Polymorphism-induced multi-functional crystal photonics achieved by a highly luminescent benzofuranyl molecule having a tetrafluorophenylene core†
Takumi
Matsuo
 *ab and
Shotaro
Hayashi
*ab and
Shotaro
Hayashi
 *ab
*ab
aSchool of Engineering Science, Kochi University of Technology, Kami, Kochi, 782-8502, Japan. E-mail: matsuo.takumi@kochi-tech.ac.jp; hayashi.shotaro@kochi-tech.ac.jp
bFOREST Center, Research Institute, Kochi University of Technology, Kami, Kochi, 782-8502, Japan
First published on 15th April 2025
Abstract
Luminescent organic single crystals are attractive as components of miniaturized photonic integrated circuits such as optical waveguides, lasers, and optical resonators. Various functions have been discovered by designing chemical or crystal structures. The optical characteristics can be modulated by designing the crystal structures. Various polymorphic compounds have been reported, and differences in luminescence colors have been well-explained by the differences in the crystal structures. However, drastic modulation of the photonics functions has not been reported. We report here multi-functional photonics achieved by a highly luminescent and polymorphic compound, 1,4-bis(benzofuran-2-yl)-2,3,5,6-tetrafluorophenylene, named BFTFP. BFTFP exhibited three types of crystal structures: BFTFP_α: a flexible fiber, BFTFP_β: a rigid block, and BFTFP_γ: a plate. Depending on each crystal's morphology or emission properties, specific photonic functions assigned for each crystal, BFTFP_α, BFTFP_β, and BFTFP_γ, respectively, were developed. BFTFP_α exhibited elastic flexibility with optical waveguiding. Although elastic organic single crystals tend to be less luminescent, the BFTFP_α crystal possessed 52% of ΦPL which was one of the highest among previously reported elastic organic single crystals. BFTFP_β exhibited amplified spontaneous emission under excitation using a nanosecond pulsed laser due to their rigidity and monomeric luminescence. Platelet crystals of BFTFP_γ exhibited intense luminescence from their basal facets, making them ideal media for highly luminant photonic devices such as vertical cavity surface emitting lasers.
Introduction
Organic electroluminescence devices relating to information technology have been well-developed in recent years. Apart from these organic electronics,1–5 organic photonics utilizing π-conjugated molecules have been well-developed due to their efficient energy transports or fast responses. Especially, organic single crystals (OSCs) are ideal media to realize photonics with high functionalities of lasers or amplified spontaneous emissions (ASEs),6–18 cavities,19–25 and optical self-waveguides.26–39 Using OSCs with these functions, photonic integrated circuits (PICs) have been constructed toward practical applications. R. Chandrasekar et al. have constructed PICs with several components of OSCs combined using a cantilever for atomic force microscopy (AFM).26 Moreover, large-scale integrations (LSIs) of OSCs with specific optical functions of lasers, cavities, or waveguiding have been constructed.8,32To investigate OSC-based photonics, highly luminescent molecules have been developed so far. trans,trans-1,4-Distyrylbenzenes (DSB) and these derivatives are frequently utilized molecules exhibiting highly luminescent performance in the solid state.6 The molecules composed of phenylene–vinylene structures such as DSBs are one of the most utilized molecular structures to develop organic lasers. Recently, to increase the stability of the molecules composed of phenylene–vinylene structures, a bridging strategy by carbon or oxygen atoms has been proposed.40,41 According to such strategies, unexpected events in molecules of OSCs such as cis–trans isomerizations or [2+2] cycloadditions in the vinylene structures can be prevented.40,42,43
Not only the chemical structure but also the molecular aggregation states also determine the optoelectronic characteristics of OSCs. To rationally develop the optoelectronic performance, crystal structures in terms of molecular conformation, packing, and alignments must be considered. Takimiya et al. have developed crystal engineering for the improvement of OSC transistors.3 To develop the photonic functions of OSCs, polymorphism has been efficiently utilized. Polymorphism is sometimes regarded as a disadvantageous contamination property because several types of crystals were unexpectedly mixed; thus, crystals with various properties were included in one batch. However, this contamination is not disadvantageous in the case of OSC μ-photonics since only one isolated OSC is utilized in the demonstration.7,16 Furthermore, the utilization of polymorphism can be a good methodology for the development of OSCs because the probability of the appearance of OSCs with high performance is increased.44
Although the rational designing toward the appearance of polymorphism is still challenging, the design strategy has been gradually constructed recently. Polymorphs are roughly categorized into packing polymorphs or conformation polymorphs. 5-Methyl-2-[(2-nitrophenyl)amino]thiophene-3-carbonitrile, called ROY, is known as one of the most polymorphic compounds. The origin of the polymorphism was interpreted as the movability of the molecular conformation, which was categorized as conformational polymorphism.41 Additionally, various intermolecular hydrogen bonding patterns can also induce polymorphism, which was categorized as packing polymorphism.45–47
Here, we designed 1,4-bis(benzofuran-2-yl)-2,3,5,6-tetrafluorophenylene, namely BFTFP, as a highly luminescent and polymorphic compound toward polymorphism-based OSC photonics.48 First of all, as a highly luminescent moiety and optically robust structure, BFP, an oxygen-bridged DSB structure, was designed as depicted in Scheme S1 (ESI†). Additionally, to induce the packing polymorphism in BFP, we introduced fluorine atoms into the central phenylene unit as displayed in Chart 1. The partially introduced heteroatoms or halogen atoms could contribute to inducing the multi-intermolecular hydrogen bonding, resulting in packing polymorphism. Furthermore, we examined the relative energy at each torsion angle for the simplified chemical structure of BFTFP (Scheme S2, ESI†). According to the literature, the conformation with a relative energy below ca. 5 kJ mol−1 is allowed to appear.49,50 Based on this fact, the torsion angle between the central phenylene unit and the benzofuran unit, θt, below 45° was expected to appear. Thus, conformational polymorphism due to this torsion movability can also be expected. In this work, we created polymorphs of BFTFP and achieved multi-functional photonics in terms of optical waveguiding, lasers, and optical resonators based on the polymorphisms in BFTFP.
