Chiral hybrid manganese(II) chloride single crystals for achieving second harmonic generation and moderate circularly polarized luminescence†
Teng
Li
a,
Yue
Wang
b,
Yanqing
Liu
a,
Guokui
Liu
 c,
Lingqiang
Meng
*d,
Yongshen
Zheng
*b and
Yangyang
Dang
c,
Lingqiang
Meng
*d,
Yongshen
Zheng
*b and
Yangyang
Dang
 *a
*a
aSchool of Physics and Physical Engineering, Qufu Normal University, Qufu 273165, P. R. China. E-mail: dyy@qfnu.edu.cn
bSchool of Materials Science and Engineering, Smart Sensing Interdisciplinary Science Center, Nankai University, Tianjin 300350, P. R. China. E-mail: yszheng@nankai.edu.cn
cSchool of Chemistry and Chemical Engineering, Linyi University, Linyi 276000, P. R. China
dSchool of Advanced Materials, Peking University Shenzhen Graduate School, Peking University, Shenzhen 518055, P. R. China. E-mail: menglingqiang@pku.edu.cn
First published on 10th March 2025
Abstract
Chiral hybrid metal halides have attracted much attention in optoelectronic fields such as encryption storage, security monitoring, and three-dimensional (3D) displays, due to their low cost, low toxicity, good stability, high luminescence efficiency, tunable emission, and chiroptical and nonlinear optical properties. Here, a pair of chiral manganese(II)-based hybrid chloride single crystals (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O (mpip = methylpiperazinium) were synthesized by a slow evaporation method. The crystal structures, X-ray photoelectron spectroscopy (XPS), second harmonic generation, band gap calculation and photoluminescence (PL) were systematically studied. (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals exhibited strong red emission characteristics caused by d–d transition of free Mn2+ when heated to 383 K. Moreover, heated (R/S-2-mpip)MnCl4·2H2O materials exhibit the obvious circular dichroism and red circularly polarized luminescence characteristics with a moderate luminescence dissymmetry factor glum value reaching ±1.2 × 10−3, which provides a new research direction for further applications from anticounterfeiting to 3D displays, as well as encryption storage.
Introduction
Organic–inorganic hybrid metal halide materials1,2 have attracted much attention due to their low exciton energy, long carrier lifetime, and high absorption efficiency.3,4 However, chiral hybrid metal halide materials exhibit unique physical properties, such as circular dichroism (CD), circularly polarized luminescence (CPL),5–8 nonlinear optical effects (NLO),9,10 ferroelectricity, and spintronics, which has extended their applications in optoelectronic devices, optical sensors, and spintronics.11–15 Among them, CD and CPL are particularly important for chiral hybrid metal halides. In 2018, Long et al. achieved spin-polarized photoluminescence in quasi-2D perovskites without external magnetism, with an asymmetry factor of about ∼3%.16 Gao et al. successfully synthesized the first chiral ferroelectric materials with circularly polarized luminescence activity, (R)- and (S)-3-(fluoropyrrolidinium)MnBr3, with glum = ±6.1 × 10−3, by introducing homochiral cations into MnBr3 inorganic chains.17 Xuan et al. reported that the enantiomeric chiral hybrid (R)- and (S)-C6H15Cl2NO·SbCl5 exhibits the strong luminescence, with a photoluminescence quantum yield (PLQY) of 71.2% and a CPL activity of 2.5 × 10−4.18 Chen et al. reported 1 D chain-like hybrid manganese bromides, (R-3-quinuclidinol)MnBr3, having a high glum of 2.3 × 10−2 and a PLQY of 50.2%.19 In 2023, they synthesized another 1 D hybrid copper iodide compound Cu4I4(R/S-3-quinuclidinol)3, which has a luminescence dissymetry factor glum of 4.0 × 10−3 and a PLQY close to 100%.20 However, the anisotropy factor of absorbance (gCD) for circularly polarized light absorption of current chiral organic–inorganic hybrid halide materials is generally low. In particular, for chiral hybrid halide materials with good circularly polarized luminescence, the luminescence dissymmetry factor (glum) is also low, while chiral halide materials with high glum usually exhibit relatively weak luminescence properties.21–26 Therefore, it is very important to design and construct ideal chiral hybrid metal halides with effective left or right CPL and high PLQY.27 Most of the previous studies were based on chiral hybrid lead halides. Based on their strong toxicity and poor stability, further optoelectronic applications of chiral lead-based halide materials were limited. Elements such as Sn,28–30 Ge,31 Bi,32–34 Cu,35 and Mn17,19 are commonly used to replace lead in the achiral and chiral lead-based halide perovskites. Among them, the non-toxicity and low cost of chiral Mn(II)-based hybrid halides, and the unique electronic structure of Mn(II) with d–d transition (4T1 → 6A1) make chiral Mn(II)-based hybrid halides exhibit some peculiar optoelectronic characteristics, which is different from the previously reported chiral Cr(III)-based complexes,36,37 such as efficient emission, large Stokes shift and longer PL lifetime.38–40By integrating the chiral characteristics with the excellent properties of Mn(II)-based halides, we synthesized a pair of chiral hybrid manganese(II)-based halide single crystals (R/S-2-mpip)MnCl4·2H2O with moderate luminescence dissymmetry factor (glum). The crystal structures, UV-Vis diffuse reflectance spectra, TGA, XPS, theoretical calculations, and optical properties of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O were studied in detail. More importantly, chiral (R/S-2-mpip)MnCl4·2H2O exhibited the obvious optical characteristics including second harmonic generation (SHG), circular dichroism (CD) and circularly polarized luminescence (CPL). These studies can provide guidance for further optoelectronic applications of these chiral Mn(II)-based halide single-crystalline materials.
