Simultaneous adjustment of afterglow wavelength and intensity in indium-substituted Ga1.99−xInxO3:0.01Cr3+†
Qingqing
Xie‡
,
Ailijiang
Tuerdi‡
,
Xiangkai
Qiao
 ,
Yalin
Zheng
,
Aikelaimu
Aihemaiti
,
Peng
Yan
and
Abdukader
Abdukayum
,
Yalin
Zheng
,
Aikelaimu
Aihemaiti
,
Peng
Yan
and
Abdukader
Abdukayum
 *
*
Xinjiang Key Laboratory of Novel Functional Materials Chemistry, College of Chemistry and Environmental Sciences, Kashi University, Kashi 844000, China. E-mail: abdukadera@sina.com
First published on 20th March 2025
Abstract
Near-infrared persistent luminescent nanoparticles (PLNPs) are widely used for deep tissue penetration in bio-imaging. Ga2O3:Cr3+, a promising phosphor material, has been extensively studied for its suitable band gap and tunable emission wavelength. Although a redshift in the fluorescence wavelength has been achieved, the persistent luminescence properties of Ga2O3:Cr3+ following this redshift have not been extensively explored. Herein, we have prepared Ga1.99−xInxO3:0.01Cr3+ (x = 0–1.99)(GIO) PLNPs utilizing a hydrothermal method followed by calcination. Replacing gallium with indium in the crystal structure of Ga2O3:Cr3+ redshifts the emission wavelength to 830 nm and induces persistent luminescence, which is attributed to lattice distortion caused by the substitution of gallium with indium, which modifies the crystal field around the Cr3+ ions. The as-prepared GIO nanoparticles show potential for future low-cost, deep-tissue bioimaging applications.
1. Introduction
Near-infrared (NIR) technology is extensively utilized in bio-imaging due to its advantages, including high sensitivity, high resolution, and the absence of harmful radiation.1 Recent advancements in technology have led to significant interest in NIR luminescent materials in both academic and industrial fields.2,3 In recent years, a diverse array of NIR luminescent materials have been developed, including quantum dots (QDs),4,5 organic luminescent materials (OLMs),6 and aggregation-induced luminescent materials (AIEs).7–9 While the luminescent characteristics of these materials can be efficiently adjusted through rigid molecular groups10,11 and the quantum size effect,12,13 many still encounter limitations, such as the requirement for in situ excitation, short delayed fluorescence durations, and unavoidable background noise.14,15Persistent luminescence nanoparticles (PLNPs) can emit long-lasting luminescence without in situ light excitation.16 In brief, these materials contain appropriate trap centers that, when exposed to light, enable electrons to absorb energy and become excited into the conduction band.17 Upon the removal of the light source, some of the electrons can be captured in the traps, thereby enabling the luminescent centers to gain energy via the recombination of electrons and holes.18 As a result, PLNPs have found applications in various fields, including bioimaging,19,20 therapy,21–23 photocatalysis,24,25 anti-counterfeiting,26–29 and data storage.30,31
Recent studies have demonstrated the tuning of emission wavelengths through ion doping, leading to the optimization of the optical properties.32–34 Among various doping options, Cr3+ has emerged as an exceptionally favorable choice for the design of red phosphors and has been extensively investigated.35 Bessiere and his colleagues reported that ZnGa2O4 doped with Cr3+ can be excited by ultraviolet radiation, exhibiting a maximum emission peak at 696 nm.36 Pan et al. synthesized β-Ga2O3:Cr3+ nanowires by a hydrothermal process followed by calcination, which also demonstrated an afterglow maximum emission peak at 696 nm.37 Incorporation of different ions alters the optical properties of the material; for instance, LiGa5O8:Cr3+ displays an emission wavelength spectrum of afterglow emission wavelength from 650 to 800 nm, peaking at 716 nm.38 Wang et al. reported In3+ doping of Ga2O3:Cr3+.39 Micro-doping of In3+ in Ga2O3:Cr3+ enhances the persistent luminescence time, although the maximum afterglow emission peak remains at 690 nm.