 | ||
| Chart 1 Schematic depiction of the chemical structure designing strategy for polymorphism-based multi-photonics. | ||
Results and discussion
As shown in Scheme 1, BFTFP was synthesized via the Suzuki–Miyaura cross-coupling reaction of 1,4-dibromotetrafluorobenzene with benzofuran-2-boronic acid in 37% yields. The target compound BFTFP was identified by measurements of 1H-NMR, 13C-NMR, and high-resolution mass-spectroscopy (HR-MS) spectra (Fig. S1–S3, ESI†).To yield polymorphs of BFTFP, three types of growth processes were performed. Each methodology is schematically displayed in Fig. 1. Fig. 1a–c show a schematic depiction of solvent diffusion, vapor diffusion, and sublimation processes, respectively. The detailed procedures are described in the Experimental section. As a result of the crystal growth, three crystal morphologies were discovered. In all processes, crystals with fiber morphology and mechanical flexibility were obtained. By the solvent diffusion process, fiber crystals were obtained as sole morphology. In the case of the vapor diffusion process, crystals with block morphology grown on the glass plates contained in the Petri dish were obtained. By the sublimation process, platelet crystals were obtained. The obtained crystal morphology was named BFTFP_α for the fiber crystals, BFTFP_β for the block crystals, and BFTFP_γ for platelet crystals.
 | ||
| Fig. 1 Schematic illustrations of crystal growth by the (a) solvent diffusion process, (b) vapor diffusion process, and (c) sublimation process. | ||
Fig. 2a–c show each fluorescence microscopy image taken for BFTFP_α, BFTFP_β, and BFTFP_γ, respectively. As the images show, each crystal exhibited drastically varied crystal morphology. Fig. 2d shows photoluminescence (PL) and its excitation (PLE) spectra recorded for each crystal. Although BFTFP_α and BFTFP_γ exhibited similar broad spectra, only BFTFP_β showed a drastically varied sharp PL spectrum exhibiting vibronic transitions. This suggested luminescence from molecules in the monomeric state in the crystal of BFTFP_β. Fig. S4 (ESI†) shows PL decay profiles measured for BFTFP_α and BFTFP_β. Each decay profile was well-fitted as a 2nd-order exponential decay curve. The fitting function f(t) is described as follows: f(t) = A1exp(−t/τ1) + A2exp(−t/τ2) where A1 and A2 are constants, τ1 and τ2 are lifetimes, and t is the decay time. In the case of BFTFP_α, A1, A2, τ1, and τ2 are 0.08, 0.044, 1.79, and 4.25, respectively. The average PL lifetime (τave) was estimated to be 2.66 ns. In the case of BFTFP_β, A1, A2, τ1, and τ2 are 0.13, 0.0060, 1.06, and 2.95, respectively. τave was estimated to be 1.14 ns. Thus, the luminescence decay is faster in the case of BFTFP_β compared to the case of BFTFP_α, which also suggested the monomeric emission for BFTFP_β rather than excimer emissions.
 | ||
| Fig. 2 Fluorescence microscopy images of (a) BFTFP_α, (b) BFTFP_β, and (c) BFTFP_γ. (d) PL and PLE spectra recorded for each polymorph. | ||
Next, crystal structures were revealed for BFTFP_α, BFTFP_β, and BFTFP_γ (CCDC No. 2420271–2420273). Fig. 3a–d show the crystal structure of BFTFP_α. Fig. 3a shows the conformation of BFTFP_α. The torsion angle estimated for the benzofuran unit to tetrafluorophenylene unit (θt) was 19.42°. Fig. 3b shows one of the packings between neighboring two molecules. Hydrogen bonding was suggested between the fluorine and hydrogen atoms. Fig. 3c shows π–π stacking suggested between two molecules packed as a face-to-face motif. Fig. 3d shows the crystal structure projected on the bc-plane with a schematic depiction of crystal morphology for indicating the crystal long axis, b-axis direction. Fig. 3e–h show the crystal structure of BFTFP_β. Fig. 3e shows the conformation of BFTFP_β. Two conformations, namely conformation 1 and conformation 2, were contained in the crystal structure. The torsion angle, θt, was estimated to be 9.26–38.53°. Fig. 2f shows one of the packings between two molecules of conformation 1. Fig. 3g shows one of the packings between two molecules of conformation 2. Hydrogen bonding was suggested between the fluorine and hydrogen atoms. Fig. 3h shows the crystal structure projected on the bc-plane with a schematic depiction of crystal morphology for indicating the crystal long axis, c-axis direction. Fig. 3i–l show the crystal structure of BFTFP_γ. Fig. 3i shows the conformation of BFTFP_γ. Two conformations, namely conformation 1 and conformation 2, were contained in the crystal structure. In conformation 2, the benzofuran units were disordered. The occupancy of the main benzofuran unit was 56.2%. The packing was displayed using the mainly occupied benzofuran units. The torsion angle, θt, was estimated to be 0.32–4.82°. Fig. 3i shows one of the packings between two conformation 1. Fig. 3k shows one of the packings between two conformation 2. Compared to the cases of BFTFP_α and BFTFP_β, different patterns of hydrogen bonding were suggested between fluorine and hydrogen. Fig. 3l shows the crystal structure projected on the bc-plane with a schematic depiction of crystal morphology for indicating crystal elongation along the a- and b-axis directions. In the preliminary examination, as depicted in Scheme S2 (ESI†), the allowed predicted torsion angles in the crystal range from 0 to ca. 45°. The experimentally appeared torsion angles were 0.32–38.53°, among BFTFP_α, BFTFP_β, and BFTFP_γ, in which angle values were well-corresponded to the preliminary examination. Thus, it was suggested that both multiple hydrogen bonding and rotatability contributed to the appearance of polymorphs. In the crystal structures, the planar π-planes of BFTFP_α and BFTFP_γ were well-overlapped while