Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diagnostic technologies for neuroblastoma
Leena
Khelifa
a,
Yubing
Hu
 *a,
Jennifer
Tall
b,
Rasha
Khelifa
c,
Amina
Ali
c,
Evon
Poon
b,
Mohamed Zaki
Khelifa
c,
Guowei
Yang
c,
Catarina
Jones
d,
Rosalia
Moreddu
e,
Nan
Jiang
*a,
Jennifer
Tall
b,
Rasha
Khelifa
c,
Amina
Ali
c,
Evon
Poon
b,
Mohamed Zaki
Khelifa
c,
Guowei
Yang
c,
Catarina
Jones
d,
Rosalia
Moreddu
e,
Nan
Jiang
 *f,
Savas
Tasoglu
*f,
Savas
Tasoglu
 g,
Louis
Chesler
bh and
Ali K.
Yetisen
g,
Louis
Chesler
bh and
Ali K.
Yetisen
 a
a
aDepartment of Chemical Engineering, Imperial College London, South Kensington, London, SW7 2BU, UK. E-mail: yubing.hu@imperial.ac.uk
bDivision of Clinical Studies, The Institute of Cancer Research, London, SM2 5NG, UK
cFaculty of Medicine, Imperial College London, South Kensington, SW7 5NH, UK
dSchool of Electronics and Computer Science, University of Southampton, Southampton, SO17 1BJ, UK
eInstitute for Life Sciences, University of Southampton, Southampton, SO17 1BJ, UK
fWest China School of Basic Medical Sciences & Forensic Medicine, Sichuan University, Chengdu 610041, China. E-mail: jiangnansophia@scu.edu.cn
gResearch Center for Translational Medicine, Koc University, Istanbul 34450, Turkey
hChildren's and Young People's Unit, Royal Marsden NHS Foundation Trust, Sutton, SM2 5PT, UK
First published on 14th July 2025
Abstract
Neuroblastoma is an aggressive childhood cancer characterised by high relapse rates and heterogenicity. Current medical diagnostic methods involve an array of techniques, from blood tests to tumour biopsies. This process is associated with long-term physical and psychological trauma. Moreover, current technologies do not identify neuroblastoma at an early stage while tumours are easily resectable. In recent decades, many advancements have been made for neuroblastoma diagnosis, including liquid biopsy platforms, radiomics, artificial intelligence (AI) integration and biosensor technologies. These innovations support the trend towards rapid, non-invasive and cost-effective diagnostic methods which can be utilised for accurate risk stratification. Point-of-care (POC) diagnostic devices enable rapid and accurate detection of disease biomarkers and can be performed at the location of the patient. Whilst POC diagnostics has been well-researched within the oncological landscape, few devices have been reported for neuroblastoma, and these remain in the early research phase and as such are limited by lack of clinical validation. Recent research has revealed several potential biomarkers which have great translational potential for POC diagnosis, including proteomic, metabolic and epigenetic markers such as MYCN amplification and microRNAs (miRNAs). Using POC devices to detect high-risk biomarkers in biofluids such as blood and urine, offers a non-invasive approach to diagnosis of neuroblastoma, enabling early screening at a population level as well as real-time health monitoring at home. This is critical to mitigating long-term morbidity associated with late diagnosis and unnecessary treatment, in turn improving outcomes for neuroblastoma patients.
1. Introduction
Neuroblastoma, a neural-crest cell-derived malignancy arising from the peripheral sympathetic nervous system,1 is the most common extra-cranial solid tumour in children, representing around 5% of all paediatric cancer diagnoses but disproportionally accounting for 15% of childhood paediatric oncology deaths.2,3 It is characterised by wide clinical heterogeneity and frequent metastasis.1 Neuroblastoma originates from neural crest progenitor cells due to disrupted differentiation processes involving pathways such as WNT, BMPs, and SWI/SNF complexes, resulting in highly heterogeneous tumours.4–6 Key genetic drivers include MYCN amplification (seen in ∼20% of cases), ALK mutations, and PHOX2B alterations, all linked to poor prognosis and aggressive disease.7–12 Children diagnosed under 1 year of age typically fall into low-risk categories with excellent survival (>95%), whereas high-risk neuroblastoma, often presenting in children over 1 year old, has poor survival and high relapse rates.13–16 Predominantly, neuroblastoma is a sporadic disease; however some familial cases have been documented.17 Most cases of neuroblastoma are diagnosed following the development of symptoms in children; since tumours arise from tissue of the sympathetic nervous system, typically the adrenal medulla, neuroblastoma is often first suspected due to mass lesions in the abdomen or associated symptoms such as weight loss and breathing difficulties.18 Metastatic disease is noted in 60% of neuroblastoma patients, and it is often not until the disease has spread that patients present with symptoms of the disease.19 Early diagnosis and accurate risk stratification are essential to the provision of appropriate treatment in patients.20 Multimodal treatment approaches include stem cell transplantation, surgery and chemotherapy which induce long-term permanent morbidity.21 For neuroblastoma, the most significant prognostic factor is age at diagnosis: earlier diagnosis of disease, whilst tumours are still localised and surgically resectable, contributes to significantly favourable outcomes.22 Several attempts at screening young infants for neuroblastoma have been unsuccessful. Elevated urinary catecholamine metabolites homovanillic acid (HVA) and vanillylmandelic acid (VMA) have been indicated for neuroblastoma diagnosis for many decades.23 In the early-to-mid 1970s, Japan pioneered a small scale screening programme to measure VMA concentrations in infants at 6 months of age.24 Although screening led to increased diagnosis of neuroblastoma, there was no improvement in outcomes for patients. This was because screening detected only favourable characteristics and led to the over-diagnosis of low-risk cases, many of which may have experienced spontaneous disease regression; this contributed to morbidity in patients following unnecessary therapy.25 Other similar studies in Canada26 and Germany27 concluded the same findings. The ability to screen for and diagnose neuroblastoma at an early stage has been a longstanding issue, and the development of highly sensitive, selective, and rapid diagnostic methods is of great importance to improving