Laura Kuehn, Mingming Huang, Udo Radius and Todd B. Marder
Org. Biomol. Chem., 2019,17, 6601-6606
DOI:
10.1039/C9OB01244C,
Paper
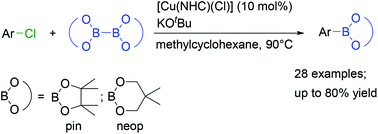
We report herein the first Cu-catalysed borylation of a wide range of aryl chlorides with different electronic and steric properties using a readily prepared NHC-stabilised Cu catalyst and KOtBu as the base with B2pin2 (pin = pinacolato) as the boron reagent. The aryl chlorides are converted into their corresponding arylboronic esters in good yields. The new procedure shows broad functional group tolerance, and B2neop2 (neop = neopentyl glycolato) can also be applied as the boron reagent.