BFTFP_β possessed highly twisted conformations and small π-plane overlapping. Fig. S5–S8 (ESI†) show the simulated molecular orbitals, absorption spectra, and oscillator strength (f) of BFTFP obtained from time-dependent density functional theory (TDDFT) calculations using a neighboring molecule model generated by a crystal structure (CIF file). Tables S1–S4 (ESI†) also summarize the results of the calculations. In the result of BFTFP_β, the molar absorption coefficient was larger than 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, which was larger than the cases of BFTFP_α and BFTFP_γ. The molecular orbitals were localized, which was ideal for high luminescence performance. These properties of crystal structures were well-corresponded to the luminescence characteristics; PL spectra shape and PL lifetime are shown in Fig. 2d and Fig. S4 (ESI†). BFTFP_α and BFTFP_γ exhibited broad excimer emission while BFTFP_β exhibited sharp monomeric emission.
000 M−1 cm−1, which was larger than the cases of BFTFP_α and BFTFP_γ. The molecular orbitals were localized, which was ideal for high luminescence performance. These properties of crystal structures were well-corresponded to the luminescence characteristics; PL spectra shape and PL lifetime are shown in Fig. 2d and Fig. S4 (ESI†). BFTFP_α and BFTFP_γ exhibited broad excimer emission while BFTFP_β exhibited sharp monomeric emission.
Before the investigations of photonic functions for each polymorph, absolute PL quantum efficiency (ΦPL) was estimated for the majorly obtained crystal form BFTFP_α. The ΦPL was estimated to be 0.52 (52%), which was one of the highest values among flexible OSCs.17 To the best of our knowledge, the pitched-π stack packing motif induces flexibility.27 However, this motif also induces less emissive properties due to the well-overlapped π-orbitals. The high ΦPL for BFTFP_α suggested that the chemical structure designed in this research worked well with high luminescence performance.
Next, we assigned specific photonic functions for BFTFP_α, BFTFP_β, and BFTFP_γ, respectively, toward multi-functional photonics based on polymorphism. To assign a specific photonic function for BFTFP_α, fluorescence waveguiding characteristics were examined as shown in Fig. 4. In this characterization, microscopic observations and PL spectral measurements of BFTFP_α were performed using a focused laser beam (λex = 405 nm) as an excitation source (set-up: Fig. S9, ESI†). When the focused laser beam irradiated an isolated fiber crystal, the luminescence was guided toward the edge of the crystal. We measured the light emission at the laser irradiation position (excitation position: Ex) and the crystal edge (guided position: WG) as a spatially resolved PL spectrum. Spatially resolved PL spectra were recorded at 4 points where the guided distance D was 23.2, 18.2, 14.1, and 11.1 from the excitation position to the waveguiding position along the long axis of the crystal (Fig. 4a). Fig. 4b and c show PL spectra taken at Ex and WG at each D. The emission intensities at the excitation and guided positions were monitored at 470 nm as IEx and IWG, respectively, and IWG/IEx were plotted at each D (Fig. 4d). Fitting according to the function, IWG/IEX = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−αD) where A is a constant and α is a loss coefficient gave α = 279 dB cm−1 was performed for the obtained plots shown in Fig. 4d. Furthermore, bending characterization studies were performed for an isolated crystal of BFTFP_α placed on a glass substrate (Fig. S10, ESI†). When the mechanical stress was applied to the straight-shaped crystal using a tweezer and a metal needle, the crystal showed bending flexibility. After releasing the stress, the crystal shape returned to its straight form. Thus, the flexibility was categorized in elasticity rather than plasticity.30
exp(−αD) where A is a constant and α is a loss coefficient gave α = 279 dB cm−1 was performed for the obtained plots shown in Fig. 4d. Furthermore, bending characterization studies were performed for an isolated crystal of BFTFP_α placed on a glass substrate (Fig. S10, ESI†). When the mechanical stress was applied to the straight-shaped crystal using a tweezer and a metal needle, the crystal showed bending flexibility. After releasing the stress, the crystal shape returned to its straight form. Thus, the flexibility was categorized in elasticity rather than plasticity.30
In general, monomeric emission shows high efficiency in luminescence, which is ideal for the application of laser media.6,12,13 With this situation in mind, ASE characteristics were examined for BFTFP_β by optical pumping using an Nd:YAG pulsed laser (λex = 355 nm, a pulse duration of ∼5 ns, and repetition frequency of 1 kHz). The measurement setup is shown in Fig. S11 (ESI†). The PL intensity (IPL) increased with increasing excitation fluence (Iex) (Fig. 5a). At a lower Iex, a broad PL band appeared. At a higher Iex (ca. ≳470 μJ cm−2), a narrow band with increased intensity was observed. Fig. 5b shows the dependence of Iex on the integrated PL intensity. The PL intensity increased sublinearly at a lower excitation power and superlinearly at a higher excitation power, resulting in the appearance of a threshold at 431 μJ cm−2. The slope of the plots was estimated to be 0.8 (IPL0.8 ∝ Iex) in the sublinear regime, while that was 1.5 (IPL1.5 ∝ Iex) in the superlinear regime. Additionally, the bandwidth was estimated as the full width at half maximum (FWHM), which was estimated as a result of peak fitting using the lorentian function. The bandwidth was also dramatically sharpened appearing as a threshold. Thus, BFTFP_β exhibited ASE with a threshold.
