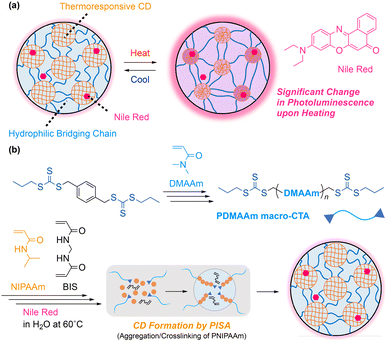
Fast and controlled thermoresponse in the photoluminescence of well-designed hydrogels of two separate nanodomains with solvatochromic dyes†
Hayato
Wakuda
a,
Shohei
Ida
 *a,
Masatoshi
Oyama
b,
Keiji
Nakajima
b,
Hiroki
Takeshita
a and
Shokyoku
Kanaoka
*a
*a,
Masatoshi
Oyama
b,
Keiji
Nakajima
b,
Hiroki
Takeshita
a and
Shokyoku
Kanaoka
*a
aDepartment of Materials Chemistry, Faculty of Engineering, The University of Shiga Prefecture, 2500 Hassaka, Hikone, Shiga 522-8533, Japan. E-mail: ida.s@mat.usp.ac.jp; kanaoka.s@mat.usp.ac.jp
bIndustrial Research Center of Shiga Prefecture, 232 Kamitoyama, Ritto, Shiga 520-3004, Japan
First published on 29th May 2025
Abstract
Stimuli-responsive hydrogels that allow simultaneous changes in multiple properties, including photoluminescence, are attractive for various applications such as sensing materials because such gels can visually represent environmental changes. In developing such novel materials, it is essential to precisely design the network structure at the nanoscale for hybridization with an appropriate dye. In this study, we designed gels with a thermoresponsive crosslinked nanodomain (CD) structure containing Nile Red, a solvatochromic dye, via the polymerization-induced self-assembly (PISA) process. The synthesis was achieved using reversible addition–fragmentation chain transfer (RAFT) polymerization of N-isopropylacrylamide (NIPAAm) from a hydrophilic bifunctional macro-chain transfer agent in an aqueous dispersion of Nile Red at a high temperature. The obtained gels appeared blue under visible light and showed faint red fluorescence under UV irradiation, while it rapidly turned reddish purple and exhibited red fluorescence without syneresis upon heating. These changes in appearance and photoluminescence were derived from changes in the microenvironment around Nile Red, induced by reversible swelling/shrinking of PNIPAAm nanodomains within the hydrogel network. Moreover, these internal structural changes simultaneously altered the mechanical properties along with the changes in appearance and photoluminescence on a similar timescale.
Introduction
Sensing systems in nature, characterized by changes in color and/or luminescence, are often composed of an environment-responsive pigment and a stimuli-responsive flexible architecture.1,2 A good case in point is a system found in bodies of squids and octopuses, which undergoes a color change in response to the state of pigments that can be governed by the contraction and relaxation of muscles on the body surface.3,4 Inspired by such systems in natural soft tissues, hybridization of dyes in artificial soft materials has been investigated: in all systems, stimuli-responsive behavior of a polymer network led to a color change of elaborately dispersed functional dyes.5–13Smart hydrogels undergo significant changes in properties and/or structure in response to a small change in environment, attracting remarkable attention for applications in various fields.14–17 In addition, this sensing ability as well as the similarity to natural soft tissues in terms of high water content and flexibility has raised expectations for future advancement in biomedical fields and soft robotics.18–21 Among them, stimuli-responsive hydrogels that permit simultaneous changes in multiple properties including luminescence properties are suitable for sensing materials since those materials can visualize environmental changes. In particular, hydrogels capable of undergoing luminescence changes in both air and water under ambient conditions are highly awaited. However, stimuli-responsive hydrogels can have potential but serious drawbacks as a sensing material: clouding after transition by dehydration and slow response for a sensor. For example, thermoresponsive hydrogels, a typical stimuli-responsive gel, start shrinking at a temperature where the solubility of network polymers decreases drastically and form aggregated moieties on a micrometer scale. The large aggregated domains scatter visible light, causing noticeable turbidity22 to blemish the transparency of a hydrogel material, which is essential for clear sensing. As mentioned above, the slow response of gels in a bulk state can also be problematic for applications. In developing novel stimuli-responsive photoluminescent hydrogels, thus, downsizing and compartmentalization of stimuli-responsive domains exhibiting the response under ambient conditions are expected to be effective means for maintaining transparency and raising the response rate.
Multiple distinct domains in a network structure of a gel have been achieved by building conetwork structures, among which, specifically, amphiphilic conetwork (APCN) structures consisting of hydrophilic and hydrophobic block segments in the network are attractive platforms for tailored functionalization of hydrogels.23–30 Recently, we successfully synthesized a new class of APCN hydrogels with crosslinked nanodomains (CDs) homogeneously dispersed.31–38 A feature of the CD gel is that CDs can be even immiscible in not only network polymer chains but also in solvents, because a single CD on a nanoscale is linked to multiple network chains. Hence, a hydrogel of thermoresponsive CDs consisting of network chains of N-isopropylacrylamide (NIPAAm) remained transparent and constant in volume even above the cloud point of the corresponding poly(NIPAAm) (PNIPAAm).34,35 In addition, upon heating, the designed hydrogels with thermoresponsive CDs responded more rapidly and sharply in water,32 and toughened in air without external water.33,34 As the CD gel has features suitable for sensing, CD gels containing fluorescent carbon dots within the thermoresponsive nanodomains were synthesized, which underwent simultaneous changes in fluorescent and mechanical properties upon heating.35 However, in terms of fluorescent properties, only a slight change in intensity was observed without any shift in the emission wavelength.