outcomes for these patients.28,29 The success of such a device would be highly dependent on the selection of appropriate biomarkers which are specific to high-risk neuroblastoma and present early in the disease.Despite the rarity of the disease, the global neuroblastoma market held a value of 1.92 billion pounds in 2016.30 The neuroblastoma treatment market was worth over £327m in 2020 and is expected to reach £418m by 2027.31 The high cost of multimodal therapy approaches in young cancer patients is a great burden to the National Health Service (NHS) and this highlights the need for initiation of lower intensity and more targeted therapy at an earlier stage.32 Further, this will facilitate treatment implementation only where necessary and avoidance of treatment for patients whose disease may spontaneously regress (stage 4S).33 Current diagnostic procedures for neuroblastoma involve biochemical profiling, multimodal imaging approaches, and histologic confirmation.34 These methods are laborious, invasive, and expensive. Furthermore, none of these methods are appropriate for routine screening of infants for high-risk neuroblastoma before symptoms present.13 The introduction of more advanced technologies would allow for more rapid and less invasive diagnosis would revolutionise medical approaches to neuroblastoma. POC tests are often referred to as near patient, bedside or extra laboratory testing.35 The main advantages of these techniques over conventional diagnostic methods are reduced length of hospital stay, reduced clinic visits, earlier discharge from hospital and fewer unnecessary hospital admissions.35 It also facilitates optimised treatment and mitigates the use of inappropriate drugs, consequently improving longevity and quality of life.35 The introduction of POC devices for neuroblastoma would significantly improve diagnosis of paediatric cancers and facilitate the move towards personalised medicine. Recently, there has been a great interest in POC devices for cancer diagnosis,36 given that cancer is the most prevalent disease in the world.37 It is well-documented that early diagnosis and targeted therapy are essential to providing good outcomes for oncology patients.38 For cancer diagnosis, POC devices have shown several limitations;39 this means that such devices are not ready to be used commercially, and these issues must be considered when developing new tools for neuroblastoma. Mainly, lateral flow assays (LFAs) and lab-on-a-chip (LoC) technologies have been researched for cancer diagnosis and monitoring.37 LFAs are rapid and cost-effective paper-based devices which have the ability to detect very low concentrations of analytes in biofluids.40 However, in the cancer field, they are limited to lack of multiplexing, commercialisation, and clinical validation.41 Further, LFAs for nucleic acid detection, which is currently an exciting avenue of research, require multiple steps for gene extraction and amplification, thus making them too complex for routine use in clinical settings and at home by patients.42 LoC technologies are highly promising, however again they are often limited by the requirement of complex steps, and most attempts to develop such devices for cancer detection will require more optimisation and testing before they can be employed for use in hospitals.43 Little to no research has been undertaken for other POC technologies, such as smartphone-based devices, leaving a gap for future research to focus.
Herein, current diagnostic approaches to neuroblastoma and their relative performances and limitations are explored. A comprehensive overview is provided of emerging technologies that are reshaping the diagnostic landscape and paving the way for POC applications. This review also explores POC platforms, and the advantages of these over conventional methods for broader cancer and neuroblastoma diagnosis are considered. Circulating biomarkers in neuroblastoma patients are also investigated and their potential for rapid disease detection. Specifically, the ability to differentially-detect high-risk neuroblastoma using POC devices is of great interest to clinicians and researchers alike, and the future potential and feasibility is explored.
2. Neuroblastoma diagnosis
2.1 Current clinical methods
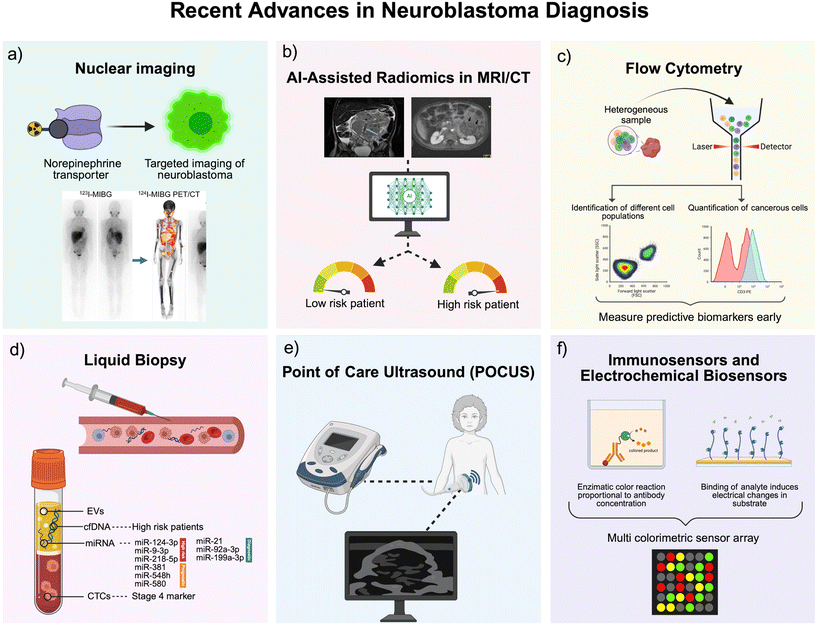
Neuroblastoma is often initially identified when a child presents with signs and symptoms of the disease.19 Biochemical analyses coupled with multimodal imaging techniques may be undertaken if a patient is suspected of suffering from the disease.44 However, ultimately, diagnosis of neuroblastoma is confirmed by either tumour tissue biopsy and histopathologic analysis, or a combination of the detection of neuroblastoma tumour cells in the bone marrow and either elevated urine or serum catecholamines34 (Table 1). Here, we have summarised current techniques utilised to diagnose patients with neuroblastoma (Fig. 1). Detailed information will be demonstrated to compare the accuracy of current diagnostic techniques and their relative convenience in diagnosing neuroblastoma. We explore the complexity, cost, advantages and disadvantages, sensitivity, and accessibility of the techniques.| Diagnostic technique | Cost | Duration | Invasive or harmful? | Is it specific to neuroblastoma? | Can it aid in staging? | Conclusive for diagnosis? |
|---|---|---|---|---|---|---|
| Patient history and physical examination | Low | POC | No | No | No | No |
| Catecholamine metabolites | Medium | Rapid | Yes | No | No | No |
| Blood cell count | Medium | Rapid | Yes | No | No | No |
| Urine cytology | Medium | Rapid | Yes | No | No | No |
| Liver and kidney function tests | Medium | Rapid | Yes | No | No | No |
| Ultrasonography (US) | Medium | Rapid | No | No | No | No |
| Computed tomography (CT) | High | Time-consuming | Yes | No | Yes | No |
| Magnetic resonance imaging (MRI) | High | Time-consuming | Yes | No | Yes | No |
| Metaiodobenzylguanidine (MIBG) scintigraphy | High | Time-consuming | Yes | Yes | No | No |
| Positron emission tomography (PET) scan | High | Time-consuming | Yes | No | No | No |
| X-ray | High | Time-consuming | Yes | No | No | No |
| Biopsy | High | Time-consuming | Yes | Yes | No | Yes |
2.2 Recent advances in neuroblastoma diagnosis