In bioimaging, longer wavelengths can reduce scattering and provide deeper tissue imaging.40 Recently, Ga1.98−x (Al0.68In0.32)xO3:0.02Cr3+ (x = 0–0.8) exhibited a fluorescence emission range from 650 to 1000 nm due to the co-doping of Al3+ and In3+.41 Additionally, Ga2−xInxO3:Cr3+ (0 ≤ x ≤ 0.5), used as a NIR LED light source ranging from 713 to 820 nm, was also prepared using high-temperature solid-state synthesis.42 However, these materials function as phosphors and lack long afterglow capabilities. Furthermore, the high-temperature solid-state synthesis often results in larger particle sizes and poor water solubility, which limits their application in biomedical settings. The co-doping of Mg2+–Ge4+ in (Ga1−xMgx)(Ga1−xGex)O3:Cr3+ leads to a notable red shift in emission peaks, with a change from 726 to 830 nm.43 However, the decay curves of these materials decrease from 221.2 to 55.9 μs, which is insufficient for long-term after-glow bioimaging. While most of the iron-doped Ga2O3 materials show a significant red shift in fluorescence, they couldn’t provide persistent luminescence.
In this work, Ga1.99−xInxO3:0.01Cr3+ (x = 0–1.99)(GIO:Cr) with an average lifetime of 17.24 s were prepared using a hydrothermal method combined with calcination. As the amount of In3+ substitution increased, the emission peak of GIO:Cr showed a significant redshift from 700 to 830 nm and persistent luminescence was induced, which is attributed to lattice distortion caused by the substitution of gallium with indium, which modifies the crystal field around the Cr3+ ions. The afterglow wavelength and intensity of GIO:Cr can be tuned by the ratio of Ga3+/In3+ and exhibits considerable potential for application in ratiometric luminescence and deep tissue bioimaging.44
2. Experimental details
2.1. Reagents and instruments
All reagents were used as received without further purification. Cr (NO3)3·9H2O (99.95%), Ga2O3 (99.99%), InCl3 (99.99%), and ethylene glycol were purchased from Aladdin (Shanghai, China). Concentrated nitric acid and aqueous ammonia (15 wt%) were obtained from Shanghai Chemical Reagent. Ultrapure water (Hangzhou Wahaha Group Co. Ltd, Hangzhou, China) was used.XRD patterns were recorded on a D/max-2500 diffractometer (Rigaku, Japan) using Cu Kα radiation (λ = 1.5418 A). TEM and high-resolution TEM images were obtained on a JEOL-100CX II microscope and a JEM-2100F field emission transmission electron microscope (JEOL, Japan), respectively. Photoluminescence spectra were recorded on an F-4500 spectrofluorometer (Hitachi, Japan).