To induce a drastic change in color or luminescence, it is imperative to select an appropriate environment-responsive dye. Promising pigments would be those exhibiting responsive behavior such as aggregation-induced emission,39,40 mechanochromism,41,42 and solvatochromism.43,44 In this study, we focused on solvatochromic dyes that change their photoluminescence in solution depending on the solvent polarity.43,44 Nile Red is a typical solvatochromic dye, which hardly fluoresces in highly polar solvents such as water, while it shows strong fluorescence in low polar solvents.45,46 This sensitivity to polarity is a good match for the temperature-sensitive transition of PNIPAAm, which produces a hydrophilic and polar environment below the cloud point, and the hydrophobic and less polar counterpart above the cloud point.47–49 The designed CD gels that we reported alter the polarity in the PNIPAAm nanodomain in response to temperature change. Thus, a CD gel combined with Nile Red is expected to become an appropriate platform for a soft material showing a significant change in photoluminescent properties. Encouraged by these backgrounds, we aimed to develop CD hydrogels that produced significant changes in photoluminescence in response to temperature changes (Fig. 1a).
In this study, the synthesis of hydrogels with homogeneously dispersed thermoresponsive CDs containing Nile Red was examined using the polymerization-induced self-assembly (PISA) process in an aqueous dispersion of Nile Red as the reaction solvent: more specifically, NIPAAm was copolymerized with a vinyl crosslinker using reversible addition–fragmentation chain transfer (RAFT) polymerization50–53 from a hydrophilic bifunctional macro-chain transfer agent (macro-CTA) in water at a temperature much higher than the cloud point of linear PNIPAAm (Fig. 1b).34,35 In the reaction, simultaneous aggregation and crosslinking of thermoresponsive PNIPAAm chains, which propagated from both ends of the hydrophilic macro-CTA, proceeded to form evenly dispersed CDs. The structure and response behavior in air and water of the obtained gels, including photoluminescence and mechanical properties, were systematically investigated.
Experimental
Materials
NIPAAm (FUJIFILM Wako Chemical, >98.0%) was purified by recrystallization from toluene/hexane. The CTA for RAFT polymerization carrying two trithiocarbonate groups in the molecule was prepared as reported in the literature.54 Distilled water was obtained using an EYELA STILL ACE SA-2100A. The following compounds, reagents, and solvents were used as received: N,N-dimethylacrylamide (DMAAm; FUJIFILM Wako Chemical, >98.0%), N,N′-methylenebisacrylamide (BIS; FUJIFILM Wako Chemical, for electrophoresis, >99.0%), Nile Red (FUJIFILM Wako Chemical, for pathology research), 2,2′-azobisisobutyronitrile (AIBN; FUJIFILM Wako Chemical, >99.0%), ammonium persulfate (APS; FUJIFILM Wako Chemical, for electrophoresis, >99.0%), N,N,N′,N′-tetramethylethylenediamine (TMEDA; FUJIFILM Wako Chemical, for electrophoresis, >99.0%), 1,2,3,4-tetrahydronaphthalene (tetralin; Sigma-Aldrich, 99.0%), 1,4-dioxane (FUJIFILM Wako Chemical, >99.0%), N,N-dimethylformamide (DMF; FUJIFILM Wako Chemical, 99.5%), paraffin oil (Hayashi Pure Chemical, >99.0%) and CDCl3 (Cambridge Isotope Laboratories, 99.5%; or Eurisotop, 99.8%).Measurement and characterization
The number-average molecular weight (Mn), weight-average molecular weight (Mw), and polydispersity index (Mw/Mn) of each polymer were determined by size-exclusion chromatography (SEC; Shimadzu Prominence system consisting of an LC-20AD precision pump and an RID-20A refractive index detector) in DMF containing 10 mM LiBr as the eluent at 40 °C (flow rate: 1.0 mL min−1) using three polystyrene gel columns (Shodex KF-805L). The columns were calibrated against standard poly(methyl methacrylate) samples (Agilent, Mn = 5.89 × 102 − 1.68 × 106).1H nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-ECS400 spectrometer operating at 399.90 MHz. The degree of polymerization (DPn) and the Mn, NMR were calculated from the integral values of the peaks derived from the CTA and the monomer units.
UV-vis absorption spectra were recorded with a Jasco V-750 in a range between 200 and 800 nm at 20 °C and 40 °C. For gel samples, rectangular specimens with the dimensions of ca. 2 × 8 × 30 mm were attached to an interior side in a measurement cell and the spectra were measured. The temperature of samples was controlled using a Jasco ETCS-761 Peltier thermostatted cell holder. For the evaluation of response behavior upon cooling, gel specimens were heated in a water bath set at 40 °C for 60 minutes. Then, they were immediately transferred to a measurement cell in the spectrometer maintained at 20 °C, and the absorption spectra were recorded at the predetermined time.
Photoluminescence spectra were recorded with a Shimadzu RF-6000. For gel samples, rectangular specimens with the dimensions of ca. 2 × 8 × 25 mm were put on a Nafion plate, which was placed in a measurement glass cell. The excitation light was exposed from 45° angles to the specimen and the spectra were measured. The samples in a heated state were measured using a sample holder connected to a constant-temperature water bath (EYELA, NTB-221). The measurements were conducted immediately after heating the samples in a water bath at 40 °C for 20 min.