Over recent years, refinements have been made to conventional diagnostic techniques for neuroblastoma, however the methods have remained largely the same and diagnosis relies on imaging and histopathology. There is an urgent need to direct research towards the development of novel technologies that can address the limitations in current methods, for rapid diagnosis and early screening, both of which would play a major role in mitigating the aggressive consequences of this disease. Herein, a summary of recent developments in the diagnosis of neuroblastoma is provided, and how these are paving the way for the creation of rapid and cost-effective tools.To address these gaps, newer PET-based radiotracers are being explored. PET offers clear advantages over SPECT: superior spatial resolution, faster acquisition times (around 15 minutes versus 90 for SPECT), better quantification of tracer uptake, and full-body tomographic coverage.105,106 Tracers like 18F-MFBG and 123I-MIBG have the same uptake mechanism as 123I-MIBG but benefit from the higher image quality of PET. For instance, 18F-MFBG PET-CT has been shown to detect all lesions seen on 123I-MIBG imaging, plus additional ones, in early human studies.107 Similarly, 124I-MIBG PET can improve lesion detection, but has drawbacks such as significantly higher radiation dose—up to 30 times higher than 123I-MIBG in children under 5 years—and more complex logistics due to the tracer's long half-life.108 Alternative PET tracers like 18F-FDG and [68Ga]Ga-DOTATATE are especially useful for MIBG-negative cases. 18F-FDG, while not tumour-specific, has shown good sensitivity in such scenarios. In one study, 18F-FDG PET had higher sensitivity (78%) and specificity (92%) than 123I-MIBG (50% and 75%, respectively) for detecting lesions in patients with MIBG-negative tumours or discordant imaging findings.109 However, 18F-FDG's use is limited by high physiological uptake in bone marrow and the brain, which can obscure small lesions and lead to false positives, especially after chemotherapy or G-CSF treatment.110–112 [68Ga]Ga-DOTATATE PET-CT, which targets somatostatin receptors (primarily SSTR2), has also demonstrated excellent performance. In a study comparing [68Ga]Ga-DOTATATE PET with 123I-MIBG scintigraphy in 42 patients with high-risk neuroblastoma, DOTATATE PET detected more bone and soft tissue lesions in the majority of cases and was positive in all patients, while 123I-MIBG failed to detect disease in two patients.113 Moreover, PET imaging with [68Ga]Ga-DOTATATE enables identification of patients eligible for peptide receptor radionuclide therapy (PRRT), offering a theragnostic pathway.114
Despite the promise of PET imaging, these newer techniques aren't yet standard in all centres due to limited availability, higher cost, and the need for further validation in larger cohorts. Still, the diagnostic and prognostic potential—especially in MIBG-negative or relapsed cases—is significant. As access to PET tracers improves and more prospective studies are published, these methods are likely to become integrated into standard neuroblastoma management. However, these methods are not likely to support early screening, staging or diagnosis of disease, and are costly and time-consuming, relying on specialised radiotracers and equipment.
Circulating tumour cells (CTCs) have also emerged as strong candidates for diagnosis and prognostication. Kojima et al.138 isolated CTCs in 8 of 10 newly diagnosed and 3 of 4 relapsed neuroblastoma patients using FACS, with key mutations like MYCN amplification detectable in 12 of 13 patients. Another study by Merugu et al.139 detected CTCs in 26 out of 42 patients, reporting a mean of 40 cells per mL at diagnosis and 6 cells per mL at relapse. Prognostic relevance is underscored by data showing that 55.6% of stage 4 patients had detectable CTCs compared to none in stage 3, and CTC-positive patients had significantly lower 5 year survival (42%) than CTC-negative patients (90%).140 Detection of CTC on several POC platforms is possible and has been achieved for other cancers.141,142 It is possible to isolate and detect CTC on point-of-care platforms for cancer diagnosis, such as portable cytometers,142 microfluidics143 and biosensors.144 Thus, research into developing and optimising such devices for neuroblastoma should be a point of focus for the future. Recent advances in molecular diagnostics have highlighted circulating RNAs as promising tools for identifying tumour presence in neuroblastoma.145 For instance, elevated levels of tyrosine hydroxylase (TH) mRNA in blood have been associated with advanced disease stages and a higher risk of relapse, supporting its role as a non-invasive marker for neuroblastoma detection.146 Moreover, the combined expression of neuroblastoma -related genes—such as PHOX2B, CHGA, DCX, DDC, and TH—in blood and bone marrow has shown strong predictive value for disease progression.147 Circulating miRNAs, especially those found within exosomes, have also gained traction as potential biomarkers. Several tumour-specific miRNAs, including miR-124-3p, miR-9-3p, and miR-218-5p, have been identified in the serum of high-risk neuroblastoma patients,148 while others such as miR-381, miR-548h, and miR-580 have demonstrated prognostic relevance.149 Notably, exosomal miRNAs—like miR-21, miR-92a-3p, and plasma miR-199a-3p—are consistently elevated in neuroblastoma cell lines and patient samples, indicating strong diagnostic potential.150,151 These exosomal signatures not only reflect tumour presence but may also shift during therapy, offering a dynamic tool for monitoring treatment response.133 Exosomes are isolated from blood using ultracentrifugation or commercial kits, followed by RNA extraction and analysis via qPCR or NGS, which are expensive, time-consuming and require trained personnel. Given the increasingly important role of miRNA as biomarkers for cancers, POC platforms for their detection have gained attention in recent years to overcome the limitations of current methods.152 However, such devices have not yet been developed for neuroblastoma. Since these biomarkers are present in the blood, and can offer selective diagnosis of high-risk disease, their contribution to next-generation POC diagnostic devices for neuroblastoma could be great.