2.2. Synthesis of Ga1.99−xInxO3:Cr3+PLNPs
Ga1.99−xInxO3:Cr3+ were synthesized using a hydrothermal followed by calcination. Typically, for Ga1.64In0.35O3:0.01Cr, 5.0 mL of ethylene glycol, 3.28 mL of Ga3+ solution (0.2 mol L−1), 0.7 of mL In3+ solution (0.2 mol L−1), and 0.4 mL of Cr3+ solution (0.01 mol L−1) were mixed and stirred for 30 minutes. The pH was adjusted to 9.0 with aqueous ammonia (15 wt%), and the mixing process was maintained for 1 h. The resulting mixture was poured into a 25 mL Teflon-lined autoclave, which was tightly sealed and heated to 170 °C for a duration of 16 h. Following naturally cooling to room temperature, the formed nanocrystals were washed with pure water and ethanol, separated by centrifugation, and subsequently dried. Finally, the nanocrystals were annealed at 700 °C for 3 h.3. Results and discussion
3.1. Structure and phase
The XRD patterns of Ga1.99−xInxO3:Cr3+ with varying In3+ contents are presented in Fig. 1a. As observed in the XRD analysis, the as-synthesized sample Ga1.99O3:0.01Cr3+ without In3+ (x = 0) exhibits a pure monoclinic phase, which is indexed to β-Ga2O3 (JCPDS: 41-1103). The diffraction peaks of Ga1.99O3:0.01Cr3+ observed at 18.75°, 30.46°, 31.69°, 35.18°, 38.39° and 64.67° correspond to the (201), (401), (202), (111), (311) and (712) planes of β-Ga2O3, respectively. The amount of Cr3+ in Ga1.99O3:0.01Cr3+ was insufficient to influence the crystal structure of β-Ga2O3. In β-Ga2O3, there are two crystallographically distinct Ga3+ positions: one tetrahedrally coordinated by oxygen (coordination number 4), and the other octahedrally coordinated by oxygen (coordination number 6).42 In3+ preferentially occupies the octahedral sites rather than the tetrahedral sites, consistent with its larger ionic radius compared to Ga3+.45 As shown in the XRD analysis, the diffraction peaks gradually shifted to lower angles upon introducing In3+ (x = 0.14–0.94) into the samples. This shift is attributed to the substitution of smaller Ga3+ (62 pm) with In3+ (92 pm) in Ga2O3, resulting in the expansion of the crystal lattice and the corresponding shift of the diffraction peaks. This expansion is attributed to substituting smaller Ga3+ with larger In3+, which prefers the octahedral sites. The crystal structure of PLNPs progressively transitioned from β-Ga2O3 to GaInO3 with increasing In content in the PLNPs. When the In content exceeded 1.04 (x), peaks corresponding to In2O3 began to emerge, whereas the peaks of Ga2O3 disappeared. The crystal structure of PLNPs gradually transfers from GaInO to In2O3. Furthermore, as the concentration of In3+ increases, In3+ exhibits a stronger energy preference for the octahedral site where Ga is completely substituted in the PLNPs, and the XRD pattern of the PLNPs (x = 1.99) well matches the structure of In2O3 (JCPDS: 1-929). The crystal structures depicted in Fig. 1b are derived from X-ray powder diffraction analysis, with structural data associated with β-Ga2O3 (ICSD: 34243), GaInO3 (ICSD: 30339), and In2O3 (ICSD: 426846). These structures represent the phase transition from β-Ga2O3 to In2O3 during In3+ doping and suggest that Ga3+ and In3+ ions are coordinated in similar octahedral environments within the β-Ga2O3 phase. | ||
| Fig. 1 (a) XRD patterns of Ga1.99−xInxO3:Cr3+ and (b) crystal structures of β-Ga2O3 (ICSD: 34243; PDF: 41-1103), β-GaInO3 (ICSD: 30339; PDF: 21-334) and In2O3 (ICSD: 426846; PDF: 1-929). | ||
3.2. Morphology of Ga0.75In1.24O3:Cr3+