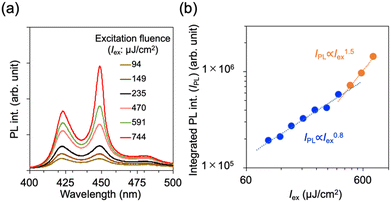 | ||
| Fig. 5 PL spectra recorded for an isolated BFTFP_β crystal taken at varied Iex values (microjoules per square centimeter) and (b) dependence of the integrated PL intensity and FWHM on the Iex. | ||
As shown in Fig. 2c, BFTFP_γ is a two-dimensional morphology. Additionally, the PL appeared from the basal facet of the crystal. This property is ideal as media for highly luminant devices such as vertical cavity surface emitting lasers (VCSELs).51,52 To characterize the vertical cavity properties, μ-PL measurements were performed for an isolated crystal of BFTFP_γ. Fig. 6a exhibits the measurement scheme. The crystal was optically excited using an LED (λex = 365 nm). The μ-PL spectrum was recorded using a set-up as shown in Fig. S9 (ESI†). Interestingly, sharp optical fringes appeared in the PL spectrum (Fig. 6b; the inset shows the PL microscopy image of the measurement sample). Considering the direction of PL detection, the fringes in the PL spectrum were derived from the strong PL confinement, resulting in interference in the vertical direction of the crystal. The thickness (T) of the measured sample was estimated to be 1.2 μm using a surface profiler. Furthermore, values of group refractive indices (ng) were estimated using the equation ng = 1/(2L*ΔE), where L is the resonator length (cm) which is the same as the estimated T, and ΔE is the mode interval value (cm−1). The ng values were estimated to be 3.6–10 (Fig. 6c), which are high and indicate the strong confinement of photons inside the crystal. This vertical cavity in BFTFP_γ exhibiting sharp fringes and ng values possesses potential for applications in highly luminant devices, VCSELs, or polariton laser media.51,52
To investigate the effectivity of the chemical structure designing strategy for BFTFP, 1,4-bis(benzothiophene-2-yl)-2,3,5,6-tetrafluorophenylene (BTTFP), 1,4-bis(benzofuran-2-yl)-phenylene (BFP) and trans,trans-1,4-bis(2-phenylethenyl)-2,3,5,6-tetrafluorophenylene (PETFP) were synthesized. Schemes S3–S5 (ESI†) show the synthesis scheme of BTTFP, BFP, and PETFP respectively. Fig. S12–S14 (ESI†) show the 1H-NMR, 13C-NMR spectra, and HR-MS recorded for BTTFP, respectively. Fig. S15–S17 (ESI†) show the 1H-NMR, 13C-NMR, and HR-MS spectra recorded for BFP, and Fig. S18–S20 (ESI†) show the 1H-NMR, 13C-NMR, and HR-MS spectra recorded for PETFP, respectively. To obtain single crystals, growth processes depicted in Fig. 1a–c, the same as the case of BFTFP, were also performed for BTTFP, BFP, and PETFP respectively. As a result, BTTFP was crystallized in flexible fiber morphology as well as BFTFP_α, in any cases. Fig. S21 (ESI†) shows the crystal structure of BTTFP (CCDC No. 2420274). The benzothiophene unit exhibited disorder based on the rotation. The major occupancy was 61.1%. As shown in Fig. S21a (ESI†), the torsion angle for the benzothiophene unit to tetrafluorophenylene unit (θt) was estimated to be 3.69°. In Fig. S21b (ESI†), π–π stacking was suggested between face-to-face stacked molecules. In different molecular interactions, hydrogen bonding was suggested between fluorine and hydrogen as shown in Fig. S21c (ESI†). Fig. S21d (ESI†) shows the crystal structure projected on the bc-plane with a schematic depiction of crystal morphology for indicating the crystal's long axis, b-axis direction. The packing motif was categorized in pitched-π stacking. Fig. S22 (ESI†) shows the PL and PLE spectra recorded for BTTFP crystals. One broad PL spectrum appeared suggesting the excimer formation in luminescence. Thus, BTTFP crystals exhibited similar packing, and luminescence properties in the case of BFTFP_α. Fig. S23 and Table S5 (ESI†) show the results of the TDDFT calculation for a model of the face-to-face stacked BTTFP crystal. The f value was smaller than that those of BFTFP_α. Fig. S25 (ESI†) shows the PL lifetime measured for the crystals of BTTFP. The profile was well-fitted as a 2nd-order exponential decay curve where A1, A2, τ1, and τ2 are 0.060, 0.040, 0.56, and 1.40, respectively. The ΦPL of crystals of BTTFP was estimated to be 0.18. The radiative rate constant (kr) was calculated to be 0.19 ns−1 using an equation: kr = ΦPL/τPL. The nonradiative rate constant (knr) was calculated to be 0.88 ns−1 using an equation: knr = (1 − ΦPL)/τPL. Similarly, kr and knr were estimated for BFTFP_α, resulting in kr = 0.20 ns−1 and knr = 0.18 ns−1, respectively. In the comparison of luminescence characteristics between crystals of BFTFP_α and BTTFP, the kr values were almost the same as each other. However, knr of BFTFP_α was drastically smaller than that of BTTFP crystals derived from the heavy atom effect of the sulfur atom in comparison to the oxygen atom,53–55 and suggested the effectivity of the oxygen atom bridging strategy as depicted in Scheme S1 (ESI†).
