Qiannan Tan, Xia Mi, Jingyu Zhang and Chao Pi
Org. Biomol. Chem., 2025,23, 2106-2110
DOI:
10.1039/D4OB02041C,
Communication
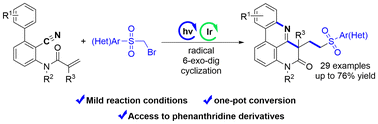
We present a visible-light-promoted radical cascade cyclization reaction via sulfonylmethylation, cyano insertion, and radical cyclization of unactivated alkenes bearing cyano groups. This strategy enables the rapid synthesis of sulfonylmethylated phenanthridines under mild conditions with broad substrate compatibility, operational simplicity, and mild reaction conditions. The developed approach provides a novel pathway for assembling complex polycyclic nitrogen-containing frameworks, addressing a critical synthetic challenge and expanding the toolbox of photochemical transformations in organic synthesis.