Despite encouraging results, liquid biopsy in neuroblastoma still faces a number of limitations that need to be addressed before routine clinical implementation. One of the main challenges is the lack of standardisation across methodologies—different studies use various kits, detection platforms, and thresholds, making it difficult to compare results or define clear diagnostic cut-offs. Additionally, many studies do not report sensitivity and specificity figures, relying instead on associations with known tumour features. Biological variability, such as inconsistent shedding of tumour DNA or cells, can lead to false negatives, particularly in early-stage disease or minimal residual disease scenarios. Furthermore, most studies have relatively small sample sizes and are often single centre, which limits generalisability. As the field moves forward, larger multicentre trials and the adoption of standardised protocols will be crucial to translating these findings into clinical practice.
In 2023, Chen et al.159 developed an electrochemical immunosensor which targeted the disialoganglioside GD2 which is expressed on neuroblastoma cells. They designed a graphene/gold nanoparticle (AuNP)/GD2 antibody-functionalised electrochemical biosensor capable of detecting GD2-positive cells in bone marrow samples. The sensor demonstrated high sensitivity and could detect cells in the range of 102 to 105 cells per mL, and the results matched 100% with gold-standard immunocytochemical staining techniques. Whilst interesting, the requirement for bone marrow samples means painful and invasive procedures for children and thus is not useful in terms of POC testing or rapid diagnostics. Nonetheless, these advancements prove how new-generation technologies are providing promising results that mitigate some of the disadvantages of conventional techniques. Graphene quantum dots (GQDs) have also been used for in vivo imaging of neuroblastoma.160 Anti-GD2/GQDs were shown to accumulate in neuroblastoma tumours in mice, which enabled fluorescence imaging of the tumours. The combination of nanotechnology with immunosensing could be an interesting avenue of research for future diagnostics, although unlikely to support POC use.
3. POC technologies
Diagnosing neuroblastoma remains a complex and resource-intensive process. Many patients, often young children, undergo invasive procedures or are exposed to ionising radiation simply to confirm a diagnosis.161,162 These methods are not only costly and time-consuming, but also emotionally and physically consuming for both patients and their families.163,164 Recent research has focused efforts in creating more rapid and convenient tests. However, even so, current diagnostic approaches often fail to differentiate between low- and high-risk forms of the disease.165 This makes them ineffective for early screening and, in some cases, may lead to overtreatment or delayed intervention. The goal has been to decrease therapy for low-risk patients to avoid long-term complications while increasing and targeting therapies for high-risk patients to improve overall survival.166 POC technologies have the potential to address these limitations. Designed to deliver diagnostic results quickly and closer to the patient, POC tools can bypass the need for centralised labs or highly specialised equipment.167 While they've gained traction in infectious disease and chronic condition monitoring, POC devices for neuroblastoma are not yet available.168,169 Still, the idea of a rapid, low-cost, and non-invasive diagnostic tool for neuroblastoma is compelling—and increasingly feasible. Such tools could help reduce diagnostic delays and limit the physical and psychological strain of the current clinical pathway. The COVID-19 pandemic accelerated interest in POC technologies by demonstrating their ability to provide rapid, decentralised testing at scale.170 Since then, advances in areas like microfluidics, biosensing, and device miniaturisation have led to new generations of portable diagnostic platforms.171 Many of these can now deliver quantitative results and are even compatible with smartphones, making them more user-friendly and accessible in non-clinical settings.172,173 With POC devices, a diagnosis can often be made in a single visit, allowing for faster treatment decisions. This is particularly beneficial in regions where access to pathology services is limited, or where time is a critical factor.174,175 Distributing POC tools through primary care providers, pharmacies, or directly to patients could expand access and reduce the overall burden on healthcare systems.176 Although there are no neuroblastoma-specific POC devices currently available, the technology landscape is diverse and growing. POC platforms now include a range of systems—from lateral flow assays (LFAs)177,178 and lab-on-a-chip (LoC) devices179,180 to electrochemical181,182 and optical biosensors,183,184 many of which are increasingly being integrated with smartphones. Each of these offers unique advantages, and their continued development could play a significant role in transforming how neuroblastoma—and other many other cancers—are diagnosed and managed in the future.3.1 Types of POC devices
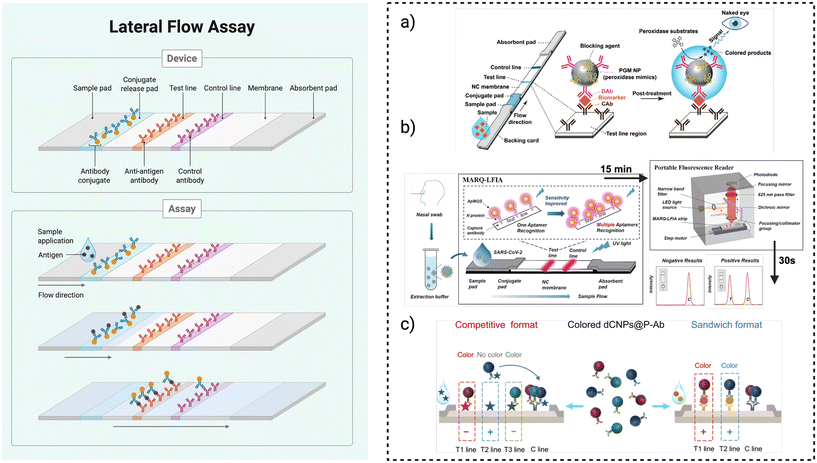 | ||
| Fig. 3 Schematic of a general LFA device and examples of application. (a) Schematic of a colorimetric LFA enhanced by platinum-group metal (PGM) nanoparticles with peroxidase-like activity. The PGM NPs are functionalised with antibodies to form detection probes that bind target analytes, enabling sensitive signal amplification at the test line via catalytic colour development after a post-treatment step. Reproduced from ref. 196 with permission from Demetrios A. Spandidos Ed. & Pub., copyright 2017. (b) MARQ-LFIA platform for SARS-CoV-2 detection. Nasal swab samples are applied to a lateral flow strip functionalised with a multiple aptamer recognition-based quantum dot fluorescent probe (ApMQS). Multiple aptamers target distinct epitopes on the viral N protein, enhancing sensitivity. A portable fluorescence reader captures test and control line intensities within 30 seconds, enabling rapid and highly sensitive point-of-care diagnosis. Reproduced from ref. 197 with permission from Royal Society of Chemistry, copyright 2025. (c) Coloured dCNPs@P-Ab used in competitive and sandwich-format multiplex lateral flow assays (mLFAs). In the competitive format (left), increasing analyte concentration reduces the test line signal, while in the sandwich format (right), signal intensity correlates with target concentration. Distinct colors enable visual differentiation of multiple targets at separate test lines. Reproduced from ref. 198 with permission from ACS Publications, copyright 2025. | ||