Results and discussion
Synthesis and crystal growth
During the synthetic process, it is difficult to form high-quality single crystals directly in HCl, in which CH3OH had been proven to be used for inducing crystallization in previous studies,41 so we used HCl–CH3OH mixed solution to provide a good growth environment for the crystals. Our research group has also synthesized high-quality single crystals using CH3OH as a co-crystal agent.42 However, different proportions of HCl and CH3OH also have an impact on the quality of the crystals. With the gradual increase of the proportion of CH3OH, the quality of crystal growth gradually improves. After a series of attempts with different proportions, the crystal quality was the best when the ratio of HCl to CH3OH was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. R/S-2-mpip and rac-2-mpip were, respectively, mixed with MnCl2 in an equimolar ratio in the air, dissolved in an equimolar ratio of HCl–CH3OH solution, and the colored precursor solutions of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O were formed and synthesized by the slow evaporation method, as shown in Fig. S1 (ESI†). Colorless plate single crystals of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O were obtained after 3–4 days, as shown in Fig. 1a, b, e, f and i, j. The phase transformation of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals and powders was observed when heated to 383 K. At room temperature, (R/S-2-mpip) MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O crystals exhibited no emission. When heated to 383 K, (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O showed the obvious red emission under the irradiation of 365 nm wavelength. The photoluminescence (PL) of their powders when exposed to ultraviolet light of different wavelengths demonstrated that the PL under 254 nm wavelength irradiation was stronger than that under 365 nm wavelength irradiation in Fig. 1c, d, g, h and k, l.
1. R/S-2-mpip and rac-2-mpip were, respectively, mixed with MnCl2 in an equimolar ratio in the air, dissolved in an equimolar ratio of HCl–CH3OH solution, and the colored precursor solutions of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O were formed and synthesized by the slow evaporation method, as shown in Fig. S1 (ESI†). Colorless plate single crystals of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O were obtained after 3–4 days, as shown in Fig. 1a, b, e, f and i, j. The phase transformation of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals and powders was observed when heated to 383 K. At room temperature, (R/S-2-mpip) MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O crystals exhibited no emission. When heated to 383 K, (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O showed the obvious red emission under the irradiation of 365 nm wavelength. The photoluminescence (PL) of their powders when exposed to ultraviolet light of different wavelengths demonstrated that the PL under 254 nm wavelength irradiation was stronger than that under 365 nm wavelength irradiation in Fig. 1c, d, g, h and k, l.
Thermogravimetric analysis (TGA) measurements were conducted on the (R/S-2-mpip)MnCl4·2H2O single crystals within the temperature range of 300 to 800 K, as shown in Fig. S2a (ESI†). The results indicate that (R/S-2-mpip)MnCl4·2H2O are stable at 371 K. Around these temperatures, the TGA curve first drops to 90%, corresponding to the loss of ligand water. The second decrease in the TGA curve can be attributed to the decomposition of the (R/S-2-mpip)MnCl4·2H2O single crystal structure. Differential scanning calorimetry (DSC) measurements were conducted on the (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals, as shown in Fig. S2b–d (ESI†). DSC curves for the (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals show broad phase transition peaks at 378 K, 380 K and 386 K as the temperature is increased from 293 K to 398 K, corresponding to the loss of ligand water. Moreover, no phase transition peaks were observed within the temperature range of 398 to 293 K, indicating that (R/S-2-mpip)MnCl4·2H2O materials undergo the irreversible phase transformation, which is consistent with the phase transformation peaks in the TGA curves.
Crystal structure related analysis
The single-crystal X-ray diffraction measurements and analysis demonstrate that (R/S-2-mpip)MnCl4·2H2O single crystals belong to the chiral monoclinic space group P21 (no. 4), while (rac-2-mpip)MnCl4·2H2O single crystals belong to the centro-symmetric monoclinic space group P21/c (no. 14). The crystal parameters are a = 8.6660(5) Å, b = 6.4956(4) Å, c = 12.0646(7) Å, and V = 665.10(7) Å3 for (R-2-mpip)MnCl4·2H2O, a = 8.6614(19) Å, b = 6.4962(13) Å, c = 12.065(3) Å, and V = 664.8(2) Å3 for (S-2-mpip)MnCl4·2H2O and a = 12.0890(8) Å, b = 6.4455(4) Å, c = 17.3538(12) Å, and V = 1321.47(15) Å3 for (rac-2-mpip)MnCl4·2H2O, as shown in Table S1 (ESI†). In all the crystal structures of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O, one Mn(II) atom coordinates with four Cl atoms and two oxygen atoms derived from two water (H2O) molecules, forming a six-coordinated deformed octahedron [MnCl4(H2O)2]2−, as shown in Fig. 2a–c, respectively. (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O complex each consist of a [MnCl4(H2O)2]2− octahedron and the organic cations [R-2-mpip2+, S-2-mpip2+ or rac-2-mpip2+], while [MnCl4(H2O)2]2− octahedra are separated by the above organic cations, forming a typical 0D