Small-angle X-ray scattering (SAXS) experiments were conducted using synchrotron radiation at beamline BL-6A of the Photon Factory at the Institute of Materials Structure Science of the High Energy Accelerator Research Organization in Tsukuba, Japan. Two-dimensional scattering images were collected on a Dectris PILATUS 1M detector. One-dimensional SAXS profiles were obtained by radial averaging of the two-dimensional images. The scattering angle was calibrated by using silver behenate having a periodical structure of 5.838 nm. The scattering vector was defined as q = (4π/λ)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ/2), where θ and λ are the scattering angle and the wavelength of the incident X-ray, respectively.
sin(θ/2), where θ and λ are the scattering angle and the wavelength of the incident X-ray, respectively.
Dynamic viscoelasticity measurements were conducted with a TA Instruments Discovery HR-2 with roughened parallel-plate geometry using columnar specimens (diameter: 8 mm, height: 1 mm). The samples were prepared in a silicone mold and coated with paraffin oil. First, the specimens were kept at 20 °C for 1 minute, and then immediately heated to 40 °C. After holding at 40 °C for 10 minutes, the specimens were rapidly cooled to 20 °C and held for a predetermined time. During this process, the storage modulus (G′) and loss modulus (G′′) were measured (strain: 10%, frequency: 1 Hz, the temperature was controlled using a Peltier plate).
The swelling degree of gels was determined by measuring the diameter of cylindrical gels. The gel samples were prepared in glass capillaries (internal diameter: 1330 μm, volume: 30 μL) in a reaction vessel, taken out from the capillaries, and washed with distilled water by immersing overnight at room temperature. The gels were immersed in water at a predetermined temperature, and the equilibrium diameter at a given temperature, d, was measured using a digital zoom microscope (Meiji Techno UNIMAC MS-40DR connected to a Shimadzu MOTICAM2000). The swelling degree was calculated from (d/d0);3d0 is the internal diameter of the glass capillary (1330 μm), which can be regarded as the diameter of the as-prepared gel.
Gel synthesis by the PISA process
Gel samples were prepared according to a procedure described in our previous report.34 In a typical preparation, DMAAm (30.8 mL, 300 mmol), CTA (406 mg, 1.00 mmol), AIBN (16.4 mg, 0.100 mmol), tetralin (5.0 mL), and 1,4-dioxane (64.2 mL) were added to a 200 mL round-bottomed flask equipped with a three-way stopcock, and bubbled with nitrogen for 10 minutes. The flask was placed in an oil bath kept at 60 °C for 24 h. The reaction was terminated by cooling the reaction mixture to −60 °C, and the reaction mixture was poured into diethyl ether to obtain the purified PDMAAm (17.8 g; DPn = 300 and Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, both of which were calculated by 1H NMR analysis). The obtained PDMAAm macro-CTA (803 mg, 0.027 mmol of macro-CTA containing 8.0 mmol of the DMAAm unit), NIPAAm (905 mg; 8.0 mmol), and BIS (24.7 mg; 0.16 mmol) was dissolved in 7.00 mL of an aqueous dispersion of Nile Red (30 μg mL−1). After bubbling nitrogen for 10 minutes into this solution, 1.0 mL of the APS solution in aqueous Nile Red dispersion (containing 0.040 mmol of APS) was added, and the reaction mixture was kept at 60 °C on a hot plate for over 24 h to reach the gelation state.
100, both of which were calculated by 1H NMR analysis). The obtained PDMAAm macro-CTA (803 mg, 0.027 mmol of macro-CTA containing 8.0 mmol of the DMAAm unit), NIPAAm (905 mg; 8.0 mmol), and BIS (24.7 mg; 0.16 mmol) was dissolved in 7.00 mL of an aqueous dispersion of Nile Red (30 μg mL−1). After bubbling nitrogen for 10 minutes into this solution, 1.0 mL of the APS solution in aqueous Nile Red dispersion (containing 0.040 mmol of APS) was added, and the reaction mixture was kept at 60 °C on a hot plate for over 24 h to reach the gelation state.
