, Z. P. Feng, J. H. Zhong, Z. L. Wang and Y. X. Tong, Macromolecules, 2010, 43, 2178–2183 CrossRef CAS.
- J. Han, G. Song and R. Guo, Adv. Mater., 2007, 19, 2993–2999 CrossRef CAS.
- H. Fan, H. Wang, J. Guo, N. Zhao and J. Xu, J. Colloid Interface Sci., 2013, 409, 255–258 CrossRef CAS PubMed.
- S. Y. Shen, Y. J. Wu, K. S. Ho, T. H. Hsieh, T. H. Ho, Y. Z. Wang, P. H. Tseng and Y. C. Hsu, Polymer, 2011, 52, 2609–2617 CrossRef CAS PubMed.
- G. Li, Y. Li, Y. Li, H. Peng and K. Chen, Macromolecules, 2011, 44, 9319–9323 CrossRef CAS.
- C. Zhou, J. Han and R. Guo, Macromolecules, 2008, 41, 6473–6479 CrossRef CAS.
- M. Yang, Z. Xiang and G. Wang, J. Colloid Interface Sci., 2012, 367, 49–54 CrossRef CAS PubMed.
- M. J. Kim, Y. D. Liu and H. J. Choi, Chem. Eng. J., 2014, 235, 186–190 CrossRef CAS PubMed.
- W. Chen, R. B. Rakhi, M. N. Hedhili and H. N. Alshareef, J. Mater. Chem. A, 2014, 2, 5236–5243 CAS.
- J. Sun and H. Bi, Appl. Surf. Sci., 2012, 258, 4276–4282 CrossRef CAS PubMed.
- W. Chen, R. B. Rakhi and H. N. Alshareef, J. Phys. Chem. C, 2013, 117, 15009–15019 CAS.
- M. M. Sk, C. Y. Yue and R. K. Jena, RSC Adv., 2014, 4, 5188–5197 RSC.
- Y. Cao and T. E. Mallouk, Chem. Mater., 2008, 20, 5260–5265 CrossRef CAS.
- B. Liang, Z. Qin, J. Zhao, Y. Zhang, Z. Zhou and Y. Lu, J. Mater. Chem. A, 2014, 2, 2129–2135 CAS.
- Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS PubMed.
- A. Caballero, L. Hernán, J. Morales, Z. González, A. J. Sánchez-Herencia and B. Ferrari, Energy Fuels, 2013, 27, 5545–5551 CAS.
- S. Sadasivan, D. Khushalani and S. Mann, Chem. Mater., 2005, 17, 2765–2770 CrossRef CAS.
- F. Venditti, R. Angelico, G. Palazzo, G. Colafemmina, A. Ceglie and F. Lopez, Langmuir, 2007, 23, 10063–10068 CrossRef CAS PubMed.
- Y. Ding, X. Liu and R. Guo, Colloids Surf., A, 2007, 296, 8–18 CrossRef CAS PubMed.
- F. Lei, B. Yan, H. H. Chen and J. T. Zhao, Inorg. Chem., 2009, 48, 7576–7584 CrossRef CAS PubMed.
- M. U. Prathap, B. Thakur, S. N. Sawant and R. Srivastava, Colloids Surf., B, 2012, 89, 108–116 CrossRef PubMed.
- B. K. Kuila and M. Stamm, J. Mater. Chem., 2010, 20, 6086–6094 RSC.
- S. Weng, Z. Lin, L. Chen and J. Zhou, Electrochim. Acta, 2010, 55, 2727–2733 CrossRef CAS PubMed.
- Q. Lu, Q. Zhao, H. Zhang, J. Li, X. Wang and F. Wang, ACS Macro Lett., 2013, 2, 92–95 CrossRef CAS.
- H. Guan, L. Z. Fan, H. Zhang and X. Qu, Electrochim. Acta, 2010, 56, 964–968 CrossRef CAS PubMed.
- R. Bleta, C. Machut, B. Léger, É. Monflier and A. Ponchel, Macromolecules, 2013, 46, 5672–5683 CrossRef CAS.
- J. Geng, J. J. Zhu, D. J. Lu and H. Y. Chen, Inorg. Chem., 2006, 45, 8403–8407 CrossRef CAS PubMed.
- M. Bláha, M. Varga, J. prokeš, A. Zhigunov and J. Vohlídal, Eur. Polym. J., 2013, 49, 3904–3911 CrossRef PubMed.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed.
- H. W. Park, T. Kim, J. Huh, M. Kang, J. E. Lee and H. Yoon, ACS Nano, 2012, 6, 7624–7633 CrossRef CAS PubMed.
- B. K. Kuila, B. Nandan, M. Böhme, A. Janke and M. Stamm, Chem. Commun., 2009, 5749–5751 RSC.
- D. S. Dhawale, R. R. Salunkhe, V. S. Jamadade, D. P. Dubal, S. M. Pawar and C. D. Lokhande, Curr. Appl. Phys., 2010, 10, 904–909 CrossRef PubMed.
- Y. G. Wang, H. Q. Li and Y. Y. Xia, Adv. Mater., 2006, 18, 2619–2623 CrossRef CAS.
- F. Huang and D. Chen, Energy Environ. Sci., 2012, 5, 5833–5841 CAS.
| This journal is © The Royal Society of Chemistry 2015 |