Nuria Aiguabella, Mareike C. Holland and Ryan Gilmour
Org. Biomol. Chem., 2016,14, 5534-5538
DOI:
10.1039/C6OB00025H,
Communication
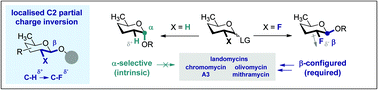
To reconcile the urgent need to access well defined β-configured 2,6-di-deoxypyranose analogues for chemical biology, with the intrinsic α-selectivity of the native system, the directing role of fluorine at C2 has been explored. Localised partial charge inversion (C–Hδ+ → C–Fδ−) elicits a reversal of the substrate-based α-stereoselectivity, irrespective of the protecting group electronics.