To improve the sensitivity and performance of LFAs, a range of new materials and technologies have been developed that enhance how targets are captured and how signals are read186 (Fig. 3a–c). Quantum dots, for example, are nano-scale fluorescent particles that can be chemically linked to several biological probes.187,188 When they bind to a specific target analyte and reach the test line, they emit a strong, stable light signal under LED or UV light, making it possible to detect even very low concentrations of the target. Magnetic nanoparticles are used earlier in the process: they are coated with molecules that bind to the target analyte and then concentrated using a magnet before the sample is applied to the strip.189–191 This “pre-enrichment” step increases the number of target molecules reaching the test line, boosting sensitivity in complex samples like blood or saliva. In addition, label-free sample enrichment methods such as dialysis with polyethylene glycol (PEG) remove interfering molecules from the sample, helping to reduce background noise and concentrate the analyte. Similarly, ion concentration polarization (ICP) uses a small electric current to trap and concentrate charged target molecules at the start of the strip before they migrate, increasing the signal strength.192,193 Lastly, modifying the shape and structure of nanomaterials—such as using star-shaped gold nanoparticles instead of spherical ones—has been shown to improve binding at the test line and produce stronger signals.194,195
 | ||
| Fig. 4 Point-of-care diagnostic devices. (a) Schematic of a lab-on-a-chip (LoC) device utilizing capillary-driven flow through microfluidic channels. Detection zones support both optical and electrochemical readouts for versatile biomarker quantification. (b) Graphene-based lab-on-a-chip (G-LOC) platform for multiplexed, non-invasive detection of kidney function biomarkers (e.g., Na+, K+, urea) in saliva. Graphene sensors are functionalised with ionophores and enzymatic interfaces, enabling real-time monitoring via a mobile electrochemical station. Reproduced from ref. 212 with permission from ACS Publications, copyright 2024. (c) Electrochemical biosensors use enzymatic reactions to induce redox or impedance changes upon target binding. These electrical changes are quantified via portable potentiostats or impedance analyzers, offering rapid and sensitive detection at the point of care. (d) Electrochemical immunosensor for the detection of α-synuclein in Parkinson's disease. The platform integrates a laser-induced graphene electrode modified with gold nanoparticles and antibodies, enabling label-free impedance-based detection with high sensitivity. Reproduced from ref. 213 with permission from Elsevier, copyright 2025. | ||
 | ||
| Fig. 5 Point-of-care diagnostic devices. (a) General schematic of an optical biosensor: a light source excites the sample at a functionalised sensing surface, where the interaction with specific analytes induces measurable changes in optical properties (e.g., absorbance, fluorescence, or refractive index). These changes are then detected by a photodetector for signal quantification. (b) Paper-based biosensor for blood urea nitrogen (BUN) detection using an integrated optical film and photodiode-based detection unit. Color intensity from enzymatic reactions is quantified digitally, enabling rapid and low-cost clinical assessment. Reproduced from ref. 229 with permission from Elsevier, copyright 2023. (c) Plasmonic glucose biosensor featuring self-assembled anisotropic gold nanoparticles on a paper substrate. Glucose-induced etching alters nanoparticle shape and color, enabling semi-quantitative visual analysis with high stability in biological fluids. Reproduced from ref. 230 with permission from Elsevier, copyright 2021. (d) Smartphone-based POC diagnostic platform using a 3D-printed chamber with integrated RGB LEDs and a smartphone camera. Fluorescent lateral flow strips can be inserted for real-time image-based quantification of nucleic acid amplification signals. Reproduced from ref. 231 with permission from Elsevier, copyright 2019. (e) Electrochemical glucose biosensor with a smartphone-connected interface. A screen-printed carbon electrode is modified with Fe3O4–poly(thiophene) nanocomposites to enable sensitive and stable glucose detection in saliva, urine, and blood. Reproduced from ref. 232 with permission from Elsevier, copyright 2022. | ||
3.2 POC technologies in cancer research
Diagnosing cancer is rarely straightforward, particularly in its early stages. Most cancers don't cause clear symptoms until the disease is already in a highly advanced state. When symptoms do appear, they're often vague or easily mistaken for more common conditions, which delays proper diagnosis.233 Once a diagnosis is suspected, patients typically go through a series of tests. These are effective but depend on skilled personnel, complex equipment, and can take days or even weeks.234 Biological variability—tumour heterogeneity—adds another layer of complexity, making it difficult to rely on a single biomarker for diagnosis or prognosis.235POC technologies offer a promising solution for these challenges (Fig. 6). They often work with easily accessible samples like blood, saliva, or urine, and can be used outside of a hospital setting.35 For patients in remote or underserved regions, this kind of access could be transformative, helping detect disease earlier and supporting more regular monitoring.176 Lateral flow assays, widely recognized for their affordability, rapidity, and ease of use, have been developed for the detection of protein biomarkers such as prostate-specific antigen (PSA), cancer antigen 125 (CA125), and carcinoembryonic antigen (CEA).236 Paper-based microfluidic devices, or μPADs, are another exciting development. These disposable, low-cost platforms can detect cancer cells or DNA using colour change or fluorescence.237 In one case, a μPAD combined with a smartphone-based reader successfully identified ROR1-positive cells in blood—bypassing the need for flow cytometry.238 More advanced technologies are also emerging. CRISPR-based diagnostics, aptamer-modified sensors, and hybrid electrochemical–optical devices are all being explored for cancer detection.239 Genetic and epigenetic markers like methylated DNA and miRNAs are gaining traction too, given their potential to offer insight into tumour subtype or treatment resistance. A recent platform was able to reach a limit of detection as low as 0.16 pM miRNA.240 Other cancer LFAs for detection of cancer biomarkers DNA methylation,74,75 single nucleotide poylmorphisms,241 and circulating tumour DNA (ctDNA) have also been developed; all of which are implicated as biomarkers for several cancers. Kalligosfyri et al.137,242,243 developed a rapid gold nanoparticle-based LFA for visual detection of KRAS gene mutations in ctDNA, relevant to colorectal cancer, using streptavidin-biotin affinity and four primer extensions at the test zone.