Fig. 2a shows the TEM image of the GIO:Cr nanoparticles. These PLNPs exhibit a particle size of approximately 60 nm. High-resolution transmission electron microscopy (HR-TEM) analysis (Fig. 2b) reveals that GIO:Cr structures exhibit two distinct types of clear lattice fringes at 0.563 nm and 0.283 nm, corresponding to the d-spacing of the spinel β-Ga2O3 (202) and rh-In2O3 (400) lattice planes, respectively. In summary, the structure of GIO:Cr consists of a mixed gallium indium-oxide incorporating both β-Ga2O3 and rh-In2O3, which aligns with the XRD results. The energy dispersive X-ray spectroscopy (EDS) of GIO:Cr (Fig. 2c–g) indicates that the elements (Ga, In, O, and Cr) are uniformly distributed in the sample, and no other elements were detected.3.3. Photoluminescence of GIO:Cr
As illustrated in Fig. 3a, the excitation spectrum of GIO:Cr, monitored at 800 nm, reveals a strong absorption peak at 300 nm, which is attributed to the combination of the Ga2O3 host excitation band and the O–Cr charge transfer band.46,47 Additionally, other absorption peaks at 460 nm and 600 nm are attributed to the 4A2 → 4T1 and 4A2 → 4T2 electron transition.48 Cr3+ serves as the luminescence center, contributing to the near-infrared emission.49 With increasing concentration of In3+, the PL of GIO:Cr shows a significant redshift of 130 nm, with the main peak shifting from 700 nm to 830 nm. This shift is associated with the crystal field strength of Cr3+ in Ga2O3.40 This phenomenon may be caused by the larger radius of In3+ compared to Ga3+, which is expected to alter the crystal field of GIO:Cr.50 The crystal field of Cr3+ can be characterized by the crystal field Dq and the Racah parameter, B, calculated using the equation:51| 10Dq = E(4T2) = E(4T2 → 4A2) | (1) |
| 11Dq + 15/2B = E(4T1 → 4A2) | (2) |
The emission transitions of Cr3+ depend on the Dq/B. In a strong crystal field, Cr3+ exhibits a narrow, intense emission at 700 nm, along with a broadband emission associated with a weaker crystal field.52,53 In this work, the main peak of GIO:Cr emission at 718 nm, generated by the 4T2 → 4A2 transition of Cr3+, was observed with excitation at 440 nm, attributed to the 4A2 → 4T1 transition of Cr3+.14 For Ga1.99O3:0.01Cr (x = 0), the Dq/B for Cr3+ was found to be 1.41. In the case of Ga1.45In0.54O3:0.01Cr (x = 0.54), the Dq/B for Cr3+ decreased to 1.28. As the concentration of In3+ increased, the crystal structure of Ga2O3 changed, indicating a transition of the crystal field experienced by Cr3+ from a strong to a weak crystal field, which resulted in a redshift and broadband emission.40
The afterglow decay curves and images of GIO:Cr (x = 0–1.24) were collected after 10 min of 254 nm UV irradiation (Fig. 3c and Fig. S2, ESI†). Although the average lifetime of GIO:Cr decreases with increasing concentration of In3+, GIO:Cr still has an obvious afterglow at an In3+ concentration of 1.24 (τav = 17.24 s). The lifetime of GIO:Cr can be accurately represented by eqn (3):39
 | (3) |
Briefly, t is the decay time of the afterglow, I0 is the initial intensity of the PL, the A are constants and depend on the rapid luminescent intensity when t = 0, and τ1, τ2, and τ3 are the time constants, indifferent to the afterglow decay lifetimes. In addition, GIO:Cr with different ratios of Ga3+/In3+ are shown in Fig. S1 (ESI†). The fitted decay curve results are shown in Table S1 (ESI†), and the decay time of afterglow was tuned by In3+.