To examine the effectiveness of the fluorination strategy on the appearance of polymorphism, characterization studies were performed for crystals of BFP. As a result of crystal growth, two polymorphs were obtained. The crystal grown by the sublimation process was named BFP_α, and that grown by the solvent diffusion or vapor diffusion process was named BFP_β. Fig. S25a–d (ESI†) show the crystal structure of BFP_α (CCDC No. 2420275). Fig. S25a (ESI†) shows the conformation of BFP_α. The torsion angle for the benzofuran unit to phenylene unit (θt) was estimated to be 0.59° and 0.81°. Fig. S25b (ESI†) shows one of the packings between two molecules. The CH–π interaction was suggested. Fig. S25c (ESI†) shows packing suggesting a herringbone packing motif. The herringbone angle was estimated to be 51.70°. Fig. S25d (ESI†) shows the crystal structure projected on the bc-plane with a schematic depiction of crystal morphology for indicating the crystal elongation direction along the a- and b-axis directions. Fig. S25e–h (ESI†) show the crystal structure of BFP_β (CCDC No. 2420276). Fig. S25e (ESI†) shows the conformation of BFP_β. The benzofuran unit exhibited disorder based on the rotation. The major occupancy was 59.4%. The torsion angle for the benzofuran unit to phenylene unit (θt) was estimated to be 0.38–1.30°. Fig. S25f (ESI†) shows one of the packings between two molecules. The CH–π interaction was suggested. Fig. S25g (ESI†) shows packing suggesting a herringbone packing motif. The herringbone angle was estimated to be 52.06°, which was quite similar to that of BFP_α. Fig. S25h (ESI†) shows the crystal structure projected on the bc-plane with a schematic depiction of crystal morphology for indicating the crystal elongation direction along the a- and c-axis directions. Fig. S26a and b (ESI†) show the PL and PLE spectra recorded for BFP_α and BFP_β, respectively. Each graph contains the fluorescence microscopy image as an inset. The PL band exhibited vibronic transitions and showed quite similar shape each other. Although the PL spectra were similar to each other, the luminescence performance was distinct each other for BFP_α and BFP_β. ΦPL was estimated to be 0.70 for BFP_α and that estimated for BFP_β was 0.30. Fig. S27 (ESI†) shows PL decay profiles taken for BFP_α and BFP_β, respectively. In the case of BFP_α, A1, A2, τ1, and τ2 are 0.110, 0.023, 2.192, and 3.807, respectively. τave was estimated to be 2.47 ns. In the case of BFP_β, A1, A2, τ1, and τ2 are 0.080, 0.058, 0.710, and 2.505, respectively. τave was estimated to be 1.46 ns. For BFP_α, kr and knr were estimated to be 0.28 ns−1 and 0.12 ns−1, respectively. For BFP_β, kr and knr were estimated to be 0.21 ns−1 and 0.41 ns−1, respectively. Although the difference in kr values of BFP_α and BFP_β was slight, knr of BFP_α was drastically smaller than that of BFP_β. The origin of the higher ΦPL estimated for BFP_α was mainly derived from this smaller knr. Interestingly, the packing in the crystal was quite similar to each other for BFP_α and BFP_β; however, the luminescence performance was drastically different, which is going to be examined in future work. Fig. S28 and S29 and Tables S6 and S7 (ESI†) show the results of the TDDFT calculation. As supporting the difference of the ΦPL, f of BFP_α was larger than that of BFP_β. BFP_α and BFP_β crystallized by the CH–π interaction exhibited almost the same conformation and packing. Thus, the crystal morphologies or PL characteristics were not different from each other. Therefore, the effect of fluorination toward a drastic change in packing the motif was revealed as discussed for BFTFP_α, BFTFP_β, and BFTFP_γ, respectively.
