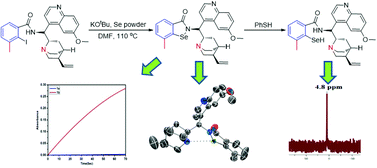
Shah Jaimin Balkrishna, Shailesh Kumar, Gajendra Kumar Azad, Bhagat Singh Bhakuni, Piyush Panini, Navjeet Ahalawat, Raghuvir Singh Tomar, Michael R. Detty and Sangit Kumar
Org. Biomol. Chem., 2014,12, 1215-1219
DOI:
10.1039/C4OB00027G,
Communication