Gel synthesis by free radical polymerization
DMAAm (0.51 mL, 5.0 mmol), NIPAAm (566 mg; 5.0 mmol) and BIS (15.4 mg; 0.10 mmol) were dissolved in 3.89 mL of an aqueous dispersion of Nile Red (38.6 μg mL−1). After bubbling nitrogen for 10 minutes into this solution, 0.50 mL of an aqueous solution of APS (containing 0.025 mmol of APS) and 0.10 mL of an aqueous solution of TMEDA (containing 0.050 mmol of TMEDA) were added, and the reaction mixture was kept at room temperature for 24 h to reach the gelation state.Results and discussion
Synthesis of crosslinked nanodomain (CD) gels containing Nile Red via the PISA process
The synthesis of gels with thermoresponsive CDs containing Nile Red was investigated using the PISA process that we have previously reported.34,35 The process involves RAFT copolymerization of NIPAAm and BIS, a crosslinker, from a bifunctional macro-CTA in water at 60 °C, where the growing PNIPAAm chains become insoluble to aggregate (Fig. 1b). In our previous report, we performed the reaction under the same conditions but without BIS, leading to the formation of a transparent physical gel.34 This gel reversibly turned to a sol state by cooling. These results indicate that the aggregated PNIPAAm nanodomain was formed via the PISA process.In this study, first, DMAAm was polymerized with a bifunctional CTA having two trithiocarbonate groups to yield a hydrophilic macro-CTA with a narrow molecular weight distribution (DPn, NMR = 300, Mn, NMR = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, Mw/Mn = 1.22; Fig. S1 and S2 in the ESI†). An aqueous dispersion of Nile Red (30 μg mL−1) prepared as the solvent of gel synthesis was nearly colorless and exhibited almost no fluorescence under UV irradiation (Fig. S3 in the ESI†). Here, the maximum concentration of Nile Red dispersion without noticeable precipitation was found to be approximately 63 μg mL−1. In the light of the photoluminescence intensity and the stability of the dispersion, we set the concentration for gel synthesis at 30 μg mL−1. Upon the addition of NIPAAm, PDMAAm macro-CTA and BIS at various concentration ratios ([NIPAAm] = 500, 750 and 1000 mM; [NIPAAm] + [DMAAm monomer unit] = 2000 mM, [BIS] = 20 mM), all dispersions turned dark blue or brown depending on the composition of the solution (Fig. S4a in the ESI†). The absorption of Nile Red at 590 nm observed in the UV-vis spectrum and fluorescence at around 660 nm significantly increased at a higher NIPAAm concentration (Fig S4b–d in the ESI†). The increased fluorescence was likely due to hydrophobic interaction between Nile Red and the NIPAAm monomer in water.
100, Mw/Mn = 1.22; Fig. S1 and S2 in the ESI†). An aqueous dispersion of Nile Red (30 μg mL−1) prepared as the solvent of gel synthesis was nearly colorless and exhibited almost no fluorescence under UV irradiation (Fig. S3 in the ESI†). Here, the maximum concentration of Nile Red dispersion without noticeable precipitation was found to be approximately 63 μg mL−1. In the light of the photoluminescence intensity and the stability of the dispersion, we set the concentration for gel synthesis at 30 μg mL−1. Upon the addition of NIPAAm, PDMAAm macro-CTA and BIS at various concentration ratios ([NIPAAm] = 500, 750 and 1000 mM; [NIPAAm] + [DMAAm monomer unit] = 2000 mM, [BIS] = 20 mM), all dispersions turned dark blue or brown depending on the composition of the solution (Fig. S4a in the ESI†). The absorption of Nile Red at 590 nm observed in the UV-vis spectrum and fluorescence at around 660 nm significantly increased at a higher NIPAAm concentration (Fig S4b–d in the ESI†). The increased fluorescence was likely due to hydrophobic interaction between Nile Red and the NIPAAm monomer in water.
Heating these solutions with APS at 60 °C, which is above the phase transition temperature of PNIPAAm, induced gelation at all concentrations (entries 1–3 in Table 1; the obtained gels are denoted as NG500, NG750, and NG1000, respectively; the subscript number in the sample code stands for the feed concentration of NIPAAm). The concentration conditions of the reaction solutions were set based on our previous study35 to ensure that the resulting gels maintain transparency at a high temperature above the transition temperature of PNIPAAm. The obtained gels appeared reddish-purple under visible light and exhibited red fluorescence under UV light immediately after the reaction (Fig. 2). Upon cooling to room temperature, these gels turned blue under visible light and exhibited faint red fluorescence under UV light. Thus, the CD gels obtained by the PISA process in the presence of Nile Red remarkably changed their appearance depending on the temperature (the details of photoluminescent properties are discussed later).
 | ||
| Fig. 2 Appearances of NG1000 at room temperature and 40 °C under (a) visible light and (b) irradiation with UV light (wavelength: 365 nm). | ||
| Entry | [NIPAAm] (mM) | [DMAAm unit] (mM) | [BIS] (mM) | Nile Red (μg per mL) | Gel codeb |
|---|---|---|---|---|---|
| a Reaction conditions: [APS] = 5.0 mM in an aqueous dispersion of Nile Red at 60 °C for 24 h. b In the sample code, NG, CG, and FG stand for “Gel with nanodomain structure containing Nile Red”, “Control Gel sample without Nile Red” and “Gel prepared by Free radical polymerization”, respectively. The subscript number stands for the feed concentration of NIPAAm (mM). c The subscript “NR63” stands for the Nile Red concentration of 63 μg mL−1. d The subscript “BIS80” stands for the BIS concentration of 80 mM. e Synthesized by free radical copolymerization: [APS] = 5.0 mM, [TMEDA] = 10 mM in an aqueous dispersion of Nile Red at room temperature for 24 h. | |||||
| 1 | 500 | 1500 | 20 | 30 | NG500 |
| 2 | 750 | 1250 | 20 | 30 | NG750 |
| 3 | 1000 | 1000 | 20 | 30 | NG1000 |
| 4 | 1000 | 1000 | 20 | 63 | NG1000NR63 |
| 5 | 1000 | 1000 | 80 | 30 | NG1000BIS80 |
| 6 | 1000 | 1000 | 20 | 0 | CG1000 |
| 7e | 1000 | 1000 | 20 | 30 | FG1000 |
The solution with the same monomer concentration as entry 3 and a greater amount of Nile Red ([NIPAAm] = [DMAAm unit] = 1000 mM; 63 μg mL−1 of Nile Red) also underwent gelation, which indicates that the presence of Nile Red produced little effect on the PISA process (entry 4; NG1000NR63). For the comparison with the sample of Entry 3, we also synthesized a gel with nanodomains much highly crosslinked ([BIS] = 80 mM; entry 5; NG1000BIS80), and a control sample without Nile Red (entry 6; CG1000) via the PISA process. A Nile Red-containing gel with a random monomer and crosslinker sequence was also prepared by free radical polymerization of DMAAm and NIPAAm in the presence of BIS (entry 7; FG1000).
SAXS measurements were performed at room temperature to examine the effect of Nile Red on the internal structure of CD gels (Fig. 3). A distinct maximum peak at q = 0.20 nm−1 was observed in the SAXS profile of NG1000, with Nile Red, and a similar maximum peak at a nearly identical q value was observed in the profile of CG1000, without Nile Red. These results demonstrate that Nile Red hardly affected the internal structure of the product CD gels. The maximum peak in the SAXS profile indicated the presence of a particle structure with high electron density, such as CDs, at an average distance, D (=2π/qmax), of 32 nm. The end-to-end distance of PDMAAm used in the synthesis of gels for SAXS measurements (DPn = 269) is 11.9 nm based on the reported relationship between DP and the end-to-end distance of polyacrylamide in water.55 Assuming that the CD is spherical, the radius of the CD is approximately 10.1 nm, and the volume of one CD is 4.2 × 10−21 L. Assuming that the volume fraction of water-containing CDs in the network corresponds to the composition ratio (PDMAAm/PNIPAAm = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the concentration of the CD is calculated as 0.19 mM using the feed concentration. These results indicated that 19 PDMAAm chains are connected to each CDs on average, and the concentration of Nile Red is lower than that of the CD (each nanodomain is calculated to contain 0.49 molecule of Nile Red); hence the aggregation of Nile Red in the CDs was negligible.
1), the concentration of the CD is calculated as 0.19 mM using the feed concentration. These results indicated that 19 PDMAAm chains are connected to each CDs on average, and the concentration of Nile Red is lower than that of the CD (each nanodomain is calculated to contain 0.49 molecule of Nile Red); hence the aggregation of Nile Red in the CDs was negligible.
Thermoresponsive photoluminescent properties
As mentioned above, the Nile Red-hybridized CD gels significantly changed their color under visible light and fluorescence emission under UV irradiation in response to temperature change (Fig. 2). These changes in color and photoluminescence were reversible with no turbidity and very little water loss after repeated heating/cooling cycles in air (maintaining 95% of the weight after 1 h heating).Absorption and fluorescence measurements were conducted with the CD gels hybridized with Nile Red in various compositions at room temperature and after heating at 40 °C for 20 minutes. Heating NG1000 caused a blue shift by approximately 15 nm in both absorption and maximum fluorescence, with fluorescence intensity increased to about 1.7 times in a reversible manner (Fig. 4). Similar changes were observed regardless of the composition of CD gels (Fig. S5 in the ESI†). These changes in appearance and photoluminescence were attributed to the alteration in the microenvironment around Nile Red within the PNIPAAm CDs, which became hydrophobic and shrunk upon heating. Since hydrophobic Nile Red has higher affinity to the PNIPAAm CDs, which were hydrophobic at the reaction temperature of the PISA process, most of Nile Red was incorporated into the PNIPAAm CDs in the gel. Thus, the change in the polarity of the PNIPAAm CDs significantly affected the properties of Nile Red, leading to notable blue shifts in the absorption and maximum fluorescence as well as fluorescence intensity increase of the CD gels. It should also be noted that the CD gel maintained its transparency due to the homogeneous dispersion of the CDs at the nanoscale without appreciable aggregation in the gel network at a high temperature, as demonstrated by SAXS analysis.
In contrast, the gel with highly crosslinked CDs containing Nile Red (NG1000BIS80) hardly exhibited changes in fluorescence intensity upon heating (Fig. S6 in the ESI†). This phenomenon suggests that the flexibility of PNIPAAm chains in the CDs played a critical role in achieving a significant change in photoluminescence. Furthermore, FG1000 without a nanodomain structure exhibited weaker fluorescence intensity and subtle thermoresponse (Fig. S7a and b in the ESI†). In addition, heating FG1000 caused significant syneresis, unsuitable for repeated use (Fig. S7c in the ESI†). This behavior contrasted sharply with the CD gels, in which the PDMAAm bridging chains retained water released from the shrunken PNIPAAm CDs upon heating and returned it to the CD upon re-cooling. Thus, the hybridization of Nile Red into CD gels with appropriate crosslinking density enabled a significant photoluminescence change in response to temperature change.
Dynamic behavior in photoluminescence and mechanical properties
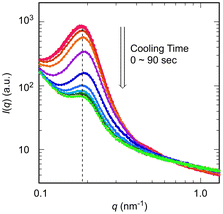
The dynamic behavior of thermoresponsive change in photoluminescence of the CD gels containing Nile Red (NG1000) was investigated by measuring the time dependence of the absorption spectra during a cooling process to 20 °C after heating at 40 °C for 60 minutes. During cooling, the maximum absorption wavelength shifted from 573 nm to a longer wavelength, and the absorption intensity gradually decreased (Fig. 5a). The wavelength returned to its original value before heating within 3 minutes, and the absorbance significantly decreased, although it remained slightly higher than the initial value even after 60 minutes of cooling (Fig. 5b and c). These results in the behavior of absorption wavelength and absorbance demonstrated a rapid response of the CD gels containing Nile Red to temperature changes. NG1000 also demonstrated a rapid response during a heating process from 20 °C to 40 °C (Fig. S8 in the ESI†), and a similar responsive behavior was observed irrespective of the composition of the CD gels (Fig. S9 in the ESI†).Such thermoresponsive behavior derived from the change in the internal structure of the CD gels, and the internal structure of network polymers is basically correlated to mechanical properties. Therefore, the time dependence of dynamic viscoelasticity was measured with NG1000 (Fig. 6). When the temperature was increased from 20 °C to 40 °C, the elastic modulus increased probably due to the restoring force upon stretching of the PDMAAm bridging chains caused by the thermoresponsive shrinkage of the PNIPAAm CDs in the network. In addition, dangling PNIPAAm segments, which were formed due to the lack of reaction with the BIS crosslinker during the PISA process, may contribute to additional physical crosslinking through thermoresponsive association with PNIPAAm CDs. A similar increase in elastic modulus was observed with an APCN with thermoresponsive segments.27 The SAXS profile of NG1000 at 40 °C exhibited a maximum peak with increasing intensity while qmax remained unchanged (Fig. 7; red curve), indicating an increase in the electron density of the CDs likely due to thermoresponsive shrinking as well as additional association of dangling PNIPAAm segments. Furthermore, rapid re-cooling to 20 °C resulted in a sharp decrease in the elastic modulus, which then remained nearly constant during the observation period (Fig. 6). The behavior of the dynamic viscoelasticity was very similar to the thermoresponsive behavior of the absorption properties. The close similarity indicates that both changes in mechanical and photoluminescent properties were induced by changes in the internal structure of a CD gel. A previous study revealed that the swelling of the PNIPAAm CDs during cooling was relatively slower than their shrinking upon heating,34 and it usually took several hours to reach equilibrium in the mechanical properties for CD gels of sizes comparable to those in this study for the tensile tests and absorption measurements. On the other hand, the present study demonstrated that the CD gels containing Nile Red achieved rapid and significant thermoresponsive changes in the photoluminescent and mechanical properties. The rapid change is possibly due to the immediate initial transition of the internal structure, as confirmed by SAXS analysis of NG1000 during cooling, which exhibited a sharp decrease in the scattering intensity associated with the CD structure (Fig. 7). Moreover, since the internal structure, photoluminescence and mechanical properties are well correlated, the mechanical properties of the CD gels may be estimated from the appearance of the gels.
 | ||
| Fig. 6 Time dependence of dynamic viscoelasticity of NG1000 during heating at 40 °C for 10 minutes and subsequent cooling at 20 °C. | ||
Thermoresponsive photoluminescence in water
Similar to the response behavior observed in air, the CD gels containing Nile Red exhibited remarkable changes in photoluminescence in response to the temperature change in water. NG1000 swelled to a large extent in water at a low temperature, and abruptly shrank at around 32 °C upon heating (Fig. 8). This behavior was very similar to that of CG1000, a CD gel without Nile Red, supporting that the presence of Nile Red had a slight effect on the network structure formed during the PISA process. In contrast, FG1000 without the nanodomain structure, which was prepared by free radical copolymerization under the same monomer and crosslinker concentration conditions as NG1000 in the presence of Nile Red, exhibited a lower swelling degree at a low temperature and gradually decreased the swelling degree upon heating. These results indicated that the PNIPAAm nanodomains of the CD gels clearly exhibited their thermoresponsive properties due to the compartmentalized structure. In addition, during this swelling measurement, NG1000 retained the color derived from Nile Red, which indicates that Nile Red remained trapped within the network. This is likely due to the immobilization of Nile Red by the crosslinking structure of the PNIPAAm nanodomain formed by the PISA process, during which Nile Red interacted strongly with PNIPAAm chains via hydrophobic interaction.Fluorescence measurements were conducted on the CD gels swollen in water at room temperature and after being heated at 40 °C for 20 minutes. NG1000 showed fluorescence in water, with a significant increase in fluorescence intensity upon heating (Fig. 9a). This thermoresponsive change in intensity was notably greater than that observed in air. Prolonged heating resulted in a remarkable visual change in color under both visible light and UV irradiation (Fig. 9b and c). At a low temperature in water, where the PNIPAAm CDs swelled within the network, the interaction between Nile Red and the gel network became weaker, resulting in a decrease in fluorescence intensity. During the shrinkage of the CDs upon heating in water, the interaction changed more significantly than it did under air, leading to a noticeable change in the appearance. On the other hand, FG1000 hardly exhibited a change in the fluorescence intensity upon heating in water (Fig. 9d–f). Thus, the CD gels containing Nile Red demonstrated large and rapid changes in fluorescence properties in response to temperature changes even in water due to their well-designed nanodomain structure.
Conclusions
Well-designed gels with a thermoresponsive CD structure containing Nile Red were demonstrated to exhibit significant changes in photoluminescence in response to temperature change both in air and water. The CD gels were successfully synthesized via the PISA process using RAFT polymerization in an aqueous dispersion of Nile Red as the solvent. The obtained gels appeared blue under visible light and showed faint red fluorescence under UV irradiation, while upon heating, the gel turned reddish purple and exhibited red fluorescence without syneresis. These behaviors contrasted sharply with those of a gel prepared by free radical copolymerization, demonstrating the importance of the CD structure in achieving significant color changes. The response of the CD gels containing Nile Red was rapid: the absorption wavelength completely altered within a few minutes. These changes in appearance and photoluminescence were derived from changes in the microenvironment around Nile Red, induced by reversible swelling/shrinking of the PNIPAAm nanodomains within the hydrogel network. Moreover, such internal structural changes simultaneously altered the mechanical properties along with the changes in appearances and photoluminescence on a similar timescale. This well-correlated change potentially allows the estimation of the changes in mechanical properties by just observing the appearance of the gels. In addition, it should be noted that this system undergoes simultaneous changes in multiple properties under ambient conditions. This study would expand the design criteria for novel soft materials exhibiting significant changes in photoluminescence properties as well as other properties, contributing to the development of advanced sensing materials and soft robotics.Author contributions
Conceptualization: S. I. and S. K.; formal analysis: H. W. and S. I.; funding acquisition: S. I.; investigation: H. W., M. O., K. N. and H. T.; supervision: S. K.; visualization: H. W. and S. I.; writing – original draft: H. W. and S. I.; writing – review & editing: S. K.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Japan Society for the Promotion of Science through a Grant-in-aid for Scientific Research (JP24K08529), by the Takahashi Industrial and Economic Research Foundation, and by JST ERATO Grant Number JPMJER2401. The SAXS experiments at Photon Factory were performed under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2023G567).References
- J. S. Sparks, R. C. Schelly, W. L. Smith, M. P. Davis, D. Tchernov, V. A. Pieribone and D. F. Gruber, PLoS One, 2014, 9, e83259 CrossRef PubMed
.
- S. Reiter, P. Hülsdunk, T. Woo, M. A. Lauterbach, J. S. Eberle, L. A. Akay, A. Longo, J. Meier-Credo, F. Kretschmer, J. D. Langer, M. Kaschube and G. Laurent, Nature, 2018, 562, 361–366 CrossRef CAS PubMed
.
- L. M. Mäthger, E. J. Denton, N. J. Marshall and R. T. Hanlon, J. R. Soc., Interface, 2009, 6, S149–S163 CrossRef PubMed
.
- M. D. Ramirez and T. H. Oakley, J. Exp. Biol., 2015, 218, 1513–1520 CrossRef PubMed
.
- S. Wu, H. Shi, W. Lu, S. Wei, H. Shang, H. Liu, M. Si, X. Le, G. Yin, P. Theato and T. Chen, Angew. Chem., Int. Ed., 2021, 60, 21890–21898 CrossRef CAS PubMed
.
- Q. Wang, G. R. Gossweiler, S. L. Craig and X. Zhao, Nat. Commun., 2014, 5, 4899 CrossRef PubMed
.
- L. Phan, R. Kautz, E. M. Leung, K. L. Naughton, Y. Van Dyke and A. A. Gorodetsky, Chem. Mater., 2016, 28, 6804–6816 CrossRef CAS
.
- N. Majstorović and S. Agarwal, ACS Appl. Polym. Mater., 2021, 3, 4992–4999 CrossRef
.
- A. Choe, J. Yeom, R. Shanker, M. P. Kim, S. Kang and H. Ko, NPG Asia Mater., 2018, 10, 912–922 CrossRef CAS
.
- S. Lim, J. E. Song, J. A. La and E. C. Cho, Chem. Mater., 2014, 26, 3272–3279 CrossRef CAS
.
- X.-Y. Du, C.-F. Wang, G. Wu and S. Chen, Angew. Chem., Int. Ed., 2021, 60, 8585–8595 CrossRef CAS PubMed
.
- A. Cayuela, S. R. Kennedy, M. L. Soriano, C. D. Jones, M. Valcárcel and J. W. Steed, Chem. Sci., 2015, 6, 6139–6146 RSC
.
- T. Enjou, S. Goto, Q. Liu, F. Ishiwari, A. Saeki, T. Uemtasu, Y. Ikemoto, S. Watanabe, G. Matsuba, K. Ishibashi, G. Watanabe, S. Minakata, Y. Sagara and Y. Takeda, Chem. Commun., 2024, 60, 3653–3656 RSC
.
- E. Caló and V. V. Khutoryanskiy, Eur. Polym. J., 2015, 65, 252–267 CrossRef
.
- M. Mahinroosta, Z. J. Farsangi, A. Allahverdi and Z. Shakoori, Mater. Today Chem., 2018, 8, 42–55 CrossRef CAS
.
- M. Karg, A. Pich, T. Hellweg, T. Hoare, L. A. Lyon, J. J. Crassous, D. Suzuki, R. A. Gumerov, S. Schneider, I. I. Potemkin and W. Richtering, Langmuir, 2019, 35, 6231–6255 CrossRef CAS PubMed
.
- X. Lin, X. Wang, L. Zeng, Z. L. Wu, H. Guo and D. Hourdet, Chem. Mater., 2021, 33, 7633–7656 CrossRef CAS
.
- A. K. Means and M. A. Grunlan, ACS Macro Lett., 2019, 8, 705–713 CrossRef CAS PubMed
.
- D. Zhalmuratova and H.-J. Chung, ACS Appl. Polym. Mater., 2020, 2, 1073–1091 CrossRef CAS
.
- J. Chen, Q. Peng, X. Peng, L. Han, X. Wang, J. Wang and H. Zeng, ACS Appl. Polym. Mater., 2020, 2, 1092–1107 CrossRef CAS
.
- L. K. Rivera-Tarazona, Z. T. Campbell and T. H. Ware, Soft Matter, 2021, 17, 785–809 RSC
.
- M. Shibayama and K. Nagai, Macromolecules, 1999, 32, 7461–7468 CrossRef CAS
.
-
C. S. Patrickios, Amphiphilic Polymer Co-networks: Synthesis, Properties, Modelling and Applications, RSC Publishing, Cambridge, UK, 2020 Search PubMed
.
- C. S. Patrickios and T. K. Georgiou, Curr. Opin. Colloid Interface Sci., 2003, 8, 76–85 CrossRef CAS
.
- G. Erdodi and J. P. Kennedy, Prog. Polym. Sci., 2006, 31, 1–18 CrossRef CAS
.
- D. G. Tsalikis, M. Ciobanu, C. S. Patrickios and Y. Higuchi, Macromolecules, 2023, 56, 9299–9311 CrossRef CAS
.
- D. E. Apostolides, G. Michael, C. S. Patrickios, B. Notredame, Y. Zhang, J.-F. Gohy, S. Prévost, M. Gradzielski, F. A. Jung and C. M. Papadakis, ACS Appl. Mater. Interfaces, 2024, 16, 23813–23825 CAS
.
- L. Fan, Z. Zeng, R. Zhu, A. Liu, H. Che and M. Huo, Chem. Mater., 2022, 34, 6408–6419 CrossRef CAS
.
- Z. Zeng, Z. Li, Q. Li, G. Song and M. Huo, Small Methods, 2023, 7, 2201592 CrossRef CAS PubMed
.
- Z. Zhao, L. Fan, G. Song and M. Huo, Chem. Mater., 2024, 36, 1436–1448 CrossRef CAS
.
- S. Ida, Polym. J., 2019, 51, 803–812 CrossRef CAS
.
- S. Ida, H. Kitanaka, T. Ishikawa, S. Kanaoka and Y. Hirokawa, Polym. Chem., 2018, 9, 1701–1709 RSC
.
- S. Ida, M. Morimura, H. Kitanaka, Y. Hirokawa and S. Kanaoka, Polym. Chem., 2019, 10, 6122–6130 RSC
.
- M. Morimura, S. Ida, M. Oyama, H. Takeshita and S. Kanaoka, Macromolecules, 2021, 54, 1732–1741 CrossRef CAS
.
- S. Ida, T. Okuno, M. Morimura, K. Suzuki, H. Takeshita, M. Oyama, K. Nakajima and S. Kanaoka, Polym. Chem., 2022, 13, 3479–3488 RSC
.
- S. Furukawa, T. Okuno, T. Shimbayashi, H. Takeshita, K.-i. Fujita and S. Ida, Polym. J., 2023, 55, 945–955 CrossRef CAS
.
- S. Furukawa, H. Takeshita, K.-i. Fujita and S. Ida, Polym. J., 2024, 56, 561–565 CrossRef CAS
.
- S. Ida, S. Toda, M. Oyama, H. Takeshita and S. Kanaoka, Macromol. Rapid Commun., 2021, 42, 2000558 CrossRef CAS PubMed
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed
.
- Y. Hu, L. Barbier, Z. Li, X. Ji, H. Le Blay, D. Hourdet, N. Sanson, J. W. Y. Lam, A. Marcellan and B. Z. Tang, Adv. Mater., 2021, 33, 2101500 CrossRef CAS PubMed
.
- Y. Chen, G. Mellot, D. van Luijk, C. Creton and R. P. Sijbesma, Chem. Soc. Rev., 2021, 50, 4100–4140 RSC
.
- E. M. Lloyd, J. R. Vakil, Y. Yao, N. R. Sottos and S. L. Craig, J. Am. Chem. Soc., 2023, 145, 751–768 CrossRef CAS PubMed
.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS
.
- A. S. Klymchenko, Acc. Chem. Res., 2017, 50, 366–375 CrossRef CAS PubMed
.
- P. Greenspan, E. P. Mayer and S. D. Fowler, J. Cell Biol., 1985, 100, 965–973 CrossRef CAS PubMed
.
- P. Greenspan and S. D. Fowler, J. Lipid Res., 1985, 26, 781–789 CrossRef CAS
.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163–249 CrossRef CAS
.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342–15367 CrossRef CAS PubMed
.
- Y. Hirokawa and T. Tanaka, J. Chem. Phys., 1984, 81, 6379–6380 CrossRef
.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS
.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2009, 62, 1402–1472 CrossRef CAS
.
- A. Gregory and M. H. Stenzel, Prog. Polym. Sci., 2012, 37, 38–105 CrossRef CAS
.
- M. D. Nothling, Q. Fu, A. Reyhani, S. Allison-Logan, K. Jung, J. Zhu, M. Kamigaito, C. Boyer and G. G. Qiao, Adv. Sci., 2020, 7, 2001656 CrossRef CAS PubMed
.
- A. M. Bivigou-Koumba, J. Kristen, A. Laschewsky, P. Müller-Buschbaum and C. M. Papadakis, Macromol. Chem. Phys., 2009, 210, 565–578 CrossRef CAS
.
- G. S. Misra and S. N. Bhattacharya, Eur. Polym. J., 1979, 15, 125–128 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic results of the macro-CTA; appearances and absorbance and photoluminescent spectra of the reaction solutions and the gels; and thermoresponsive fluorescent behavior of the gels. See DOI: https://doi.org/10.1039/d5py00331h |
| This journal is © The Royal Society of Chemistry 2025 |