Fig. S30 (ESI†) shows the crystal structure of PETFP (CCDC No. 2420277). The distance between the neighboring vinylene units was estimated to be 3.809 Å. According to the literature, the reaction between two vinylene units occurs even in the solid state of the molecules.42 To discuss the effectivity of the oxygen-bridging strategy of BFTFP, photostability was compared between BFTFP_α and PETFP. Fig. S31 (ESI†) shows PL spectra at each UV irradiation time recorded for isolated crystals of BFTFP_α and PETFP, respectively (excitation: λ = 365 nm). The PL intensity from BFTFP_α hardly decreased while that measured for PETFP almost disappeared. The origin of this low optical durability was attributed to the reaction between neighboring vinylenes. Thus, it was revealed that the oxygen bridging strategy plays an important role in increasing the photostability.
Experimental
Chemicals
Palladium(II) acetate (Wako), tris-(dibenzylideneacetone)dipalladium(0) (Pd2dba3; Sigma-Aldrich), 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (S-Phos; Sigma-Aldrich), 1,4-dibromotetrafluorobenzene (TCI), 1,4-dibromobenzene (TCI), benzofuran-2-boronic acid (TCI), benzo[b]thiophene-2-boronic acid (TCI), and (E)-styrylboronic acid (TCI) were used as received. All solvents were used as received.Synthesis
Crystal growth
Measurements
Unless otherwise noted, all the experiments were conducted at room temperature (25 ± 3 °C). Liquid-state 1H (400 MHz) and 13C (100 MHz) nuclear magnetic resonance (NMR) spectra were recorded using a JEOL ECZ400S spectrometer. Chemical shifts (δ) were expressed relative to the resonances of the residual non-deuterated solvents for 1H [CDCl3: 1H(δ) = 7.26 ppm] or 1H [CD2Cl2: 1H(δ) = 5.33 ppm] and 13C [CDCl3: 13C(δ) = 77.16 ppm]. Absolute values of the coupling constants were given in Hertz (Hz), regardless of their sign. Multiplicities were abbreviated as singlet (s), doublet (d), triplet (t), and multiplet (m). Mass spectral analysis was performed using a compact QTOF spectrometer (Bruker) and the data were analyzed with DataAnalysis 5.1. Data collection for X-ray crystal analysis was performed using Rigaku/XtaLAB Synergy-S/Cu (Mo-Kα λ = 0.7107 Å) diffractometers. The X-ray measurement was performed at −170 °C or −160 °C. The structures were solved by direct methods (SHELXT) and refined through full-matrix least-squares techniques on F2 using SHELXL and OLEX2 crystallographic software packages. PL and those excitation spectra were recorded using a JASCO model FP-8500 spectrophotometer. The absolute quantum yield (ΦPL) was estimated using Hamamatsu C9920-02 system equipped with an integrating sphere. The spectra used for the estimation of ΦPL were included in Appendix S1 in the ESI.† Optical microscopy fluorescence images were obtained using a SHIMADZU moticam 1080BMH with WUBEN E19 UV 365 nm. WUBEN E19 was also used for the examinations of photostability. Spatially resolved μ-PL spectra were recorded using an analyzing system [405 nm UV laser OptoSigma LDU33-405-3.5 (Sigma-Koki), micro-PL spectra using a USB400 and a R200-7-UV-vis probe recorded using a CCD (detector: Sony ILX511B linear siliconCCD array). The excitation laser was filtered with a band-pass filter (YIF-BA460IFS) and focused on the crystal samples with an objective lens (50×, NA = 0.80 or 20×, NA = 0.45). The crystal samples were excited at 5.0 μW. The collected emission was then guided to a spectrometer (Lambda Vision SA-100A) and recorded using a CCD (detector: Hamamatsu Photonics S11151-2048 CCD linear image sensor). Optically pumped lasing was measured using a fiber-coupled spectrometer (HRS-300S-NI-KKAS) equipped with a CCD detector (PIXIS-256E-KKAS), and a Nd:YAG laser (λ3ω = 355 nm, > 5 ns pulse duration, 1 kHz repetition) as an optical excitation source. The crystal thickness was measured using a surface profiler (KLA-Tencor, Alpha-Step D-500).Theoretical calculations
Density functional theory (DFT) calculations were performed using the Gaussian 03 suite of programs using the B3LYP/6-31G+ methods. The orbital diagrams were generated using the GaussView program.56Conclusions
We newly designed a highly luminescent and polymorphic compound, BFTFP. Polymorphs with drastically varied morphologies and crystal structures were obtained due to the modified crystal growth methodologies. Owing to the variations of the crystal structures of BFTFP, specific photonic functions relating to the flexible fluorescence waveguiding, ASE, and vertical cavity resonators were successively assigned for each polymorph. The effectivity was also supported by comparison to crystals of similar chemical structures.Author contributions
T. M. designed the research, performed the experiments, and wrote the manuscript. S. H. supervised these processes.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data have been deposited at the CCDC under 2420271–2420277.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. H. acknowledges the JST FOREST Program (no. JPMJFR211W) and a KAKENHI (Grant-in-Aid for Scientific Research B: no. 24K01574) of the Japan Society for the Promotion of Science (JSPS). T. M. acknowledges a KAKENHI (Grant-in-Aid for Young Scientific Research: no. 22K14671), and a Scientific Research Grant from the “Iketani Science and Technology Foundation”. The authors acknowledge Prof. Dr Akitaka Ito at the Kochi University of Technology for measurements of PL lifetimes and evaluation of ΦPL. They also acknowledge Mr Nakabayashi and Mr Shimada at the Kochi University of Technology for the measurements and analysis of MS spectra.Notes and references
- S. Sandanayaka, T. Matsushima, F. Bencheikh, S. Terakawa, W. J. Potscavage Jr, C. Qin, T. Fujihara, K. Goushi, J. Ribierre and C. Adachi, Appl. Phys. Express, 2019, 12, 061010 CrossRef.