structure. In the crystal structure of (R-2-mpip)MnCl4·2H2O, the shortest distance between adjacent Mn–Mn is 6.4956(5) Å, the Mn–Cl bond lengths range from 2.5185(6) to 2.5981(7) Å, the Mn–O bond lengths range from 2.1811(17) to 2.1913(18) Å, the Cl–Mn–Cl bond angles range from 88.05(2) to 178.16(3)°, the O–Mn–Cl bond angles range from 85.49(5) to 93.18(5)°, and the O–Mn–O bond angle is 176.73(8)°. In the crystal structure of (S-2-mpip)MnCl4·2H2O, the shortest distance between adjacent Mn–Mn is 6.4962(14) Å, the Mn-Cl bond lengths range from 2.5169(9) to 2.5983(8) Å, the Mn–O bond lengths range from 2.1795(18) to 2.193(2) Å, the Cl–Mn–Cl bond angles range from 88.05(2) to 178.13(3)°, the O–Mn–Cl bond angles range from 85.49(6) to 93.19(5)°, and the O–Mn–O bond angle is 176.68(9) °. In the crystal structure of (rac-2-mpip)MnCl4·2H2O, the shortest distance between adjacent Mn–Mn is 6.4455(5) Å, the Mn–Cl bond lengths range from 2.5180(6) to 2.6024(6) Å, and the Mn–O bond lengths range from 2.1744(14) to 2.1750(14) Å, the Cl–Mn–Cl bond angles range from 85.775(19) to 176.43(2)°, the O–Mn–Cl bond angles range from 86.23(4) to 92.38(4)°, and the O–Mn–O bond angle is 178.21(6)°. From the above data, it can be seen that the Mn–Cl bond lengths are roughly the same as the previously reported Mn–Cl bond lengths,43,44 but the Mn–O bond lengths are shorter, and hence the [MnCl4(H2O)2]2− octahedron is not an ideal octahedron and exhibits slight distortion. The powder X-ray diffraction (PXRD) patterns of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O match well with the simulated XRD patterns from their single-crystal X-ray diffraction, as shown in Fig. 2d–f, respectively. When heated to 383 K, (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals still exhibit the relatively good stability. To verify the chemical elements and composition of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O, XPS is used to analyze the chemical and band state in Fig. S3–S5 (ESI†). The core Mn 2p peaks of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O located at ≈640, 644, and 651 eV are attributed to Mn 2p3/2, Mn–O, and Mn 2p1/2, respectively. Besides, the bond valence sum (BVS) for (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O is depicted in Tables S2–S4 (ESI†). The bond valence Sij of Mn element is +2, which further verifies the accuracy of the above crystal structures.To verify the accuracy of these chiral crystal structures, second harmonic generation (SHG) of (R/S-2-mpip)MnCl4·2H2O was demonstrated. Using a femtosecond laser device as a pump,45 the SHG signals of these chiral manganese halides were collected. The wavelength-dependent SHG spectra collected from the (R-2-mpip)MnCl4·2H2O single crystals under the same laser power (50 mW) are shown in Fig. 3a, demonstrating a clear SHG response of (R-2-mpip)MnCl4·2H2O when excited with a broadband laser from 800 to 1040 nm. Meanwhile, SHG measurements were also performed on (S-2-mpip)MnCl4·2H2O (Fig. S6, ESI†). In particular, these SHG signals are highly dependent on the pump excitation wavelength, with the peak positions varying with the pump wavelength and always remaining at half the position of the pump excitation wavelength.45–47 When the pump wavelength is at 1020 nm, the (R-2-mpip)MnCl4·2H2O single crystal exhibits the strongest SHG signal at 510 nm. The wavelength-dependent SHG spectra of Y-cut quartz in the range of 800–1040 nm are shown in Fig. 3b. In contrast, the SHG intensity of (R-2-mpip)MnCl4·2H2O at an excitation wavelength of 1000 nm can reach up to 1/20 of that of Y-cut quartz. To further investigate the nonlinear optical properties of (R-2-mpip)MnCl4·2H2O single crystals, we conducted polarization-dependent measurements. We selected several laser wavelengths of 920 nm, 1000 nm and 1020 nm to carry out the polarization-dependent spectra to reveal the relationship between SHG intensity and polarization angle, as shown in Fig. 3d–f. By changing every 10° polarization angle, we obtained the distribution relationship between the SHG intensity and the polarization angle. The profile of this quadrupolar curve was accurately fitted using the cos4(θ) function. A slight deviation of the polarization angle dependence of SHG intensity from the ideal cos4(θ) behavior was observed. These deviations could be caused by crystal imperfections and wavelength-dependent effects. The results indicate that the material exhibits significant anisotropy. For (R-2-mpip)MnCl4·2H2O, the maximum SHG intensity under 920 nm excitation occurs at polarization angles of approximately 10° and 190°, while under 1000 nm and 1020 nm excitation, the maximum SHG intensity occurs at polarization angles of approximately 100° and 280°. We quantified the anisotropy in each case using the formula ρ = (Imax − Imin)/(Imax + Imin). The (R-2-mpip)MnCl4·2H2O exhibits pronounced anisotropic behavior, with the 920 nm excitation showing a strong alignment with the expected anisotropic response, as demonstrated by a polarization ratio of 60%. Power-dependent tests were conducted with an incident wavelength of 800 nm, as shown in Fig. 3c. (S-2-mpip)MnCl4·2H2O exhibited NLO characteristics, which was similar to the above results, as shown in Fig. S6 (ESI†). Therefore, the above experimental results demonstrate that the SHG intensity shows a slope of approximately 2.0 with respect to the incident laser power, confirming second-order nonlinear optical properties of (R/S-2-mpip)MnCl4·2H2O, which further verifies their chiral structures.