Trapping levels play an important role in PLNPs.54,55 An appropriate density of traps in persistent phosphors can effectively preserve the excitation energy and primarily determines the intensity and lifetime of persistent luminescence.56–58Fig. 3d presents the thermoluminescence (TL) spectra of GIO:Cr (x = 1.24). The TL peak temperature, Tm, GIO:Cr (x = 1.24), was recorded as 383 K. The expression used to approximate the trap-level model is as per the following equation (eqn (4)):59
| E = Tm/500 | (4) |
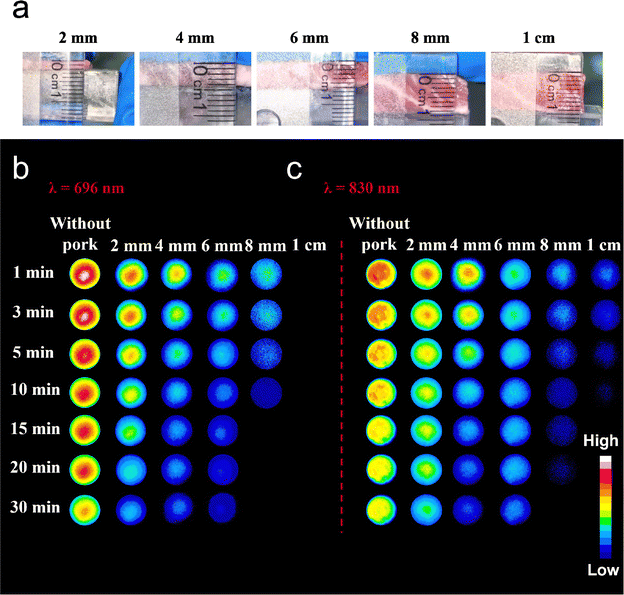
As shown in Fig. 4a, different thicknesses of pork were placed over Ga2O3:Cr and Ga0.75In1.24O3:0.01Cr3+. Fig. S3a and b (ESI†) demonstrate that the persistent luminescence intensity decreases with increasing pork tissue thickness, regardless of whether the emission wavelength is 696 nm or 830 nm. Fig. 4b and c show persistent luminescence images of Ga2O3:Cr (λem = 696 nm) and Ga0.75In1.24O3:0.01Cr3+ (λem = 830 nm) captured at different time intervals. Specifically, for the emission peak at 696 nm, the afterglow signal sharply declines at 8 mm, and no detectable afterglow is observed beyond this thickness. In contrast, for the emission peak at 830 nm, a detectable afterglow signal remains even at a thickness of 1 cm. These results highlight the potential of GIO:Cr for deep tissue imaging, offering valuable non-invasive imaging for early diagnosis and disease monitoring.
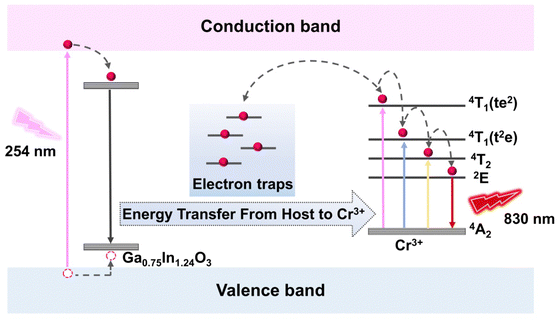
The persistent luminescence mechanism of GIO:Cr is depicted in Fig. 5. In GIO:Cr (x = 1.24), the trap levels are broadly distributed across a wide energy range, as evidenced by the extensive thermoluminescence spectra in Fig. 3d. Upon 254 nm UV excitation, electrons are promoted from the valence band to the conduction band, with some being captured by electron traps through non-radiative transitions, while others transition to deeper traps via interstitial crossing. After the UV excitation ceases, these trapped electrons are slowly released, and return to the excited state via interstitial crossing and subsequently relax to the ground state, emitting NIR light at 830 nm.
4. Conclusions
We have successfully synthesized Ga1.99−xInxO3:0.01Cr3+ (x = 0–1.99) PLNPs using a hydrothermal method combined with calcination. The fine-tuning of the Ga3+/In3+ ratio has been demonstrated to markedly alter the luminescence properties of the PLNPs, representing a breakthrough in the design of deep-red emissive materials. Notably, the optimized nanoparticles exhibit a maximum emission peak at 830 nm with an extended lifetime of 17.24 s. These deep-red nanoscale PLNPs show great potential for biomedical applications, particularly in deep-tissue imaging, where deeper penetration and longer persistence are crucial.Author contributions
Qingqing Xie: data curation, writing – original draft preparation; AilijiangTuerdi: data curation, writing – original draft preparation; Xiangkai Qiao: data curation; YalinZheng: data curation; AikelaimuAihemaiti: data curation; Peng Yan: data curation; AbdukaderAbdukayum: methodology, conceptualization, supervision, funding acquisition, writing – reviewing and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work is supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region, China (2022D01E16), the National Natural Science Foundation of China (22364016), the Xinjiang Tianshan Talent Training Program, China (2024TSYC), and the Tianshan Innovation Team Plan of Xinjiang Uygur Autonomous Region, China (2023D14002).References
- L.-L. Chen, L. Zhao, Z.-G. Wang, S.-L. Liu and D.-W. Pang, Small, 2022, 18, 2104567 CrossRef CAS PubMed.