Commercial examples of cancer POC tests are still rare, but a few exist. A one-step PSA test has been developed, and Abbott's NMP22 BladderCheck test can detect a urinary bladder cancer marker in under 30 minutes using just a few drops of urine.244,245 However, both have faced criticism due to poor sensitivity and specificity, highlighting the need for further optimization, namely through adding washing steps to reduce background noise, Additionally, relying on a single biomarker is often not sufficient for reliable cancer detection.235 As a result, interest is growing in multiplexed lateral flow assays that can detect several targets simultaneously.246 When paired with smartphone-based readers, these multiplexed devices could offer more accurate and accessible diagnostics. LoC has been used to measure miRNAs,247,248 detect circulating tumour cells, and analyse markers such as CEA and EGFR in lung cancer.249 Compared to traditional LFAs, LoCs can handle more complex assays and often don't require amplification steps—making them ideal for molecular diagnostics. One recent study demonstrated a microfluidic chip that could detect tumour-associated miRNAs in serum without amplification, achieving high specificity and a detection limit below 40 pmol L−1.247 Wearable cancer diagnostics, however, are still in early stages, with some interesting proof of concepts developed, but with few in vivo and clinical validation. Calderón-Santiago et al. used sweat to screen for lung cancer,250 while the GILUPI CellCollector® captured circulating tumour cells (CTCs) directly from the blood.251 Lai et al.252 enhanced CTC capture using a graphene oxide nano-net, and Park et al. developed a microfluidic device to isolate CTCs based on mechanical properties, while Pekin et al. detected ctDNA mutations with a microfluidic digital PCR platform.253 Beyond initial diagnosis, POC devices hold significant potential for monitoring treatment response, predicting therapeutic efficacy, and identifying early signs of relapse.254 For instance, phosphoCDK levels could be tracked during trials involving CDK inhibitors, giving real-time insight into drug activity.255 POC devices also lend themselves to time-point sampling, allowing clinicians to track changes in biomarker levels throughout treatment. In the future, they could help predict aggressive disease, monitor resistance patterns, or even anticipate metastasis.
In the context of neuroblastoma, POC detection for the detection of VMA and HVA in urine has been achieved through the use of nickel-doped zinc oxide nanomaterials.158 Other POC immunosensors have been developed that detect GD2 (ref. 159) and dopamine release.157 As POC platforms continue to evolve and integrate with AI, 3D printing, and mobile technology, they are steadily moving toward a new standard: diagnostics that are not only fast and portable, but also personalised, predictive, and deeply embedded in everyday care. However, while advances in engineering and device miniaturisation are critical, the success of POC technologies ultimately depends on our understanding of the disease itself. In cancer, this means uncovering the molecular events that drive tumour development, progression, and resistance to treatment.256 Without reliable biomarkers—whether genetic mutations, epigenetic changes, protein expression patterns, or metabolic signatures—POC tools lack meaningful targets to measure.257 As such, progress in cancer biology, including the identification of early indicators of malignancy or predictors of therapeutic response, is essential for informing the design of clinically useful POC diagnostics. The more precisely we understand tumour heterogeneity, microenvironmental cues, and disease evolution, the more targeted and effective these tools can become.