UV-vis diffuse reflectance spectra and theoretical calculations
The UV-Vis diffuse reflectance spectra of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O powders in air were measured before and after heating to 383 K. And the optical band gap of these crystals was calculated using the Kubelka–Munk function.48,49 The optical band gaps of (R/S-2-mpip)MnCl4·2H2O are 5.42 eV and 5.48 eV, as shown in Fig. 4a and d. It can be observed from Fig. 4g that (rac-2-mpip)MnCl4·2H2O exhibits a characteristic peak around 350 nm, the optical band gap of which is 5.20 eV. To further understand the electronic properties of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O, density functional theory (DFT) calculations were performed. As shown in Fig. 4b and e, the indirect band gaps of (R/S-2-mpip)MnCl4·2H2O are both 2.38 eV. As for (R/S-2-mpip)MnCl4·2H2O, the valence band maximum (VBM) is located at the R2 point, while the conduction band minimum (CBM) is situated at the Z point.50 In contrast to the aforementioned cases, the direct band gap of (rac-2-mpip)MnCl4·2H2O is found to be 2.45 eV, and interestingly, both VBM and CBM are situated at the Z point, which can be clearly observed in Fig. 4h. Due to the limitations of density functional theory (DFT), the optical band gap is often larger than the theoretical band gap.51,52 We also calculated the projected density of states (PDOS) diagrams for all the systems studied and selected the key components, as shown in Fig. 4c, f, and i. The PDOS diagrams reveal that the VBM of (R-2-mpip)MnCl4·2H2O is primarily composed of the spin-up Mn 3d atomic orbitals, spin-up Cl 3p atomic orbitals, and some spin-up O 2p atomic orbitals, while the CBM is mainly constituted by the spin-down Mn 3d atomic orbitals. The PDOS composition in (S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O is similar to that in (R-2-mpip)MnCl4·2H2O. These results indicate that the absorption and excitation processes of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals primarily occur within the inorganic framework.Based on the above phase transformation when exposed to 383 K, the optical band gaps of (R/S-2-mpip)MnCl4·2H2O (heated) and (rac-2-mpip)MnCl4·2H2O (heated) are respectively 2.58 eV, 2.58 eV and 2.67 eV, as can be observed in Fig. S7a–c (ESI†). Characteristic peaks for (R-2-mpip)MnCl4·2H2O (heated) appear around 244 nm, 264 nm, and 420 nm. In the case of (S-2-mpip)MnCl4·2H2O (heated), distinct characteristic peaks are noted around 256 nm, 325 nm, and 422 nm. As for (rac-2-mpip)MnCl4·2H2O (heated), its characteristic peaks are roughly located at 280 nm and 330 nm. From the above results, The optical band gaps of (R/S-2-mpip)MnCl4·2H2O (heated) and (rac-2-mpip)MnCl4·2H2O (heated) are different from those of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O.
Photoluminescence (PL) properties
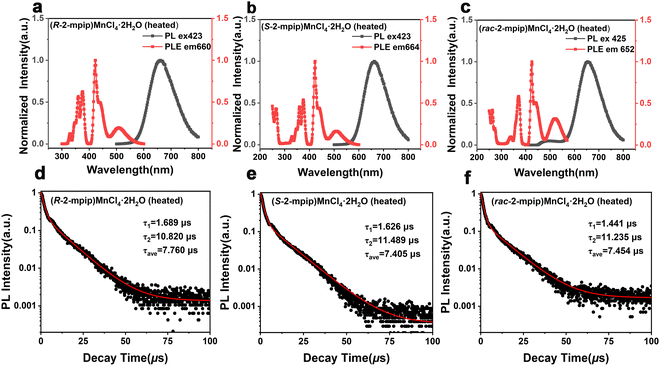
In order to further understand the optical properties of chiral hybrid Mn(II)-based materials, PL measurements of (R/S-2-mpip)MnCl4·2H2O (heated) and (rac-2-mpip)MnCl4·2H2O (heated) were performed, such as photoluminescence excitation (PLE), photoluminescence (PL), and PL decay lifetime and photoluminescence quantum yields (PLQY). The PLE spectra of (R/S-2-mpip)MnCl4·2H2O (heated) in Fig. 5a and b show multiple peaks at 344 nm, 360 nm, 374 nm, 420 nm, and 505 nm, which corresponds to 6A1(6S) → 4Eg(4D), 6A1(6S) → 4T2g(4D), 6A1(6S) → 4A1g,4Eg(4G), 6A1(6S) → 4T2g(4G), and 6A1(6S) → 4T1g(4G) transitions, respectively.53,54 The only red emission peak of (R/S-2-mpip)MnCl4·2H2O (heated) single crystals located at about 650 nm was confirmed when exposed to 423 nm irradiation. However, (rac-2-mpip)MnCl4·2H2O single crystals exhibit a PL peak located at 652 nm when excited at 425 nm, as depicted in Fig. 5c, which exhibits the multiple distinct PLE peaks similar to those of (R/S-2-mpip)MnCl4·2H2O (heated), and also show a significant Stokes shift of approximately 227 nm attributed to self-trapped excitons (STEs).55,56 A second-order exponential decay function was used for fitting their PL decay lifetime and the average PL decay lifetimes of (R/S-2-mpip)MnCl4·2H2O (heated) and (rac-2-mpip)MnCl4·2H2O (heated) were calculated to be 7.760 μs, 7.405 μs, and 7.454 μs, respectively, as shown in Fig. 5d–f. This indicates that the three crystals have relatively long average PL decay lifetimes. From Fig. S8a–c (ESI†), it can be seen that the PLQY values of (R/S-2mpip)MnCl4·2H2O (heated) are approximately 13% and 11%. However, the PLQY of (rac-2mpip)MnCl4·2H2O (heated) reaches over 50%, indicating a high PLQY. The higher orange-red emission PLQY observed in (rac-2mpip)MnCl4·2H2O (heated) is possibly attributed to the regular arrangement in the centrosymmetric structure of (rac-2mpip)MnCl4·2H2O.57Given that the crystalline powders of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O possess no PL properties at room temperature, they exhibit red light emissions of varying intensities under irradiation at 254 nm and 365 nm when heated to 383 K, as illustrated in Fig. S9 (ESI†). This phenomenon corresponds to the disappearance of coordinated water during the heating processes, thereby indicating that the three types of crystals demonstrate the thermochromic phase transformations and hygroscopic sensitivity.