- Z. Hu, W. H. Chen, J. Tian and Z. Cheng, Trends Mol. Med., 2020, 26, 469–482 CrossRef CAS PubMed.
- W.-T. Huang, V. Rajendran, M.-H. Chan, M. Hsiao, H. Chang and R.-S. Liu, Adv. Opt. Mater., 2023, 11, 2202061 CrossRef CAS.
- C. Ding, Y. Huang, Z. Shen and X. Chen, Adv. Mater., 2021, 33, 2007768 CAS.
- F. P. García de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, M. Bayer and E. H. Sargent, Science, 2021, 373, eaaz8541 Search PubMed.
- Q. Dang, Y. Jiang, J. Wang, J. Wang, Q. Zhang, M. Zhang, S. Luo, Y. Xie, K. Pu, Q. Li and Z. Li, Adv. Mater., 2020, 32, 2006752 Search PubMed.
- J. Lin, X. Zeng, Y. Xiao, L. Tang, J. Nong, Y. Liu, H. Zhou, B. Ding, F. Xu, H. Tong, Z. Deng and X. Hong, Chem. Sci., 2019, 10, 1219–1226 RSC.
- W. Xu, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 7476–7487 CAS.
- Z. Zhang, W. Wang, Y. Jiang, Y. X. Wang, Y. Wu, J. C. Lai, S. Niu, C. Xu, C. C. Shih, C. Wang, H. Yan, L. Galuska, N. Prine, H. C. Wu, D. Zhong, G. Chen, N. Matsuhisa, Y. Zheng, Z. Yu, Y. Wang, R. Dauskardt, X. Gu, J. B. Tok and Z. Bao, Nature, 2022, 603, 624–630 CrossRef CAS PubMed.
- B. Li, M. Liu, L. Sang, Z. Li, X. Wan and Y. Zhang, Adv. Opt. Mater., 2023, 11, 2202610 Search PubMed.
- G. Feng and B. Liu, Acc. Chem. Res., 2018, 51, 1404–1414 CrossRef CAS PubMed.
- M. J. Afshari, C. Li, J. Zeng, J. Cui, S. Wu and M. Gao, ACS Nano, 2022, 16, 16824–16832 Search PubMed.
- T. Yuan, T. Meng, Y. Shi, X. Song, W. Xie, Y. Li, X. Li, Y. Zhang and L. Fan, J. Mater. Chem. C, 2022, 10, 2333–2348 RSC.
- A. Abdukayum, J. T. Chen, Q. Zhao and X. P. Yan, J. Am. Chem. Soc., 2013, 135, 14125–14133 CrossRef CAS PubMed.
- S. K. Sun, H. F. Wang and X. P. Yan, Acc. Chem. Res., 2018, 51, 1131–1143 CrossRef CAS PubMed.
- T. Matsuzawa, Y. Aoki, N. Takeuchi and Y. Murayama, J. Electrochem. Soc., 1996, 143, 2670–2673 CrossRef CAS.
- F. Clabau, X. Rocquefelte, S. Jobic, P. Deniard, M.-H. Whangbo, A. Garcia and T. Le Mercier, Chem. Mater., 2005, 17, 3904–3912 CrossRef CAS.
- Q. le Masne de Chermont, C. Chaneac, J. Seguin, F. Pelle, S. Maitrejean, J. P. Jolivet, D. Gourier, M. Bessodes and D. Scherman, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9266–9271 CrossRef CAS PubMed.
