Qiang Sha and Haixuan Liu
Org. Biomol. Chem., 2019,17, 7547-7551
DOI:
10.1039/C9OB01422E,
Paper
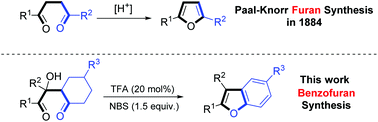
A facile, one-pot method for the de novo synthesis of benzofurans from 2-hydroxy-1,4-diones is described. Using trifluoroacetic acid (TFA) as the catalyst and N-bromobutanimide (NBS) as the oxidant, 2-hydroxy-1,4-diones underwent a cyclization/oxidative aromatization cascade reaction to afford a variety of benzofuran derivatives in moderate to good yields. This is a practically useful method for the synthesis of benzofuran derivatives starting from 2-hydroxy-1,4-diones which is also a supplement of the Paal–Knorr furan synthesis. A preliminary mechanism study was undertaken to support the proposed mechanism during which a novel 1,6-conjugate addition reaction was revealed.