Alessandra Casnati, Marco Fontana, Elena Motti and Nicola Della Ca’
Org. Biomol. Chem., 2019,17, 6165-6173
DOI:
10.1039/C9OB01085H,
Paper
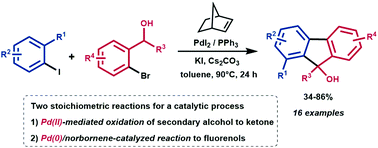
Herein, we report a novel catalytic synthesis of substituted 9H-fluoren-9-ols starting from aryl iodides and secondary ortho-bromobenzyl alcohols in the presence of palladium/norbornene as a catalytic system. The present protocol exhibits high functional group tolerance, mild reaction conditions and moderate to good yields. This transformation is based on two sequential pathways: (i) Pd(II)-mediated oxidation of the secondary alcohol to the corresponding ketone and (ii) Pd(0)/norbornene-catalyzed reaction of the in situ generated ortho-bromoacetophenone with the aryl iodide.