To verify the stability of (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O in different solvents (distilled water and anhydrous ethanol), we opted for spraying distilled water or anhydrous ethanol evenly onto the heated crystalline powders, and it was found that under 254 nm and 365 nm wavelength irradiation, red emission almost completely vanished. After allowing the samples to air dry, (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O exhibited no luminescence under subsequent irradiation with light of various wavelengths. Interestingly, upon reheating the samples at 383 K, the red emission of these samples was recovered, as demonstrated in Fig. S10 and S11 (ESI†), which proved that the thermochromic phase transformation was reversible in distilled water and anhydrous ethanol. Therefore, these Mn(II)-based hybrid halide materials can provide guidance for optoelectronic applications, such as anti-counterfeiting encryption, thermal detection and alcohol tests.58
Circular dichroism (CD) and circularly polarized luminescence (CPL)
To verify the intrinsically chiroptical activity, (R/S-2-mpip)MnCl4·2H2O (heated) single-crystalline powders were prepared in KBr pellets and subjected to related measurement parameters such as circular dichroism (CD), circularly polarized luminescence (CPL), glum, direct current (DC) voltage, and ΔI. The circular dichroism and absorption spectra of (R/S-2-mpip)MnCl4·2H2O are shown in Fig. 6a and b. The microcrystalline powders of (R/S-2-mpip)MnCl4·2H2O exhibit distinct CD spectra in Fig. 6a, with opposite signals in the wavelength range from 210 nm to 230 nm. The CD peaks of (R/S-2-mpip)MnCl4·2H2O are located 215 nm before the edge of the absorption spectra's extinction band, indicating the moderate chiroptical characteristics. Apparently, the spectra of (R/S-2-mpip)MnCl4·2H2O show the mirror-like opposite CPL signals in the 575–750 nm region,59,60 as shown in Fig. 6c, which corresponds to the DC spectra in Fig. 6d. Anisotropy changes the polarization state of the CPL, leading to changes in the polarization ellipse parameters and affecting accurate characterizations. The absorption and scattering of LCP and RCP are different, leading to changes in the intensity of the CPL signal and affecting the evaluation of optical activity. | ||
| Fig. 6 The circularly polarized luminescence spectra of the (R/S-2-mpip)MnCl4·2H2O (heated). (a) CD, (b) absorbance, (c) CPL, (d) DC(V), (e) glum and (f) ΔI of the (R/S-2-mpip)MnCl4·2H2O (heated). | ||
Moreover, the interaction of anisotropic structures with the CPL can enhance or weaken the optical activity of the CPL, increasing the measurement uncertainty. Birefringence alters the phase relationship between the LCP and RCP, leading to a change in the polarization state and a deviation in the measurement of the optical rotation angle. It generates interference phenomena, which interferes with the CPL signal and masks the signal characteristics, resulting in measurement errors. Furthermore, it is wavelength-dependent, changing the shape and intensity distribution of the CPL spectra and affecting the analysis of the spectral properties. Due to the difficulty in directly describing CPL materials, the dissymmetry factor glum was introduced,61,62 with the expression formula, glum = 2(IL − IR)/(IL + IR). In Fig. 6e, glum value of (R/S-2-mpip)MnCl4·2H2O is approximately ±1.2 × 10−3, with opposite signals, which is close to the value of the other Mn(II)-based halides in Table S5 (ESI†). ΔI represents the difference between IL (LCP) and IR (RCP), and Fig. 6f shows the opposite signals. Intrinsic chirality of (R/S-2-mpip)MnCl4·2H2O was formed via the chiral organic cations and inorganic framework through hydrogen bond interactions. These results reveal that the chiral cations have successfully imparted their chirality to the inorganic structural framework, thereby endowing the inorganic framework with the inherent chiral characteristics of the chiral cations.63
Experimental
Raw materials and reagents
All the analytical raw materials and reagents of MnCl2 (99%, AR, J&K), R/S-2-mpip (99%, AR, J&K), rac-2-mpip (99%, AR, J&K), HCl (AR, J&K) and CH3OH (99.5%, J&K) were purchased from Sinopharm Co., Ltd and directly utilized without further purification in the experiment.Synthesis and crystal growth
(R-2-mpip)MnCl4·2H2O was synthesized with a reaction of R-2-mpip and MnCl2 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in a HCl–CH3OH mixed solution. R-2-mpip (0.9172 g, 9 mmol) and MnCl2 (1.1490 g, 9 mmol) were dissolved in HCl (2 mL) and CH3OH (2 mL) solution at 60 °C to form a light green solution. And then the solution was cooled to room temperature. Finally, the solution was placed in a quiet position to be grown by a slow evaporation method. Colourless (R-2-mpip)MnCl4·2H2O single crystals were formed after 3–4 days, as shown in Fig. 1a.
1 in a HCl–CH3OH mixed solution. R-2-mpip (0.9172 g, 9 mmol) and MnCl2 (1.1490 g, 9 mmol) were dissolved in HCl (2 mL) and CH3OH (2 mL) solution at 60 °C to form a light green solution. And then the solution was cooled to room temperature. Finally, the solution was placed in a quiet position to be grown by a slow evaporation method. Colourless (R-2-mpip)MnCl4·2H2O single crystals were formed after 3–4 days, as shown in Fig. 1a.
(S-2-mpip)MnCl4·2H2O was synthesized with a reaction of S-2-mpip and MnCl2 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in HCl–CH3OH mixed solution. S-2-mpip (0.9194 g, 9 mmol) and MnCl2 (1.1347 g, 9 mmol) were dissolved in HCl (2 mL) and CH3OH (2 mL) solution at 60 °C to form a light green solution. And then the solution was cooled to room temperature. Finally, the solution was placed in a quiet position to be grown by a slow evaporation method. Colourless (S-2-mpip)MnCl4·2H2O single crystals were formed after 3–4 days, as shown in Fig. 1e.