3.3 Biomarkers for POC neuroblastoma diagnosis
POC offer the promise of rapid testing and immediate clinical decision support directly at the site of patient care. The potential for POC systems to utilise nanotechnology for biomarker detection is gaining traction within the oncological landscape, including neuroblastoma diagnosis.258 These innovations facilitate timely and accurate diagnoses, which are critical given the aggressive nature of neuroblastoma and the time-sensitive nature of paediatric oncology interventions. Only a few POC devices have been developed for neuroblastoma diagnosis, and these aim to detect catecholamine metabolites. These are still in the early stages of development and, whilst not invasive, do not differentiate between high risk and low risk disease – something which will be essential to the development of an effective screening and relapse monitoring tool. In this section, possible biomarkers for neuroblastoma are explored, and the potential to apply these to different POC platforms, and the concentrations at which these should be measured, is investigated (Table 2).| Biomarker type | Example biomarker | Sample type | Detection method | Stage (early/late) | Key features |
|---|---|---|---|---|---|
| Genetic biomarkers | MYCN amplification | Blood | FISH, PCR, cfDNA analysis | Early | Associated with high-risk NB, poor prognosis |
| ALK mutation/amplification | Blood | FISH, PCR, cfDNA analysis | Early | Promotes MYCN activity; poor survival | |
| NSE (neuron-specific enolase) | Spinal fluid, serum or urine | Blood assay | Late | Decreased survival indicator | |
| Circulating tumour DNA (ctDNA) | Blood | Next-generation sequencing (NGS), dPCR | Early and late | Detection of tumour-specific mutations; useful for monitoring and relapse prediction | |
| cfDNA | Blood | qPCR, ddPCR-method, whole genome sequencing | Early | Common alterations detected non-invasively; correlated with NB stage | |
| Ferritin | Blood | Blood assay | Early | Associated with worse prognosis | |
| LDH | Blood | Blood assay | Late | Increased metastatic burden | |
| ATRX | Tissue biopsy | IHC, genome sequencing, FISH | Late | Loss of ATRX associated with poor prognosis | |
| TERT | Tissue biopsy, blood | FISH, RT-qPCR, NGS | Late | TERT mutations linked with aggressive tumour features | |
| RNA biomarkers | TH mRNA | Blood | RT-qPCR | Early | High in advanced stages, relapse predictor |
| PHOX2B mRNA | Early | Strong prognostic marker | |||
| Circulating MiRNA | miR-124-3p | Blood | RT-qPCR | Early | Regulates PI3K/AKT, TGF-β, and p53 signalling, impacting cell proliferation, apoptosis, and metastasis |
| miR-9-3p | Early | Prognostic for high-risk NB | |||
| miR-21 | Late | Increased to enable escape from immune detection | |||
| miR-16-5p | Early | Regulates apoptosis | |||
| miR-21-5p | Late | Similar to miR-21, cancer-related, late progression | |||
| miR-125b-5p | Early | Promote tumour cell proliferation and inhibit p53-dependent apoptosis | |||
| miR-199a-3p | Early | Facilitate proliferation and migration | |||
| Circulating cells | CTCs expressing GD2, CD90, CD45, and CD235a | Blood | Blood assays | Late | Associated with metastasis, relapse |
| Metabolic biomarkers | VMA | Urine | HPLC, LC-MS/MS | Early | Classical NB markers; good sensitivity |
| HVA | Early | ||||
| 3-Methoxytyramine (3-MTS), NMN | Early | Additional urinary markers | |||
| ODC1 | Blood, saliva or urine | Western blot, ELISA, IHC | Late | Tumour progression, aggressiveness, poor prognosis | |
| HLA-G | Blood | ELISA | Late | Increased to enable escape from immune detection | |
| Polyamines | Blood, urine | HPLC, fluorescence | Early | Elevated urine levels can suggest cancer | |
| Serum cholesterol | Blood | Lipid profile, enzyme colorimetric method | Early | Elevated levels associated with poor prognosis | |
| NLR | Blood | Full blood count | Early | Elevated NLR suggests inflammation, associated with poor prognosis | |
| HALP | Blood | Blood tests | Early | Elevated HALP score associated with better prognosis | |
| Exosomal proteins | NCAM, NCL, MYH9, FN1, LTBP1 | Blood | LC-MS/MS, ELISA, Western blot | Early and late | High risk patients, role in cell migration, and metastasis |
| Proteomic markers | Vitronectin | Blood | ELISA, western blot | Early | Elevated levels associated with poor prognosis |
| Butyrycholinesterase | Blood | Fluorescent activity assay | Early | Decreased levels associated with poor prognosis | |
| Neuropeptide Y | Sweat, blood | Electrochemical detection, GMR biosensors | Early | Elevated levels linked to stress | |
| GD2 | Blood | Immunoassay | Early | Can distinguish between high and low risk disease | |
| Trk | Tissue | IHC, FISH | Early | Trk A associated with good prognosis; Trk B associated with poor prognosis |
3.3.1.1 MYCN amplification. MYCN amplification is the most significant poor prognostic marker in neuroblastoma patients.270MYCN amplification is present in around 20–30% of neuroblastoma patients, and survival in these patients is less than 50%.18,271 Low risk patients lacking MYCN amplification can never progress to high-risk disease.272,273 In high-risk patients with upregulation of MYCN, amplification is always present at diagnosis, thus, amplification of MYCN is an early factor which is perhaps driving development of aggressive phenotypes in young patients. Traditionally, fluorescent in situ hybridisation (FISH) and southern blot analysis are used to measure MYCN copy numbers in tissue samples.11 This presents with many technical difficulties, such as interference of cells and other tissue components leading to false negative results, as well as lack of reliability of results due to small DNA quantities. More recently, real-time PCR has been used to quantify MYCN copy number in ctDNA.132 Detection of MYCN amplification is thus difficult, time consuming and requires biopsy samples, however PCR-based techniques are adaptable to portable microfluidic POC devices. Currently, its measurement is only indicated once patients have been diagnosed with neuroblastoma, for the purpose of risk and treatment stratification. Early detection of MYCN amplification for screening could be achieved through the detection of MYCN protein in biofluids such as blood.274 However, there are currently no confirmed reference ranges for the concentration of the protein in healthy patients. This would be essential to know before a device to detect it could be developed. Furthermore, it is not yet known whether levels of this protein are elevated in neuroblastoma patients, and studies would be required to determine this, although it can be hypothesised that patients with elevated expression of MYCN would have higher levels of the protein within circulating tumour cells. When these cells lyse, they may release their contents into the blood stream thus leading to elevated concentrations of the protein in blood. Another issue would be that MYCN protein has a short half-life of around 30 minutes,274 and this may present difficulties in determining accurate reference ranges for the protein. One study in 2016 by Smith et al.275 described the optimisation of an ELISA test for MYCN protein measurement in cell lysates derived from patient bone marrow samples. Devices such as ELISA tests are commercially available for this; however, their use is limited to research purposes and have not been employed for routine clinical use.
3.3.1.2 ALK mutations. Anaplastic lymphoma kinase (ALK) codes for a receptor tyrosine kinase and mutations are present in around 14% of high risk forms of neuroblastoma.276 Along with PHOX2B, ALK is associated with some forms of familial neuroblastoma. ALK is important for neural development but postnatally, it has little to no expression in healthy tissues. In mouse and zebrafish models, ALK mutations have been correlated with MYCN amplification. ALK status is mainly tested for in lung cancer, using techniques such as FISH or other immunohistochemical methods, which are not suitable for POC testing.277 Combaret et al.278 used a droplet digital PCR system to test ALK status in neuroblastoma patients using peripheral blood. This showed promising results with sensitivity and specificity both around 90–100%. Despite this, the process is not suitable for POC testing but is a good alternative to test for ALK status, if there is no tumour tissue available. Developing low cost POC platforms for ALK mutation screening could facilitate early identification of disease.