- K. Yoshida, J. Gong, A. L. Kanibolotsky, P. J. Skabara, G. A. Turnbull and I. D. W. Samuel, Nature, 2023, 621, 746–752 CrossRef CAS.
- K. Takimiya, K. Bulgarevich, M. Abbas, S. Horiuchi, T. Ogaki, K. Kawabata and A. Ablat, Adv. Mater., 2021, 33, 2102914 CrossRef CAS PubMed.
- T. He, M. Stolte, C. Burschka, N. H. Hansen, T. Musiol, D. Kälblein, J. Pflaum, X. Tao, J. Brill and F. Würthner, Nat. Commun., 2015, 6, 5954 CrossRef CAS PubMed.
- T. Kanagasekaran, H. Shimotani, K. Kasai, S. Onuki, R. D. Kavthe, R. Kumashiro, N. Hiroshiba, T. Jin, N. Asao and K. Tanigaki, arXiv, 2019, arXiv:1903.08869.
- J. Gierschner, S. Varghese and S. Y. Park, Adv. Opt. Mater., 2016, 4, 348–364 CrossRef CAS.
- H. Huang, Q. Zheng, B. Yu, Y. Yang, Y. Li, Y. Li, Y. Ji, Q. Liao and H. Fu, J. Phys. Chem. Lett., 2024, 15, 4890–4897 CrossRef CAS.
- H.-H. Fang, R. Ding, S.-Y. Lu, Y.-D. Yang, Q.-D. Chen, J. Feng, Y.-Z. Huang and H.-B. Sun, Laser Photonics Rev., 2013, 7, 281–288 CrossRef CAS.
- T. Matsuo, Y. Ueda, H. Mizuno, F. Sasaki, K. Yamashita and H. Yanagi, ACS Photonics, 2022, 9, 2015–2023 CrossRef CAS.
- J. Tang, J. Zhang, Y. Lv, H. Wang, F. F. Xu, C. Zhang, L. Sun, J. Yao and Y. S. Zhao, Nat. Commun., 2021, 12, 3265 CrossRef CAS PubMed.
- T. Matsuo, F. Sasaki and H. Yanagi, Appl. Phys. Express, 2022, 15, 051002 CrossRef CAS.
- T. Matsuo and S. Hayashi, J. Phys. Chem. Lett., 2024, 15, 3968–3974 CrossRef CAS.
- T. Matsuo, S. Azuma, M. Nakabayashi, F. Sasaki and S. Hayashi, Adv. Opt. Mater., 2025, 2402701 CrossRef CAS.
- T. Matsuo, C. Rössiger, J. Herr, R. Göttlich, D. Schlettwein, H. Mizuno, F. Sasaki and H. Yanagi, RSC Adv., 2020, 10, 24057 RSC.
- S. Chen, X.-D. Wang, M.-P. Zhuo, G.-Q. Wei, J.-J. Wu and L.-S. Liao, Adv. Opt. Mater., 2022, 10, 2101931 CrossRef CAS.
- H. Liu, Z. Lu, B. Tang, Z. Zhang, Y. Wang and H. Zhang, Dyes Pigm., 2018, 149, 284–289 CrossRef CAS.
- R. Huang, Y. Wang, Y. Huang, M. Wang and S. Liao, Dyes Pigm., 2025, 235, 112639 CrossRef CAS.
- Y. Tanaka, K. Goto, K. Yamashita, T. Yamao, S. Hotta, F. Sasaki and H. Yanagi, Appl. Phys. Lett., 2015, 107, 163303 CrossRef.
- S. M. Yoon, J. Lee, J. H. Je, H. C. Choi and M. Yoon, ACS Nano, 2011, 5, 2923–2929 CrossRef CAS PubMed.
- S. Zhao, H. Yamagishi, O. Oki, Y. Ihara, N. Ichiji, A. Kubo, S. Hayashi and Y. Yamamoto, Adv. Opt. Mater., 2022, 10, 2101808 CrossRef CAS.
- T. Matsuo, J. Kuwabara, T. Kanbara and S. Hayashi, J. Phys. Chem. Lett., 2023, 14, 6577–6582 CrossRef CAS.
- X. Feng, R. Lin, S. Yang, Y. Xu, T. Zhang, S. Chen, Y. Ji, Z. Wang, S. Chen, C. Zhu, Z. Gao and Y. S. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202310263 CrossRef CAS.
- T. Yamao, Y. Taniguchi, K. Yamamoto, T. Miki, S. Ota, S. Hotta, M. Goto and R. Azumi, Jpn. J. Appl. Phys., 2007, 46, 7478–7482 CrossRef CAS.
- M. Nakabayashi, T. Matsuo and S. Hayashi, Chem. – Eur. J., 2023, 29, e2023023 CrossRef.
- K. Takazawa, J. Inoue, K. Mitsuishi and T. Takamasu, Adv. Mater., 2011, 23, 3659–3663 CrossRef CAS PubMed.
- J. Ravi and R. Chandrasekar, Adv. Opt. Mater., 2021, 9, 2100550 CrossRef CAS.
- S. Hayashi, S. Yamamoto, D. Takeuchi, Y. Ie and K. Takagi, Angew. Chem., Int. Ed., 2018, 57, 17002 CrossRef CAS PubMed.