- X. Fu, L.-X. Yan, X. Zhao, L.-J. Chen and X.-P. Yan, Chem. Eng. J., 2024, 480, 147740 Search PubMed.
- X. Fu, X. Zhao, L.-J. Chen, P. Ma, T. Liu and X.-P. Yan, Biomater. Sci., 2023, 11, 5186–5194 RSC.
- Z. Zhou, Y. Li and M. Peng, Chem. Eng. J., 2020, 399, 125688 CrossRef CAS.
- N. Liu, X. Chen, X. Sun, X. Sun and J. Shi, J. Nanobiotechnol., 2021, 19, 113 CrossRef CAS PubMed.
- X. Liu, R. Xi, Y. Hu, Y. Wang and A. Abdukayum, Dalton Trans., 2024, 53, 6601–6608 RSC.
- A. Tuerdi and A. Abdukayum, RSC Adv., 2019, 9, 17653–17657 RSC.
- F. He, A. Abulimiti, B. Li, Y. Wu, Y. Chen, M. Zhang and A. Abdukayum, ACS Appl. Nano Mater., 2023, 6, 12871–12881 CrossRef.
- X. Zhang, Z. Wang, C. Xu, D. Gao, Q. Pang, J. Xu and X. Wang, Laser Photonics Rev., 2025, 19, 2401376 CrossRef CAS.
- Q. Kuang, X. Hou, C. Du, X. Wang and D. Gao, Phys. Chem. Chem. Phys., 2023, 25, 17759–17768 RSC.
- D. Gao, C. Du, Y. Wang, W. Xu, W. Gao, Q. Pang and Y. Wang, J. Mater. Chem. C, 2024, 12, 19487–19497 RSC.
- X. Qiao, L. Kasim, A. Tuerdi, G. Hu and A. Abdukayum, ACS Appl. Nano Mater., 2023, 6, 20831–20840 CrossRef CAS.
- D. Gao, Z. Wang, Q. Pang, Q. Kuang, F. Gao, X. Zhang, S. Yun and X. Wang, Adv. Opt. Mater., 2023, 11, 2300303 CrossRef CAS.
- D. Gao, Z. Wang, X. Zhang, Q. Pang and X. Wang, ACS Appl. Mater. Interfaces, 2025, 17, 3587–3596 CrossRef CAS PubMed.
- F. Kang, M. Peng, X. Yang, G. Dong, G. Nie, W. Liang, S. Xu and J. Qiu, J. Mater. Chem. C, 2014, 2, 6068–6076 RSC.
- F. Kang, H. Zhang, L. Wondraczek, X. Yang, Y. Zhang, D. Y. Lei and M. Peng, Chem. Mater., 2016, 28, 2692–2703 CrossRef CAS.
- F. Kang, G. Sun, P. Boutinaud, F. Gao, Z. Wang, J. Lu, Y. Y. Li and S. Xiao, J. Mater. Chem. C, 2019, 7, 9865–9877 RSC.
- M. N. da Silva, J. M. de Carvalho, M. C. de Abreu Fantini, L. A. Chiavacci and C. Bourgaux, ACS Appl. Nano Mater., 2019, 2, 6918–6927 CAS.
- A. Bessiere, S. Jacquart, K. Priolkar, A. Lecointre, B. Viana and D. Gourier, Opt. Express, 2011, 19, 10131–10137 CAS.
- Y. Y. Lu, F. Liu, Z. J. Gu and Z. W. Pan, J. Lumin., 2011, 131, 2784–2787 CAS.
- F. Liu, W. Yan, Y. J. Chuang, Z. Zhen, J. Xie and Z. Pan, Sci. Rep., 2013, 3, 1554 Search PubMed.
- L. Li, K. Xu, Y. Wang, Z. Hu and H. Zhao, Opt. Mater. Express, 2016, 6, 1122–1130 Search PubMed.