1 in HCl–CH3OH mixed solution. S-2-mpip (0.9194 g, 9 mmol) and MnCl2 (1.1347 g, 9 mmol) were dissolved in HCl (2 mL) and CH3OH (2 mL) solution at 60 °C to form a light green solution. And then the solution was cooled to room temperature. Finally, the solution was placed in a quiet position to be grown by a slow evaporation method. Colourless (S-2-mpip)MnCl4·2H2O single crystals were formed after 3–4 days, as shown in Fig. 1e.
(rac-2-mpip)MnCl4·2H2O was synthesized by the reaction of rac-2-mpip and MnCl2 with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in HCl–CH3OH mixed solution. rac-2-mpip (0.9208 g, 9 mmol) and MnCl2 (1.1352 g, 9 mmol) were dissolved in HCl (2 mL) and CH3OH (2 mL) solution at 60 °C to form a light yellow solution. And then the solution was cooled to room temperature. Finally, the solution was placed in a quiet position to be grown by a slow evaporation method. Colourless (rac-2-mpip)MnCl4·2H2O single crystals were formed after 3–4 days, as shown in Fig. 1i.
1 in HCl–CH3OH mixed solution. rac-2-mpip (0.9208 g, 9 mmol) and MnCl2 (1.1352 g, 9 mmol) were dissolved in HCl (2 mL) and CH3OH (2 mL) solution at 60 °C to form a light yellow solution. And then the solution was cooled to room temperature. Finally, the solution was placed in a quiet position to be grown by a slow evaporation method. Colourless (rac-2-mpip)MnCl4·2H2O single crystals were formed after 3–4 days, as shown in Fig. 1i.
The above synthesis and growth were performed in an ambient atmosphere.
Conclusions
In summary, chiral manganese(II)-based hybrid halide single crystals (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O were successfully obtained at room temperature by the slow evaporation method. Interestingly, (R/S-2-mpip)MnCl4·2H2O single crystals exhibited the moderate chiroptical activity and second-harmonic generation, which showed the stable and effective CPL behaviours with glum reaching ± 1.2 × 10−3. (R/S-2-mpip)MnCl4·2H2O and (rac-2-mpip)MnCl4·2H2O single crystals when heated up to 383 K exhibited the red luminescence with higher PLQY. These characteristics provide guidance for the structural design and further optical applications of these chiral hybrid Mn(II)-based materials.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 51802215 and 22401157), the Natural Science Foundation of Shandong Province (No. ZR2023ME095 and ZR2022MA067), and the Fundamental Research Funds for the Central Universities, Nankai University (No. 63241411). All the authors thank Dr Chunlong Li (Qilu University of Technology), Dr Jing Wei (Beijing Institute of Technology), Dr Ruiheng Pan (Chongqing University of Posts and Telecommunications), and Prof. Kang Wang (ICCAS) for their help with UV-vis spectra, XPS, PLE/PL spectra and PLQY measurements, respectively.Notes and references
- C. K. Zhou, H. R. Lin, Q. Q. He, L. J. Xu, M. Morku, M. Chaaban, S. Lee, X. Q. Shi, M. H. Du and B. W. Ma, Mater. Sci. Eng., R, 2019, 137, 38–65 Search PubMed.
- C. K. Zhou, H. R. Lin, Y. Tian, Z. Yuan, R. Clark, B. H. Chen, L. J. van de Burgt, J. C. Wang, Y. Zhou, K. Hanson, Q. J. Meisner, J. Neu, T. Besara, T. Siegrist, E. Lambers, P. Djurovich and B. W. Ma, Chem. Sci., 2018, 9, 586–593 CAS.
- C. K. Zhou, H. R. Lin, H. L. Shi, Y. Tian, C. Pak, M. Shatruk, Y. Zhou, P. Djurovich, M. H. Du and B. W. Ma, Angew. Chem., Int. Ed., 2018, 57, 1021–1024 CAS.
- Y. Zhang, Y. G. Zhang, Y. Y. Zhao, H. Jia, Z. W. Yang, B. P. Yin, Y. C. Wu, Y. P. Yi, C. Zhang and J. N. Yao, J. Am. Chem. Soc., 2023, 145, 12360–12369 CAS.
- D. D. Nuzzo, L. Cui, J. L. Greenfield, B. Zhao, R. H. Friend and S. C. J. Meskers, ACS Nano, 2020, 14, 7610–7616 Search PubMed.
- J. Q. Ma, C. Fang, C. Chen, L. Jin, J. Q. Wang, S. Wang, J. Tang and D. H. Li, ACS Nano, 2019, 13, 3659–3665 CrossRef CAS PubMed.
- Y. Liu, Z. Luo, Y. Wei, C. Li, Y. Chen, X. He, X. Chang and Z. Quan, Angew. Chem., Int. Ed., 2023, 62, e202306821 CrossRef CAS PubMed.
- X. Niu, Y. Li, L. Lu, Z. Wang, Y. Zhang, T. Shao, H. Wang, S. Gull, B. Sun, H.-L. Zhang, Y.-S. Chen, K. Wang, Y. Du and G.-K. Long, Nat. Commun., 2025, 16, 2525 Search PubMed.
- Y. S. Zheng, J. L. Xu and X.-H. Bu, Adv. Opt. Mater., 2022, 10, 2101545 CrossRef CAS.
- J. J. Zhao, H. Huo, Y. J. Zhao, Y. W. Guo, M. Q. Dong, Y. Fu, J. Y. Zhang, Z. X. Gao and L. Kang, Chem. Mater., 2023, 35, 4347–4354 CrossRef CAS.