- X. Cao, J. Peng, W. Wang, W. Xu and S. Xu, Dyes Pigm., 2023, 219, 111531 CrossRef CAS.
- Z. Lu, Y. Zhang, H. Liu, K. Ye, W. Liu and H. Zhang, Angew. Chem., Int. Ed., 2020, 59, 4299–4303 CrossRef CAS.
- L. Catalano, D. P. Karothu, S. Schramm, E. Ahmed, R. Rezgui, T. J. Barber, A. Famulari and P. Naumov, Angew. Chem., Int. Ed., 2018, 57, 17254–17258 CrossRef CAS.
- H. Liu, Z. Bian, Q. Cheng, L. Lan, Y. Wang and H. Zhang, Chem. Sci., 2019, 10, 227–232 RSC.
- Y. Wu, J. Feng, X. Jiang, Z. Zhang, X. Wang, B. Su and L. Jiang, Nat. Commun., 2015, 6, 6737 CrossRef CAS PubMed.
- M.-P. Zhuo, J.-J. Wu, X.-D. Wang, Y.-C. Tao, Y. Yuan and L.-S. Liao, Nat. Commun., 2019, 10, 3839 CrossRef.
- J. M. Halabi, E. Ahmed, L. Catalano, D. P. Karothu, R. Rezgui and P. Naumov, J. Am. Chem. Soc., 2019, 141, 14966–14970 CrossRef CAS PubMed.
- M. Annadhasan, A. V. Kumar, P. Giri, S. Nandy, M. K. Panda, K. V. J. Jose and R. Chandrasekar, Angew. Chem., Int. Ed., 2023, 62, e202302929 CrossRef CAS PubMed.
- M. Rohullah, V. V. Pradeep, S. Singh and R. Chandrasekar, Nat. Commun., 2024, 15, 4040 CrossRef CAS PubMed.
- S. Hayashi and T. Koizumi, Angew. Chem., Int. Ed., 2016, 55, 2701–2704 CrossRef CAS PubMed.
- T. Seki, N. Hoshino, Y. Suzuki and S. Hayashi, CrystEngComm, 2021, 23, 5686–5696 RSC.
- T. Matsuo, K. Ikeda and S. Hayashi, Aggregate, 2023, 4, e378 CrossRef CAS.
- Y. Oyama, M. Mamada, A. Shukla, E. G. Moore, S.-C. Lo, E. B. Namdas and C. Adachi, ACS Mater. Lett., 2020, 2, 161–167 CrossRef CAS.
- X. Zhu, H. Tsuji, J. T. L. Navarrete, J. Casado and E. Nakamura, J. Am. Chem. Soc., 2012, 134, 19254–19259 CrossRef CAS PubMed.
- G. W. Coates, A. R. Dunn, L. M. Henling, J. W. Ziller, E. B. Lobkovsky and R. H. Grubbs, J. Am. Chem. Soc., 1998, 120, 3641–3649 CrossRef CAS.
- H. Wang, P. Chen, Z. Wu, J. Zhao, J. Sun and R. Lu, Angew. Chem., 2017, 129, 9591–9595 CrossRef.
- G. J. O. Beran, I. J. Sugden, C. Greenwell, D. H. Bowkill, C. C. Pantelides and C. S. Adjiman, Chem. Sci., 2022, 13, 1288–1297 RSC.
- E. Parisi, E. Santagata, E. Simone, F. Borbone and R. Centore, J. Am. Chem. Soc., 2024, 146, 19405–19413 CrossRef CAS PubMed.
- G. H. Roche, D. Flot, J. J. E. Moreau, O. J. Dautel, J.-S. Filhol and A. Lee, Cryst. Growth Des., 2021, 21, 3850–3863 CrossRef CAS.
- E. J. Wu, A. W. Kelly, L. Iuzzolino, A. Y. Lee and X. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202406214 CrossRef CAS PubMed.
- S. Ye, G. Liu, S. Pu and J. Wu, Org. Lett., 2012, 14, 70–73 CrossRef CAS PubMed.
- J. Bernstein, Polymorphism in Molecular Crystals, Oxford University Press, New York, 2002 Search PubMed.
- C. Liu, J. G. Brandenburg, O. Valsson, K. Kremer and T. Bereau, Soft Matter, 2020, 16, 9683–9692 RSC.
- S. K. Cohen and S. Forrest, Nat. Photonics, 2010, 4, 371–375 CrossRef.
- K. Yamashita, T. Nakahata, T. Hayakawa, Y. Sakurai, T. Yamao, H. Yanagi and S. Hotta, Appl. Phys. Lett., 2014, 104, 253301 CrossRef.
- O. Gidron, N. Varsano, L. J. W. Shimon, G. Leitus and M. Bendikov, Chem. Commun., 2013, 49, 6256–6258 RSC.
- P. A. Praveen, T. Kanagasekaran, C. Ma, M. Terada, T. Jin, Y. Wakabayashi and H. Shimotani, J. Mater. Chem. C, 2024, 12, 15995–16003 RSC.
- P. A. Praveen, P. Muthuraja, P. Gopinath and T. Kanagasekaran, J. Phys. Chem. A, 2022, 126, 600–607 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao and H. Nakaiet al.Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford, CT, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2420271–2420277. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5tc00817d |
| This journal is © The Royal Society of Chemistry 2025 |