- T. Jia and G. Chen, Coord. Chem. Rev., 2022, 471, 214724 CAS.
- C.-Y. Chang, N. Majewska, K.-C. Chen, W.-T. Huang, T. Leśniewski, G. Leniec, S. M. Kaczmarek, W. K. Pang, V. K. Peterson, D.-H. Cherng, K.-M. Lu, S. Mahlik and R.-S. Liu, Chem. Mater., 2022, 34, 10190–10199 CAS.
- J. Zhong, Y. Zhuo, F. Du, H. Zhang, W. Zhao and J. Brgoch, ACS Appl. Mater. Interfaces, 2021, 13, 31835–31842 CAS.
- J. Zhong, L. Zeng, W. Zhao and J. Brgoch, ACS Appl. Mater. Interfaces, 2022, 14, 51157–51164 CAS.
- L. M. Pan, X. Zhao, X. Wei, L. J. Chen, C. Wang and X. P. Yan, Anal. Chem., 2022, 94, 6387–6393 CAS.
- W. T. Chen, H. S. Sheu, R. S. Liu and J. P. Attfield, J. Am. Chem. Soc., 2012, 134, 8022–8025 CAS.
- F. Shi and H. Qiao, CrystEngComm, 2020, 22, 7794–7799 CAS.
- H. Kwak, J.-S. Kim, D. Han, J. S. Kim, J. Park, G. Kwon, S.-M. Bak, U. Heo, C. Park, H.-W. Lee, K.-W. Nam, D.-H. Seo and Y. S. Jung, Nat. Commun., 2023, 14, 2459 CAS.
- J. Zhang, W. Mu, K. Zhang, J. Sun, J. Zhang, N. Lin, X. Zhao, Z. Jia and X. Tao, CrystEngComm, 2020, 22, 7654–7659 CAS.
- B. Viana, S. K. Sharma, D. Gourier, T. Maldiney, E. Teston, D. Scherman and C. Richard, J. Lumin., 2016, 170, 879–887 CAS.
- M. G. Brik, S. J. Camardello, A. M. Srivastava, N. M. Avram and A. Suchocki, ECS J. Solid State Sci. Technol., 2016, 5, R3067 CAS.
- B. Struve and G. Huber, Appl. Phys. B, 1985, 36, 195–201 Search PubMed.
- Y. Jin, Y. Hu, L. Yuan, L. Chen, H. Wu, G. Ju, H. Duan and Z. Mu, J. Mater. Chem. C, 2016, 4, 6614–6625 Search PubMed.
- B. Bai, P. Dang, D. Huang, H. Lian and J. Lin, Inorg. Chem., 2020, 59, 13481–13488 CrossRef CAS PubMed.
- M. Allix, S. Chenu, E. Véron, T. Poumeyrol, E. A. Kouadri-Boudjelthia, S. Alahraché, F. Porcher, D. Massiot and F. Fayon, Chem. Mater., 2013, 25, 1600–1606 CrossRef CAS.
- Y. Zhuang, L. Wang, Y. Lv, T.-L. Zhou and R.-J. Xie, Adv. Funct. Mater., 2018, 28, 1705769 CrossRef.
- X. Chen, Y. Li, K. Huang, L. Huang, X. Tian, H. Dong, R. Kang, Y. Hu, J. Nie, J. Qiu and G. Han, Adv. Mater., 2021, 33, 2008722 CrossRef CAS PubMed.
- J. Du, A. Feng and D. Poelman, Laser Photonics Rev., 2020, 14, 2000060 CrossRef CAS.
- L. Liang, J. Chen, K. Shao, X. Qin, Z. Pan and X. Liu, Nat. Mater., 2023, 22, 289–304 CrossRef CAS PubMed.
- K. Van den Eeckhout, P. F. Smet and D. Poelman, Materials, 2010, 3, 2536–2566 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc05353b |
| ‡ Qingqing Xie and Ailijiang Tuerdi contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |