Open Access Article
Open Access ArticleTi3C2Tx MXenes and Mn3O4 nanoparticles synergistically promote the electrochemical synthesis of ammonia under ambient conditions†
Lanxiang Huang *ab,
Renchuan Denga,
Xiang Wang
*ab,
Renchuan Denga,
Xiang Wang a,
Qin Wanga and
Yuan Lianga
a,
Qin Wanga and
Yuan Lianga
aSchool of New Energy Materials and Chemistry, Leshan Normal University, Leshan, Sichuan 614000, China. E-mail: 120678486@qq.com
bWest Silicon Photovoltaic New Energy Industry Technology Research Institute, Leshan, Sichuan 614000, China
First published on 15th July 2025
Abstract
Ammonia serves as a hydrogen energy carrier and a renewable, zero-carbon fuel alternative that is safely transportable. The electrochemical catalytic reduction of N2 to NH3 in aqueous electrolytes at ambient temperature and pressure (eNRR) using electricity generated from renewable energy sources such as solar and wind power can provide an environmentally friendly approach. To effectively suppress the occurrence of hydrogen evolution side reactions, it is necessary to design and synthesize catalysts with high selectivity for N2 adsorption. Owing to the ability of transition metals with unoccupied d orbitals to significantly promote the adsorption of N2 molecules and the activation of inert bonds, researchers have explored manganese-oxide catalysts through both experimental and theoretical studies. However, manganese oxides are semiconductor materials with poor conductivity. To solve this problem, the Ti3C2Tx MXene material can be introduced as a carrier for manganese oxide particles. In this study, the Ti3C2@Mn3O4 composite was used as an electrocatalyst for ammonia synthesis under ambient conditions using a simple method. Benefiting from the synergistic catalytic effect of MXene and Mn3O4, the composite exhibits excellent catalytic performance for ammonia synthesis, with an NH3 yield rate of 53.7 μg h−1 mgcat.−1 and satisfactory FE of 10.4% at −0.6 V (vs. RHE) under ambient conditions. The composite catalyst exhibits excellent stability, durability, and selectivity, with outstanding synergistic effects, surpassing most reported NRR electrocatalysts. This simple and versatile strategy may offer researchers inspiration for rationally designing highly efficient NRR electrocatalysts.
1. Introduction
Ammonia is a hydrogen energy carrier that can be easily and safely transported, as well as a renewable and zero-carbon fuel. However, the Haber–Bosch process for producing ammonia is a high-temperature, high-pressure process with high energy consumption in the chemical industry, consuming about 2% of the world's energy annually. At the same time, the H2 required for the reaction is mainly produced through the steam reforming of natural gas, with the required energy mainly derived from fossil fuels, resulting in annual CO2 emissions of approximately 320 million tons.1 Therefore, finding a green, environmentally friendly, and energy-efficient method for ammonia synthesis is of great significance for the sustainable development of the economy and ecology.The electrochemical catalytic reduction of N2 in aqueous electrolytes under ambient conditions to synthesize ammonia (eNRR), utilizing electricity generated from renewable energy sources such as solar and wind power, can provide an alternative and environmentally benign method. This method not only overcomes the limitations of thermodynamic equilibrium for the thermodynamically unfavorable NH3 synthesis reaction with the driving force of electricity but also eliminates or reduces the constraints of thermodynamic equilibrium, thereby avoiding the reliance on temperature and high pressure conditions and reducing CO2 emissions, becoming a highly researched field.2–4 In acidic aqueous electrolytes, the reaction occurring at the cathode is5
| N2 (g) + 6H+ (aq.) + 6e− → 2NH3 (g) (E0 = 0.0577 V vs. SHE) | (1) |
At the same time, a side reaction occurs:
| 2H+ (aq.) + 2e− → H2 (g) (E0 = 0 V vs. SHE) | (2) |
During the eNRR process, N2 molecules need to be adsorbed and activated on the catalyst surface, followed by hydrogenation reactions. N2 molecules are highly inert, so active H+ ions are more easily adsorbed onto the catalyst surface, leading to strong competitive hydrogen evolution reactions (HER), with low faradaic efficiency (FE) for eNRR and slow ammonia synthesis rates.6,7 Therefore, suppressing the hydrogen evolution reaction is key to improving the performance of eNRR.
To effectively suppress the occurrence of hydrogen evolution side reactions, it is necessary to design and prepare a catalyst with high selectivity for N2 adsorption. In light of the fact that transition metals with unoccupied d orbitals can significantly promote the adsorption of N2 molecules and the activation of inert bonds, researchers have explored manganese-based catalysts through experimental and theoretical studies.8–11 Kong et al. used MnO2 as an NRR electrocatalyst and achieved an NH3 yield rate of 34.12 μg h−1 mgcat.−1 in 0.1 M HCl;12 Wu et al. synthesized Mn3O4 nanocubes as an NRR electrocatalyst with an NH3 yield rate of 11.6 μg h−1 mgcat.−1 and FE of 3.0% in 0.1 M Na2SO4;13 Zheng et al. reported a MnO-based composite, which displayed a low FE of 1.5% in 0.1 M Na2SO4.14
As a transition metal oxide, manganese oxides are semiconductor materials with poor conductivity. In order to improve its conductivity, the Ti3C2Tx MXene material can be introduced as a carrier for manganese oxide particles. MXenes (Mn+1Xn, n = 1, 2, and 3)are 2D layered transition metal carbides and nitrides that exhibit excellent conductivity, good hydrophilicity, and a variety of surface terminal groups (Tx, such as ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH, and –F).15–17 The synthesized manganese oxide nanoparticles can be firmly anchored on the surface and interlayers of Ti3C2Tx MXene materials due to the electronegativity of the surface terminals (Tx). In addition, recent research suggests that Ti3C2Tx exhibits good NRR activity.18–21
O, –OH, and –F).15–17 The synthesized manganese oxide nanoparticles can be firmly anchored on the surface and interlayers of Ti3C2Tx MXene materials due to the electronegativity of the surface terminals (Tx). In addition, recent research suggests that Ti3C2Tx exhibits good NRR activity.18–21
In this study, a Ti3C2@Mn3O4 composite was used as an electrocatalyst for ammonia synthesis under ambient conditions in a simple method. Benefiting from the synergistic catalytic effect of MXene and Mn3O4, the composite exhibits excellent catalytic performance for ammonia synthesis with an NH3 yield rate of 53.7 μg h−1 mgcat.−1 and satisfactory FE of 10.4% at −0.6 V (vs. RHE) under ambient conditions. The composite catalyst exhibits excellent stability, durability, and selectivity, with outstanding synergistic effects, surpassing most reported NRR electrocatalysts. This simple and versatile strategy may offer researchers inspiration for rationally designing highly efficient NRR electrocatalysts.
2. Experimental section
2.1 Material preparation
First, an appropriate amount of Ti3AlC2 MAX material was weighed and etched with hydrofluoric acid (HF) to remove the Al layer. The mixture was then filtered, washed, and vacuum dried to obtain the layered Ti3C2Tx MXene material.22 Subsequently, 0.1 g of Ti3C2Tx MXenes was ultrasonically dispersed in 50 mL of deionized water until a uniform dispersion was formed, followed by the addition of 15 mL of a MnSO4 solution (0.05 g mL−1) into the dispersion with continuous stirring. Due to the abundant terminal groups (Tx, such as O, –F, and –OH) of Ti3C2Tx MXene and electronegativity, positively charged manganese ions (Mn2+) will be electrostatically attracted to intercalate between the Ti3C2Tx MXene layers and firmly adsorb on its surface; meanwhile, the intercalation of manganese ions will effectively increase the interlayer spacing of Ti3C2Tx MXenes.23Then, 10 mL of KOH solution with a concentration of 0.2 g mL−1 was added dropwise to the dispersion under continuous stirring for 30 min. The added hydroxide ions (OH−) reacted with Mn2+ to form manganese hydroxide (Mn(OH)2), which was immediately oxidized to manganese tetroxide (Mn3O4) in the air atmosphere. The resulting Mn3O4 was firmly anchored to the surface of the Ti3C2Tx MXene material to form the Ti3C2@Mn3O4 composite materials, thereby preventing further growth of Mn3O4 nanoparticles. The resulting dispersion was vacuum-filtered and washed until the pH was 7–8, then dried under vacuum at 100 °C for 12 h. After cooling to room temperature, the material was used for electrode preparation. At the same time, pure Mn3O4 particles were synthesized using the same method for comparative experiments.
2.2 Material characterization
The phase structure of the prepared materials was characterized using an X-ray diffractometer (XRD, DX-600X, Cu-Kα1). The surface elemental composition and the corresponding chemical structure of the prepared materials were analyzed using an X-ray photoelectron spectrometer (XPS, Thermo Scientific NEXSA, Al-Kα). The micro-morphology of the materials was observed using a field emission scanning electron microscope (SEM, FEI Inspect F50), transmission electron microscope (TEM), and high-resolution transmission electron microscope (HRTEM, JEM2100). The manganese (Mn) content in the Ti3C2@Mn3O4 composite material was analyzed using an inductively coupled plasma emission spectrometer (ICP-OES, AGILENT 730).2.3 Electrochemical analysis
The electrochemical tests were conducted in a two-compartment electrolytic cell, separated by a Nafion membrane. A platinum electrode and saturated Hg/Hg2SO4 electrode were applied as the counter electrode and reference electrode, respectively. For the working electrode, an appropriate amount of catalyst and Nafion solution (5 wt%) were ultrasonically dispersed in a mixed solution of pure water and anhydrous ethanol to form a uniform dispersion. Then, a small amount of the dispersion was loaded onto a carbon cloth with an area of 1 × 1 cm2. The final catalyst loading is about 0.5 mg cm−2. The electrolysis cell (H2SO4, pH = 2) was continuously purged with high-purity N2 for 30 min under sealed conditions to exclude air while ensuring that the N2 was saturated. The corresponding electrochemical tests were performed at room temperature and atmospheric pressure using an electrochemical workstation. All electrode potentials were converted to those corresponding to the reversible hydrogen electrode (E (vs. RHE) = E (vs. Hg/Hg2SO4) + 0.616 + 0.059 × pH).The linear sweep voltammetry (LSV) curves were determined at a scan rate of 5 mV s−1 and the corresponding chronoamperometry tests were carried out at a series of negative potentials. Throughout the experiment, a continuous flow of high-purity nitrogen gas was maintained to the cathodic compartment. Cyclic voltammetry (CV) curves were conducted in the range of 0.35 and 0.45 V with the scan rates of 20, 40, 60, 80, and 100 mV s−1.
2.4 Determination of NH3
The yield of ammonia (NH3) produced by the electrocatalytic reduction of N2 was determined using the indophenol blue method.24,25 4 mL of the electrolyte after the reaction was mixed with 500 μL of sodium salicylate (C7H5NaO3) solution (0.4 M C7H5NaO3, 0.32 M NaOH), 50 μL of sodium hypochlorite (NaClO) solution (0.05 M NaClO, 0.75 M NaOH), and 50 μL of sodium nitroprusside (C5FeN6Na2O) solution (1 wt%). The mixed solution was then kept in the dark for 2 h. Then, the absorbance of the mixed solution was measured using a UV-visible spectrophotometer. The concentration–absorbance curve was plotted using a standard NH4Cl solution of 0∼2 μg mL−1. The yield of ammonia was calculated using eqn (3):| rNH3 = (cNH3V)/(tm) | (3) |
2.5 Determination of N2H4
The Watt–Chrisp method was used for hydrazine detection.24,25 First, p-dimethylaminobenzaldehyde (0.998 g) was dissolved in ethanol (50 mL) to act as the colorimetric reagent. Then, the electrolyte (2 mL) was thoroughly mixed with the colorimetric reagent (2 mL) for 20 min, and the absorbance was detected using a UV-visible spectrophotometer (λ = 455 nm). The absorbance curve was plotted using standard hydrazine solutions (0–1 μg mL−1).2.6 FE of NH3 production
The FE for ammonia synthesis is the ratio of the charge transferred for ammonia production to the total consumed charge, which is given by eqn (4):| FE = 3FnNH3/Q | (4) |
F is the Faraday constant (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1), nNH3 is the molar amount of produced ammonia (mol), and Q is the total consumed charge (C).
485 C mol−1), nNH3 is the molar amount of produced ammonia (mol), and Q is the total consumed charge (C).
3. Results and discussions
3.1 Morphology and structure
Fig. 1a shows the XRD patterns of the samples. When Mn3O4 nanoparticles were in situ grown on the surface of Ti3C2Tx MXene layers to form the Ti3C2@Mn3O4 composites, the original XRD characteristic peak at 8.85° shifted to a lower angle at 6.9°, and the interlayer spacing of Ti3C2Tx MXene increased from 0.998 to 1.280 nm, indicating manganese ions were effectively intercalated into the Ti3C2Tx MXene layers; the diffraction peaks at 18.0°, 2.9°, 32.3°, 36.1°, 36.4°, 44.4°, 59.8° matched with the standard card of Mn3O4 (PDF# 24-0734), corresponding to the diffraction peaks of (1 0 1), (1 1 2), (1 0 3), (2 1 1), (2 02), (2 2 0), and (2 2 4), respectively. The ICP-OES analysis revealed that the manganese content in Ti3C2@Mn3O4 was 0.456 g, and thus the calculated content of Mn3O4 in the Ti3C2@Mn3O4 composite was 63.3%. Fig. 1b–f show the XPS spectra of the Ti3C2@Mn3O4 material. The Ti2p spectrum (Fig. 1c) exhibits three sets of resolved peaks at 455.3 and 461.0, 456.8 and 462.4, 458.6 and 464.2 eV, corresponding to Ti–C, Ti3+, and Ti4+(Ti–O),23 respectively. The C 1 s spectrum (Fig. 1d) can be deconvoluted into four Gaussian peaks, with the strongest peak at 284.8 eV corresponding to the C–C bonds, while the other peaks at 285.2, 286.4 and 288.3 eV correspond to the C–Ti, C–O and C–F bonds, respectively.23 As for Mn 3s (Fig. 1e), the peaks at 83.2 and 88.9 eV correspond to Mn 3s3/2 and Mn 3s1/2, respectively, and the binding energy difference (ΔE) between these two peaks is 5.7 eV, which is lower than that of Mn2+ (ΔE = 6 eV) and higher than that of Mn3+ (ΔE = 5.3 eV), indicating that the valence state of Mn in the Ti3C2@Mn3O4 composites is a mixed valence state of Mn2+ and Mn3+.26 The O 1s spectrum (Fig. 1f) can be deconvoluted into three peaks at 529.7, 530.1, and 531.4 eV, corresponding to O–C, Mn–O–Mn (Ti–O), and O–H,26 respectively. All the above analysis indicates that the prepared Ti3C2Tx MXene materials indeed have rich surface termination structures, such as![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OH, and –F, thereby endowing Ti3C2Tx MXenes with electronegativity.
O, –OH, and –F, thereby endowing Ti3C2Tx MXenes with electronegativity.
 | ||
| Fig. 1 XRD patterns of (a) Ti3C2Tx MXenes, Mn3O4 and Ti3C2@Mn3O4; (b) overall survey, (c) Ti 2p, (d) C 1s, (e) Mn 3s, and (f) O 1s XPS spectra of Ti3C2@Mn3O4. | ||
The acid treatment (Fig. 2a and b) results in the etching of Al, yielding Ti3C2Tx MXenes with a smooth accordion-like surface and a significantly increased layer spacing. The prepared Ti3C2@Mn3O4 composites were observed and analyzed using TEM and HRTEM. As can be seen Fig. 2c and d, due to the strong electrostatic attraction between the surface terminals Tx (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –F, and –OH) of Ti3C2Tx MXenes and Mn2+, Mn2+ will be inserted into the interlayer of Ti3C2Tx MXenes and adsorbed on its surface, thus anchoring the generated Mn3O4 particles firmly in the interlayer and on the surface of Ti3C2Tx MXenes, inhibiting their growth or aggregation into secondary large particles. Meanwhile, the presence of interlayer Mn3O4 nanoparticles can effectively expand the interlayer spacing of Ti3C2Tx MXenes, which increased from 0.998 to 1.280 nm, as revealed by the aforementioned XRD (Fig. 1a). The planar spacings of the generated nanoparticles are 0.313, 0.294, and 0.254 nm, corresponding to the (1 1 2), (2 0 0), and (2 1 1) planes of Mn3O4, respectively, analyzed by HRTEM (Fig. 2d). Meanwhile, the laminar structure of Ti3C2Tx MXenes can be clearly seen. The distributions of Mn, O, Ti, and C in the Ti3C2@Mn3O4 composites are shown in Fig. 2e, indicating these elements are uniformly dispersed in the material. This result demonstrates that Mn3O4 nanoparticles are evenly grown on Ti3C2Tx MXenes.
O, –F, and –OH) of Ti3C2Tx MXenes and Mn2+, Mn2+ will be inserted into the interlayer of Ti3C2Tx MXenes and adsorbed on its surface, thus anchoring the generated Mn3O4 particles firmly in the interlayer and on the surface of Ti3C2Tx MXenes, inhibiting their growth or aggregation into secondary large particles. Meanwhile, the presence of interlayer Mn3O4 nanoparticles can effectively expand the interlayer spacing of Ti3C2Tx MXenes, which increased from 0.998 to 1.280 nm, as revealed by the aforementioned XRD (Fig. 1a). The planar spacings of the generated nanoparticles are 0.313, 0.294, and 0.254 nm, corresponding to the (1 1 2), (2 0 0), and (2 1 1) planes of Mn3O4, respectively, analyzed by HRTEM (Fig. 2d). Meanwhile, the laminar structure of Ti3C2Tx MXenes can be clearly seen. The distributions of Mn, O, Ti, and C in the Ti3C2@Mn3O4 composites are shown in Fig. 2e, indicating these elements are uniformly dispersed in the material. This result demonstrates that Mn3O4 nanoparticles are evenly grown on Ti3C2Tx MXenes.
 | ||
| Fig. 2 (a) and (b) SEM images of Ti3C2Tx MXenes; TEM (c) and HRTEM (d) images of Ti3C2@Mn3O4; (e) corresponding elemental mappings of Mn, O, Ti, and C. | ||
3.2 Electrocatalytic performance
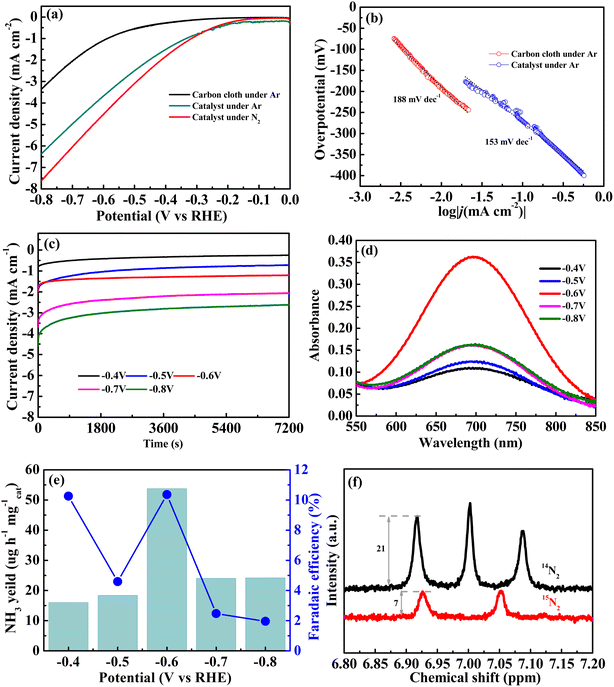
Firstly, the linear sweep voltammetry (LSV) curves were determined to evaluate the NRR activity of Ti3C2@Mn3O4 in N2- and Ar-saturated H2SO4 solutions (pH = 2) (tests confirm that neither Ar nor N2 contained any other nitrogen species (Table S1†)). As shown in Fig. 3a, the two curves of the catalyst under N2 and Ar conditions exhibit similar trends but different current densities. Apparently, at the same voltage, the current density is higher in N2 atmosphere, indicating a more active NRR reaction. In general, the more negative the voltage, the more pronounced the reduction reaction. However, based on the competitive hydrogen evolution side reaction of the NRR reaction, the hydrogen evolution becomes more intense as the voltage increases, leading to a gradual decrease in FE. To further evaluate the HER activity of Ti3C2@Mn3O4, the LSV curve of the carbon cloth without the electrocatalyst was determined under an Ar atmosphere as a control experiment. The current density with the catalyst is higher than that of carbon cloth at the same potential, indicating that the catalyst possesses HER catalytic activity. The corresponding Tafel analysis is presented in Fig. 3b.In acidic electrolytes, the HER process primarily involves three steps: electrochemical hydrogen adsorption (Volmer step), electrochemical desorption (Heyrovsky step), and chemical desorption (Tafel step), as shown in the following equations:27
| Volmer: H3O+ + e−→ Hads + H2O (120 mV dec−1) | (5) |
| Heyrovsky: Hads + H3O+ + e− → H2 + H2O (40 mV dec−1) | (6) |
| Tafel: 2Hads → H2 (30 mV dec−1) | (7) |
H3O+ combines with electrons on the catalyst surface to readily form Hads, which constitutes the Volmer step, with a Tafel slope of 120 mV dec−1. Subsequently, the generated Hads may follow two distinct pathways: The adsorbed Hads combines with another H3O+ and gains an electron to form H2, which then desorbs from the active site (Heyrovsky step), with a Tafel slope of 40 mV dec−1. Alternatively, two adjacent Hads species directly combine to produce H2, followed by desorption (Tafel step), with a Tafel slope of 30 mV dec−1. Tafel slope serves as a crucial indicator of the kinetics in electrochemical reactions, particularly in HER. Notably, under an argon atmosphere, the Tafel slope recorded with just a carbon cloth or in the presence of the catalyst is 188 and 153 mV dec−1, respectively. Both values significantly exceed the 120 mV dec−1 threshold, strongly suggesting that the Volmer step serves as the rate-determining step in the HER mechanism.
In order to determine the optimal working voltage for the NRR reaction, a series of NRR experiments were carried out using the comparative potentials of −0.4 to −0.80 V, and the resulting time-dependent current density curves are shown in Fig. 3c. The UV-vis absorption spectrum of the electrolytes after 2 h of electrocatalysis (Fig. 3d) shows an obvious absorption, which proves the NRR catalytic performance of Ti3C2@Mn3O4. With the increase in voltage, the absorption peak intensity shows a trend of first increasing and then decreasing, with the strongest peak at −0.6 V. The yield of ammonia tested by the indophenol method (Fig. S1†) also shows a trend of first increasing and then decreasing with the rise in voltage, with the highest yield (53.7 μg h−1 mgcat.−1) at −0.6 V, while FE shows a gradually declining trend (Fig. 3e). Although a high FE is observed at −0.4 V, the NH3 yield rate is too low; therefore, −0.6 V was chosen as the optimal operating voltage, at which the highest ammonia production rate was obtained and the FE was also high (10.4%). Hydrazine (N2H2) is a potential secondary product in the electrocatalytic NRR process, which was barely detected by the Watt and Crisp methods (Fig. S2 and S3†).
Subsequently, isotopic labeling measurements with 14N2 and 15N2 gas feeds were conducted to confirm the true source of NH3. 14N2 exhibits a reaction duration of 6 hours (h), whereas 15N2 completes the same process within 2 h. The results (Fig. 3f) of the 1H-NMR spectrum display the characteristic triplet and doublet peaks of 14NH4+ and 15NH4+, corresponding to the used 14N2 and 15N2 gas feeds, respectively.28–30 This indicates that the nitrogen in the ammonia indeed comes from the nitrogen gas used. As evidenced by the 1H-NMR spectrum analysis, the nitrogen isotopic composition exhibits a distinct temporal pattern with the 14N2 peak intensity (21 a.u.) demonstrating a three-fold enhancement compared to the 15N2 counterpart (7 a.u.). This proportional relationship aligns with the temporal progression of the reaction, providing quantitative evidence for the time-dependent accumulation of ammonia products.
In order to verify that N2 is the main nitrogen source for the ammonia synthesis reaction, the following comparative experiments were conducted. (i) The Ti3C2@Mn3O4 catalyst was electrolyzed at the open-circuit voltage in a N2-saturated electrolyte for 2 h. (ii) The black CP was electrolyzed at −0.6 V in a N2-saturated electrolyte for 2 h. (iii) The Ti3C2@Mn3O4 catalyst was electrolyzed at −0.6 V in an Ar-saturated electrolyte for 2 h. The UV-vis absorption spectra of the electrolytes obtained from the above three comparisons, treated with indophenol indicator, are shown in Fig. 4a, which shows that only a slight amount of NH3 was detected in cases (i), (ii), and (iii) compared to Ti3C2@Mn3O4, indicating that electrocatalytic NRR occurs only under a N2-saturated atmosphere and a specific applied voltage. The durability and stability of catalysts are also important indicators for evaluating the performance of electrocatalysts. Fig. 4b shows the NH3 yield rates and corresponding FEs of Ti3C2@Mn3O4 with alternating 2 h-cycles in N2-saturated electrolytes (Fig. S4†). As can be seen, during the 8 cycles of intermittent operation, the NH3 yield rates remained largely unchanged despite minor fluctuations and the corresponding FEs also remained nearly stable. The NH3 yield rates and corresponding FEs of the Ti3C2@Mn3O4 composite material during alternating 2 hour cycles in N2- and Ar-saturated electrolytes, respectively, are shown in Fig. 4c and S5.† In the N2 atmosphere, the NH3 yield rate remained stable at approximately 55 μg h−1 mgcat.−1, with the FE maintained at around 7%. In contrast, under an Ar atmosphere, the detected NH3 yield was negligible (∼2 μg h−1 mgcat.−1), and the FE was essentially 0% due to no appreciable NH3 production. These results demonstrate that the catalyst shows effective catalytic performance for eNRR under ambient conditions. After a long cycle of 22 h (Fig. 4d), there was no significant difference in the current density of catalysis at an applied potential of −0.6 V. Moreover, the XRD patterns of the electrode before and after cycling are identical (Fig. S6†). The excellent catalytic performance of the Ti3C2@Mn3O4 material is attributed to the synergistic catalytic effect of Mn3O4 and MXenes, as evident from Fig. 4e and S7.† The amount of ammonia produced at the Mn3O4, Ti3C2 and Ti3C2@Mn3O4 electrodes after electrolysis for 2 h under ambient conditions at −0.6 V is 22.5, 15.4 and 53.7 μg h−1 mgcat.−1, respectively.
As summarized in Table 1, the present work demonstrates superior NH3 yield and FE compared with state-of-the-art electrocatalysts reported in the literature. Although ref. 38 demonstrates a high FE of 73.3%, it employs the noble metal gold as the catalyst and achieves only a modest NH3 yield of 23 μg h−1 mgcat.−1. Similarly, ref. 39 reports an FE of 56.5%, but with a considerably lower NH3 yield of merely 7.4 μg h−1 mgcat.−1. In comparison, our work achieves a superior NH3 yield of 53.7 μg h−1 mgcat.−1 while maintaining competitive FE performance.
| Materials | Optimal operating voltage (V vs. RHE) | NH3 yield rate (ug h−1 mgcat.−1) | FE (%) | References |
|---|---|---|---|---|
| −0.6 | 53.7 | 10.4 | This work | |
| SnS2 | −0.5 | 3.3 | 10.8 | 8 |
| MnO2-Ti3C2Tx | −0.55 | 34.1 | 11.3 | 12 |
| Mn3O4 nanocube | −0.8 | 11.6 | 3 | 13 |
| MnO-carbon nanofibers | −1.25 | 35.9 | 1.5 | 14 |
| Ti3C2Tx MXene nanosheets | −0.4 | 20.4 | 9.3 | 20 |
| Fluorine-free Ti3C2Tx nanosheets | −0.3 | 36.9 | 9.1 | 21 |
| Mn3O4 nanoparticles | −0.5 | 25.9 | 5.5 | 31 |
| CuAg/Ti3C2 | −0.5 | 4.1 | 9.7 | 32 |
| Molybdenum carbide | −0.5 | 7.7 | 1.8 | 33 |
| Pd films grown on Ni foam | −0.1 | 18.2 | 10.3 | 34 |
| SnO2 | −0.6 | 25.2 | 11.4 | 35 |
| BiOCl-modified 2D titanium carbide MXene | −0.1 | 4.0 | 11.9 | 36 |
| 1T/2H mixed-phase MoSSe | −0.45 | 32.3 | 12.6 | 37 |
| Gold nanoparticles | −0.3 | 23.0 | 73.3 | 38 |
| FeSA–N–C | 0 | 7.4 | 56.5 | 39 |
To understand the intrinsic advantages of Ti3C2@Mn3O4 for NRR catalytic activity, the electrochemical active surface area was estimated by the double-layer capacitance (Cdl) from CV experiments in the range of 0.35–0.45 V (Fig. 5a–c). The Cdl value of Ti3C2Tx is calculated to be 0.59 mF cm−2 (Fig. 5d), which is larger than that of Mn3O4 (0.19 mF cm−2), and the value of Ti3C2@Mn3O4 is calculated to be 0.23 mF cm−2. As revealed from this data, the high specific surface area of MXene and its interaction with Mn3O4 can indeed effectively increase the electrochemical surface area of Mn3O4, thereby improving its catalytic activity. In order to investigate the intrinsic reason for the high electrocatalytic performance of Ti3C2@Mn3O4, N2 temperature-programmed desorption (N2-TPD) experiments were carried out, and the results are displayed in Fig. 5e. The chemical desorption peaks of Mn3O4 and Ti3C2 were located at 90 °C and 164 °C, respectively, in the N2-TPD spectra. As for Ti3C2@Mn3O4, there were two characteristic peaks at around 90 °C and 169 °C, which are associated with the peak for the chemical adsorption of nitrogen for Mn3O4 and Ti3C2, respectively. This additionally confirms that the synergistic effect of Mn3O4 and Ti3C2 promoted the NRR catalytic performance of the Ti3C2@Mn3O4 material.
4. Conclusion
The Ti3C2@Mn3O4 composite was used as an electrocatalyst for ammonia synthesis under ambient conditions via a very simple method. Benefiting from the synergistic catalytic effect of MXenes and Mn3O4, the composite exhibits excellent catalytic performance for ammonia synthesis with an NH3 yield rate of 53.7 μg h−1 mgcat.−1 and satisfactory FE of 10.4% at −0.6 V under ambient conditions. The composite catalyst exhibits excellent stability, durability, and selectivity, with outstanding synergistic effects, surpassing most reported NRR electrocatalysts. This simple and versatile strategy may offer researchers inspiration for rationally designing highly efficient NRR electrocatalysts.Data availability
All data included in this study are available from the corresponding author upon request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported by the Sichuan Science and Technology Program (2024NSFSC0236).References
- G. Soloveichik, Electrochemical synthesis of ammonia as a potential alternative to the Haber–Bosch process, Nat. Catal., 2019, 2, 377–380 CrossRef CAS.
- X. W. Lv, Y. Liu, R. Hao, W. Tian and Z. Y. Yuan, Urchin-like Al-doped Co3O4 nanospheres rich in surface oxygen vacancies enable efficient ammonia electrosynthesis, ACS Appl. Mater. Interfaces, 2020, 12, 17502–17508 CrossRef CAS PubMed.
- X. He, H. Guo, T. Liao, Y. Pu, L. Lai, Z. Wang and H. Tang, Electrochemically synthesized SnO2 with tunable oxygen vacancies for efficient electrocatalytic nitrogen fixation, Nanoscale, 2021, 13, 16307–16315 RSC.
- Y. Zha, M. Liu, J. Wang, J. Feng, D. Li, D. Zhao, S. Zhang and T. Shi, Electrochemical ammonia synthesis by reduction of nitrate on Au doped Cu nanowires, RSC Adv., 2023, 13, 9839–9844 RSC.
- H. Shen, C. Choi, J. Masa, X. Li, J. Qiu, Y. Jung and Z. Sun, Electrochemical ammonia synthesis: mechanistic understanding and catalyst design, Chem, 2021, 7, 1–47 Search PubMed.
- P. Huang, Z. Cheng, L. Zeng, J. Yu, L. Tan, P. Mohapatra, L. Fan and Y. Zhu, Enhancing nitrogen electroreduction to ammonia by doping chlorine on reduced graphene oxide, ACS Catal., 2020, 10, 14928–14935 CrossRef CAS.
- G. Y. Arif, L. Luo, W. Ye, M. A. Mushtaq, X. Fang, X. Xiang, S. Jia and D. Yan, Hierarchical hollow nanotubes of NiFeV-layered double hydroxides@CoVP heterostructures towards efficient, pH-universal electrocatalytical nitrogen reduction reaction to ammonia, Appl. Catal., B, 2020, 265, 118559–118569 CrossRef.
- X. Chen, Y. T. Liu, C. Ma, J. Yu and B. Ding, Self-organized growth of flower-like SnS2 and forest-like ZnS nanoarrays on nickel foam for synergistic superiority in electrochemical ammonia synthesis, J. Mater. Chem. A, 2019, 7, 22235–22241 RSC.
- M. A. Légaré, G. Bélanger-Chabot, R. D. Dewhurst, E. Welz, I. Krummenacher, B. Engels and H. Braunschweig, Nitrogen fixation and reduction at boron, Science, 2018, 359, 896–900 CrossRef PubMed.
- Z. H. Xue, S. N. Zhang, Y. X. Lin, H. Su, G. Y. Zhai, J. T. Han, Q. Y. Yu, X. H. Li, M. Antoniett and J.-S. Chen, Electrochemical education of N2 into NH3 by donor-acceptor couples of Ni and Au nanoparticles with a 67.8% faradaic efficiency, J. Am. Chem. Soc., 2019, 141, 14976–14980 CrossRef CAS PubMed.
- Y. Liu, M. Han, Q. Xiong, S. Zhang, C. Zhao, W. Gong, G. Wang, H. Zhang and H. Zhao, Dramatically enhanced ambient ammonia electrosynthesis performance by in-operando created Li-S interactions on MoS2 electrocatalyst, Adv. Energy Mater., 2019, 9, 1803935 CrossRef.
- W. Kong, F. Gong, Q. Zhou, G. Yu, L. Ji, X. Sun, A. M. Asiri, T. Wang, Y. Luo and Y. Xu, An MnO2-Ti3C2Tx MXene nanohybrid: an efficient and durable electrocatalyst toward artificial N2 fixation to NH3 under ambient conditions, J. Mater. Chem. A, 2019, 7, 18823–18827 RSC.
- X. Wu, L. Xia, Y. Wang, W. Lu, Q. Liu, X. Shi and X. Sun, Mn3O4 nanocube: an efficient electrocatalyst toward artificial N2 fixation to NH3, Small, 2018, 14, 1803111 CrossRef PubMed.
- X. Zheng, Z. Zhang, X. Li and C. Ding, MnO-carbon nanofiber composite material toward electrochemical N2 fixation under ambient conditions, New J. Chem., 2019, 43, 7932–7935 RSC.
- S. Bi, Y. Wu, A. Cao, J. Tian, S. Zhang and Z. Niu, Free-standing three-dimensional carbon nanotubes/amorphous MnO2 cathodes for aqueous zinc-ion batteries with superior rate performance, Mater. Today Energy, 2020, 18, 100548–100558 CrossRef CAS.
- H. Tong, T. Li, J. Liu, D. Gong, J. Xiao, L. Shen, B. Ding and X. Zhang, Fabrication of the oxygen vacancy amorphous MnO2/carbon nanotube as cathode for advanced aqueous zinc-ion batteries, Energy Technol., 2021, 9, 2000769–2000775 CrossRef CAS.
- X. Chen, W. Li, Z. Zeng, D. Reed, X. Li and X. Liu, Engineering stable Zn-MnO2 batteries by synergistic stabilization between the carbon nanofiber core and birnessite-MnO2 nanosheets shell, Chem. Eng. J., 2021, 405, 1–12 Search PubMed.
- C. Zhang, B. Anasori, A. Seral-Ascaso, S.-H. Park, N. McEvoy, A. Shmeliov, G. S. Duesberg, J. N. Coleman, Y. Gogotsi and V. Nicolosi, Transparent, flexible, and conductive 2D titanium carbide (MXene) films with high volumetric capacitance, Adv. Mater., 2017, 29, 1702678 CrossRef PubMed.
- Q. Tang, Z. Zhou and P. Shen, Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer, J. Am. Chem. Soc., 2012, 134, 16909–16916 CrossRef CAS PubMed.
- J. Zhao, L. Zhang, X. Xie, X. Li, Y. Ma, Q. Liu, W. H. Fang, X. Shi, G. Cui and X. Sun, Ti3C2Tx (T = F, OH) MXene nanosheets: conductive 2D catalysts for ambient electrohydrogenation of N2 to NH3, J. Mater. Chem. A, 2018, 6, 24031–24035 RSC.
- T. Li, X. Yan, L. Huang, J. Li, L. Yao, Q. Zhu, W. Wang, W. Abbas, R. Naz, J. Gu, Q. Liu, W. Zhang and D. Zhang, Fluorine-free Ti3C2Tx (T = O, OH) nanosheets (50–100 nm) for nitrogen fixation under ambient conditions, J. Mater. Chem. A, 2019, 7, 14462–14465 RSC.
- W. Chen, G. Li, A. Pei, Y. Li, L. Liao, H. Wang, J. Wan, Z. Liang, G. Chen, H. Zhang, J. Wang and Y. Cui, A manganese–hydrogen battery with potential for grid-scale energy storage, Nat. Energy, 2018, 3, 428–437 CrossRef CAS.
- D. Chao, W. Zhou, C. Ye, Q. Zhang, Y. Chen, L. Gu, K. Davey and S.-Z. Qiao, An electrolytic Zn–MnO2 battery for high-voltage and scalable energy storage, Angew. Chem., Int. Ed., 2019, 58, 7823–7828 CrossRef CAS PubMed.
- X. He, H. Guo, T. Liao, Y. Pu, L. Lai, Z. Wang and H. Tang, Electrochemically synthesized SnO2 with tunable oxygen vacancies for efficient electrocatalytic nitrogen fixation, Nanoscale, 2021, 13, 16307–16315 RSC.
- X. Wang, X. Chi, Z. Fu, Y. Xiong, S. Li, Y. Yao, K. Zhang, Y. Li, S. Wang, R. Zhao, Z. Yang and Y. M. Yan, Interfacial electric field triggered N2 activation for efficient electrochemical synthesis of ammonia, Adv. Funct. Mater., 2022, 32, 2202820 CrossRef.
- M. Liu, Q. Zhao, H. Liu, J. Yang, X. Chen, L. Yang, Y. Cui, W. Huang, W. Zhao, A. Song, Y. Wang, S. Ding, Y. Song, G. Qian, H. Chen and F. Pan, Tuning phase evolution of β-MnO2 during microwave hydrothermal synthesis for high-performance aqueous Zn ion battery, Nano Energy, 2019, 64, 103942–103948 CrossRef CAS.
- J. Ye, Z. Yu, W. Chen, Q. Chen and L. Ma, Ionic-liquid mediated synthesis of molybdenumdisulfide/graphene composites: an enhanced electrochemical hydrogen evolution catalyst, Int. J. Hydrogen Energy, 2016, 28, 12049–12061 CrossRef.
- A. Kafle, D. Gupta, A. Bordoloi and T. C. Nagaiah, Self-standing Fe3O4 decorated paper electrode as a binder-free trifunctional electrode for electrochemical ammonia synthesis and Zn-O2 batteries, Nanoscale, 2022, 14, 16590–16601 RSC.
- X. Fu, J. Zhang and Y. Kang, Recent advances and challenges of electrochemical ammonia synthesis, Chem Catal., 2022, 10, 2590–2613 Search PubMed.
- M. Han, M. Guo, Y. Yun, Y. Xu, H. Sheng, Y. Chen, Y. Du, K. Ni, Y. Zhu and M. Zhu, Effect of heteroatom and charge reconstruction in atomically precise metal nanoclusters on electrochemical synthesis of ammonia, Adv. Funct. Mater., 2022, 32, 2202820 CrossRef CAS.
- C. Wang, X. Zhua and P. Zuo, Novel confinement of Mn3O4 nanoparticles on two-dimensional carbide enabling high-performance electrochemical synthesis of ammonia under ambient conditions, Chem. Eng. J., 2020, 396, 125163 CrossRef CAS.
- A. Liu, Q. Yang, X. Ren, M. Gao, Y. Yang, L. Gao, Y. Li, Y. Zhao, X. Liang and T. Ma, Two-dimensional CuAg/Ti3C2 catalyst for electrochemical synthesis of ammonia under ambient conditions: a combined experimental and theoretical study, Sustainable Energy Fuels, 2020, 4, 5061–5071 RSC.
- K. P. Ramaiyan, S. Ozden, S. Maurya, D. Kelly, S. K. Babu, A. Benavidez, F. G. Garzon, Y. S. Kim, C. R. Kreller and R. Mukundan, Molybdenum carbide electrocatalysts for electrochemical synthesis of ammonia from nitrogen: activity and stability, J. Electrochem. Soc., 2020, 167, 044506 CrossRef CAS.
- Z. Wang, Z. Dai, H. Yu, H. Zhang, W. Tian, Y. Xu, X. Li, L. Wang and H. Wang, Pore-size-tuned Pd films grown on Ni foam as an advanced catalyst for electrosynthesis of ammonia, ACS Sustainable Chem. Eng., 2020, 31, 11827–11833 CrossRef.
- X. He, H. Guo, T. Liao, Y. Pu, L. Lai, Z. Wang and H. Tang, Electrochemically synthesized SnO2 with tunable oxygen vacancies for efficient electrocatalytic nitrogen fixation, Nanoscale, 2021, 13, 16307–16315 RSC.
- Y. Wang, M. Batmunkh, H. Mao, H. Li, B. Jia, S. Wu, D. Liu, X. Song, Y. Sun and T. Ma, Low-overpotential electrochemical ammonia synthesis using BiOCl-modified 2D titanium carbide MXene, Chin. Chem. Lett., 2022, 33, 394–398 CrossRef CAS.
- M. Sun, C. Ma, M. Ma, Y. Wei, S. Dong, X. Zhang, J. Tian and M. Shao, Electrochemical ammonia synthesis via nitrogen reduction reaction on 1T/2H mixed-phase MoSSe catalyst: Theoretical and experimental studies, Mater. Today Phys., 2023, 30, 100945 CrossRef CAS.
- L. Tan, N. Yang, X. Huang, L. Peng, C. Tong, M. Deng, X. Tang, L. Li, Q. Liao and Z. Wei, Synthesis of ammonia via electrochemical nitrogen reduction on high-index faceted Au nanoparticles with a high faradaic efficiency, Chem. Commun., 2019, 55, 14482 RSC.
- M. Wang, S. Liu, T. Qian, J. Liu, J. Zhou, H. Ji, J. Xiong, J. Zhong and C. Yan, Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential, Nat. Commun., 2019, 10, 341 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01799h |
| This journal is © The Royal Society of Chemistry 2025 |