- J. Ahn, S. Ma, J.-Y. Kim, J.-H. Kyhm, W. Yang, J. A. Lim, N. A. Kotov and J. Moon, J. Am. Chem. Soc., 2020, 142, 4206–4212 Search PubMed.
- H. Duim and M. A. Loi, Mater, 2021, 4, 3835–3851 Search PubMed.
- H. Lu, Z. V. Vardeny and M. C. Beard, Nat. Rev. Chem., 2022, 6, 470–485 CrossRef PubMed.
- H. Li, R. Cao, M. Tao, J. Jiang and Y. Xiao, Chem. Sci., 2025, 16, 4057–4065 Search PubMed.
- G. R. Chen, Z.-K. Zhu, J. B. Wu, P. P. Yu, Y. Zeng, H. L. Dai, H. W. Yang, W. H. Wu, Y. Y. Wang and J. H. Luo, ACS Appl. Mater. Interfaces, 2024, 16, 67970–67978 CrossRef CAS PubMed.
- G. K. Long, C. Y. Jiang, R. Sabatini, Z. Y. Yang, M. Y. Wei, L. N. Quan, Q. M. Liang, A. Rasmita, M. Askerka, G. Walters, X. W. Gong, J. Xing, X. L. Wen, R. Quintero-Bermudez, H. F. Yuan, G. C. Xing, X. R. Wang, D. T. Song, O. Voznyy, M. T. Zhang, S. Hoogland, W. B. Gao, Q. H. Xiong and E. H. Sargent, Nat. Photonics, 2018, 12, 528–533 CrossRef CAS.
- J. X. Gao, W. Y. Zhang, Z. G. Wu, Y. X. Zheng and D. W. Fu, J. Am. Chem. Soc., 2020, 142, 4756–4761 CrossRef CAS PubMed.
- H. L. Xuan, J. L. Li, L. J. Xu, D. S. Zheng and Z. N. Chen, Adv. Opt. Mater., 2022, 10, 2200591 CrossRef CAS.
- J. Chen, S. Zhang, R. Q. Li, S. Ye, A. K. Cheetham and L. L. Mao, Angew. Chem., Int. Ed., 2022, 61, e202205906 Search PubMed.
- J. Chen, X. Pan, X. Y. Zhang, C. Sun, C. C. Chen, X. Q. Ji, R. Chen and L. L. Mao, Small, 2023, 19, 2300938 CrossRef CAS PubMed.
- J. Wang, C. Fang, J. Q. Ma, S. Wang, L. Jin, W. C. Li and D. H. Li, ACS Nano, 2019, 13, 9473–9481 Search PubMed.
- B. B. Wang, C. Wang, H. Y. Zhang, M. J. Sun, H. Wang, S. P. Wang and G. J. Zhao, J. Alloys Compd., 2022, 910, 164892 CrossRef CAS.
- H. L. Xuan, Y. F. Sang, L. J. Xu, D. S. Zheng, C. M. Shi and Z. N. Chen, Chem. – Eur. J., 2022, 28, e202201299 CrossRef CAS PubMed.
- M. Z. Wang, X. M. Wang, B. T. Zhang, F. Y. Li, H. X. Meng, S. J. Liu and Q. Zhao, J. Mater. Chem. C, 2023, 11, 3206–3212 RSC.
- M. P. Davydova, L. Q. Meng, M. I. Rakhmanova, I. Y. Bagryanskaya, V. S. Sulyaeva, H. Meng and A. V. Artem’ev, Adv. Opt. Mater., 2023, 11, 2202811 Search PubMed.
- X. X. Yu, S. B. Zhong, Z. Y. Guo, J. Guan, H. Tang, X. L. He, Y. H. Chen and S. Pan, J. Mater. Chem. C, 2025, 13, 2190–2197 Search PubMed.
- H. Y. Liu, G. Yu, P. H. Huo, R. Y. Guo, Y. J. Li, H. Qi, J. Y. Zheng, T. Jin, Z. F. Zhao, Z. Q. Bian and Z. W. Liu, Mater. Horiz., 2025 10.1039/D4MH01760A.
- X. T. Wang, T. Y. Zhang, Y. B. Lou and Y. X. Zhao, Mater. Chem. Front., 2019, 3, 365–375 RSC.
- X. S. Wang, J. J. Yang, J. Zhong, J. S. Yu and X. J. Pan, Polymers, 2024, 16, 3053 CrossRef CAS PubMed.
- X. Y. Chen, J. Cheng, L. F. He, L. J. Zhao, C. Q. Zhang, A. Y. Pang and J. M. Li, Molecules, 2023, 28, 3787 CrossRef CAS PubMed.
- L. S. Liang and P. Gao, Adv. Sci., 2018, 5, 1700331 CrossRef PubMed.
- C. D. Liu, C. C. Fan, B. D. Liang and W. Zhang, Inorg. Chem. Front., 2024, 11, 4611–4618 RSC.
- M. Zhang, D. J. Liu, Y. Wei, P. P. Dang, G. G. Guo and J. Lin, Chin. J. Lumin., 2023, 44, 2098–2119 CAS.
- C. C. Wu, Q. H. Zhang, G. H. Liu, Z. H. Zhang, D. Wang, B. Qu, Z. J. Chen and L. X. Xiao, Adv. Energy Mater., 2020, 10, 1902496 CrossRef CAS.
- J. S. Wen, K. Rong, L. Q. Jiang, C. L. Wen, B. Wu, B. Sa, Y. Qiu and R. Ahuja, Nano Energy, 2024, 128, 109802 CrossRef CAS.
- J. R. Jimenez, S. Miguez-Lago, M. Poncet, Y. T. Ye, C. L. Ruiz, C. M. Cruz, A. G. G. Campana, E. Colacio, C. Piguet and J. M. Herrera, J. Mater. Chem. C, 2023, 11, 2582–2590 RSC.
- P. Maxime, B. Celine, G. Laure, J. J. Ramon and P. Claude, Front. Chem., 2024, 12, 1472943 Search PubMed.
- Z. Zhou, T. Jiang, Y. Yang, Y. Deng, M. Wang, Y. Ma, S. Liu and Q. Zhao, Adv. Opt. Mater., 2024, 12, 2302185 Search PubMed.
- X. Han, P. Cheng, H. Yang, J. Guan, M. Xin, G. Li, X. Li, Y. Zheng, J. Xu and X.-H Bu, Angew. Chem., Int. Ed., 2025, 64, e202419776 Search PubMed.
- J. Li, Q. Luo, J. Wei, L. Zhou, P. Chen, B. Luo, Y. Chen, Q. Pang and J. Z. Zhang, Angw. Chem., Int. Ed., 2024, 63, e202405310 CrossRef CAS PubMed.
- R. Hagihara, T. Umeno, S. Ueki, D. Yoshihara, Y. Fuchi, K. Usui, M. Sakuma, K. Yamada and S. Karasawa, Chem. Eur. J., 2021, 27, 3039–3046 CrossRef CAS PubMed.
- Z. Song, X. Liu, C. Yang, Q. Wu, X. Guo, G. Liu, Y. Wei, L. Meng and Y. Dang, Adv. Opt. Mater., 2024, 12, 2301272 CAS.
- W. Zhang, W. Zheng, L. Li, P. Huang, J. Xu, W. Zhang, Z. Shao and X. Chen, Adv. Mater., 2024, 36, 2408777 CAS.
- D. Y. Wang, S. X. Wang, C. Y. Tian, L. Wang, R. Y. Wang, W. L. Zhang, X. Y. Li, L. H. Wang, Z. C. Du, X. W. Lei and F. Yu, J. Lumin., 2025, 277, 120960 CrossRef CAS.
- B. Li, Y. Yu, M. Y. Xin, J. L. Xu, T. Z. Zhao, H. M. Kang, G. X. Xing, P. S. Zhao, T. Y. Zhang and S. Jiang, Nanoscale, 2023, 15, 1595–1601 RSC.
- C. Q. Yuan, X. Y. Li, S. Semin, Y. Q. Feng, T. Rasing and J. L. Xu, Nano Lett., 2018, 18, 5411–5417 CrossRef CAS PubMed.
- D. Chen, P. Wang, Y. Liu, G. Liu, J. Wei, Y. Zheng and Y. Dang, Inorg. Chem., 2024, 63, 24022–24029 CrossRef CAS PubMed.
- S. Landi jr, I. R. Segundo, E. Freitas, M. Vasilevskiy, J. Carneiro and C. J. Tavares, Solid State Commun., 2022, 341, 114573 CrossRef.
- H. Jamil, I. M. Dildar, U. Ilyas, J. Z. Hashmi, S. Shaukat, M. N. Sarwar and M. Khaleeq-ur-Rahman, Thin Solid Films, 2021, 732, 138796 CrossRef CAS.
- W. W. Meng, X. M. Wang, Z. W. Xiao, J. B. Wang, D. B. Mitzi and Y. F. Yan, J. Phys. Chem. Lett., 2017, 8, 2999–3007 CrossRef CAS PubMed.
- P. R. Jubu, O. S. Obaseki, F. K. Yam, A. A. Avaa, A. A. McAsule, Y. Yusof and D. A. Otor, J. Opt., 2023, 52, 1426–1435 CrossRef.
- M. te Vrugt, H. Löwen and R. Wittkowski, Adv. Phys., 2020, 69, 121–247 CrossRef.
- S. Y. Yan, W. L. Tian, H. Chen, K. X. Tang, T. T. Lin, G. Y. Zhong, L. Z. Qiu, X. Y. Pan and W. Z. Wang, Adv. Funct. Mater., 2021, 31, 2100855 CrossRef CAS.
- Z. L. He, J. H. Wei, L. B. Luo, Z. Z. Zhang and D. B. Kuang, J. Mater. Chem. C, 2023, 11, 1251–1257 RSC.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CrossRef CAS PubMed.
- S. Kahmann, E. K. Tekelenburg, H. Duim, M. E. Kamminga and M. A. Loi, Nat. Commun., 2020, 11, 2344 CrossRef CAS PubMed.
- M. Z. Li and Z. G. Xia, Chem. Soc. Rev., 2021, 50, 2626–2662 RSC.
- D. Y. Li, J. H. Song, Y. Cheng, X. M. Wu, Y. Y. Wang, C. J. Sun, C. Y. Yue and X. W. Lei, Angew. Chem., Int. Ed., 2022, 61, e202206437 CrossRef CAS PubMed.
- H. Tanaka, Y. Inoue and T. Mori, ChemPhotoChem, 2018, 2, 386–402 CrossRef CAS.
- J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1–16 CrossRef CAS.
- T. H. Zhao, J. L. Han, P. F. Duan and M. H. Liu, Acc. Chem. Res., 2020, 53, 1279–1292 CrossRef CAS PubMed.
- X. He, Y. T. Zheng, Z. S. Luo, Y. Wei, Y. L. Liu, C. L. Xie, C. Li, D. F. Peng and Z. W. Quan, Adv. Mater., 2024, 36, 2309906 CrossRef CAS PubMed.
- S. R. Dan, S. Paramanik and A. J. Pal, ACS Nano, 2024, 18, 35644–35653 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray diffraction XPS, theoretical calculations, TGA/DSC and optical measurements. CCDC 2418218–2418220. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5tc00279f |
| This journal is © The Royal Society of Chemistry 2025 |