3.3.1.3 5hmC. 5hmC profiling in cfDNA is a recently discovered biomarker for neuroblastoma which could distinguish high risk disease with high sensitivity and specificity. Currently, this technology requires sequencing-based analysis, however there is potential to miniaturise this, for example via nanopore sequencing or electrochemical biosensors, thus eventually enabling POC-based risk stratification.
3.3.1.4 ATRX mutations and TERT rearrangements. Like MYCN, mutations in the alpha-thalassemia/mental retardation, X-linked (ATRX) and telomerase reverse transcriptase (TERT) genes are associated with high-risk forms of neuroblastoma.279 The majority of these genetic mutations lead to telomere lengthening. ATRX and TERT mutations are more commonly found in patients older than the age of 18 months, with TERT being associated with stage 4 disease and ATRX being associated with a refractory clinical subtype – all of which are unfavourable signs of neuroblastoma. No POC testing of these genes are evident in current literature. The current method for detecting ATRX status is through biopsy or surgery, which are invasive, and so currently it is not feasible to transform this into POC.
3.3.1.5 Novel gene biomarkers. Other genes, such as H2AFX, DDX39A, BCL11A and S100A9 have been shown to be associated with high-risk neuroblastoma and unfavourable prognoses.280–283 Current research has not covered POC detection of these genes but this could be a potential avenue for research in the future.
3.3.1.6 MicroRNAs. MiRNAs are made of 20–22 non-coding nucleotide molecules and are mainly found in the introns of protein-coding genes, and can control the expression of genes, such as MYCN and TERT.284 Studies have found there to be an upregulation of several miRNAs in aggressive forms of neuroblastoma.285 MiRNA proves to be resilient in harsh pHs and resistant to RNase activity, which allows it to be an effective detection tool.286 Several POC platforms have been developed for miRNA detection, including LFAs, electrochemical sensors, and portable qPCR systems. This shows the high feasibility of developing such a device for neuroblastoma in the near future.
3.3.2.1 Neuro-specific enolase. Another biomarker that holds valuable prognostic information for neuroblastoma is neuron-specific enolase (NSE); a glycolytic protein found in neurons. NSE is elevated in patients with neuroblastoma, among other neuroendocrine tumours such as small-cell carcinomas and islet cell tumours.287 There is still a need to establish normal ranges for NSE, however it has been suggested that concentrations of this marker in serum remain between 16.95–26.71 ng mL−1 in healthy new-borns and 13.94–22.19 ng mL−1 in healthy infants.288 Such low concentrations make accurately measuring such biomarkers difficult, however given that NSE is not specific to neuroblastoma, it can be used as a prognostic predictor in patients with confirmed disease.289 NSE can be measured using assays such as enzyme-linked immunosorbent assay (ELISA). ELISA is a plate-based technique for detection and quantification of analyte measurement. While these devices are not POC and require trained professionals, they are very rapid and are widely used for the detection of disease biomarkers. NSE can be measured in biofluids including serum and pleural effusion fluid. Fan et al. developed a wireless POC testing system that involved testing NSE using microfluidic paper-based analytical devices, an electrochemical detector and smartphone.290 Comparing their results to ELISA, their device showed results within an acceptable range and has good selectivity towards NSE. Detection limits range from 1 to 500 ng mL−1 with a LOD of 10 pg mL−1, which would be suitable for NSE in neuroblastoma. Although this was developed for the detection of NSE in small cell lung cancer, this POC testing system proves to be promising for other tumours like neuroblastoma too. This suggests that POC assays, such as LFAs, are highly feasible for NSE detection. In terms of diagnosis of neuroblastoma, NSE cannot be used in isolation but could be used alongside the measurement of other biomarkers to confirm a diagnosis.
3.3.2.2 Lactate dehydrogenase. Serum lactate dehydrogenase (LDH) is also used as a tumour biomarker for several cancers since it is indicative or tissue damage and has been shown to be elevated in MYCN-amplified neuroblastoma patients.291 High LDH values have been shown to correlate with poorer prognosis in both localised and metastatic disease states.292 In metastatic patients over the age of 18 months, LDH has recently been implicated as a predictor of poor event-free and overall survival rate.293 In new-borns, normal serum total LDH concentrations lie between 160 and 540 IU L−1.294 In children, serum LDH should be around 60–170 IU L−1.294 Studies have shown LDH to increase with increased tumour burden; thus, levels of the protein remain low in early stages of the disease.15,49 Separate studies have confirmed the strength of serum LDH in prognostication.295 Therefore, serum LDH measurement could be used as a diagnostic tool for high-risk neuroblastoma, although its ability to detect disease as an early stage is unknown. LDH can be measured using protein assays in samples of blood, cerebrospinal fluid or biopsies, as well as urine which provides a much less invasive alternative which is especially important for younger patients.296 However, assaying of biomarkers takes around 1–2 hours, and this does not qualify as POC; nonetheless, it is highly feasible that cheaper and more rapid devices such as LFAs could be developed for its detection.
3.3.3.1 Plasma-derived exosome proteins. Plasma-derived exosome proteins (exo-pro) show different properties depending on if the neuroblastoma is high or low risk.299 These differences can be seen in the proteins involved in cell migration, proliferation and metastasis. Exo-pro of neuroblastoma carry vital information about the primary origins of the neuroblastoma and has potential to be both a diagnostic and prognostic biomarker. Lateral flow immunoassays (LFIA) have been developed to test for exo-pros. Oliveira-Rodriguez et al.300 successfully developed an LFIA to analyse exo-pros from patient plasma. Each test can be done within 15 minutes. This was tested on metastatic melanoma cells but can be adapted to other cancers such as neuroblastoma.
3.3.3.2 Vitronectin. Vitronectin is a glycoprotein important for cell migration. It is found in the plasma and extracellular matrix.301 Studies show higher levels of vitronectin are associated with poor neuroblastoma prognosis. The range of vitronectin in neuroblastoma patients range from 52.4 to 870 μg mL−1, with a suggested cut-off of 361 μg mL−1 as this is when patients over the age of 18 months had worse prognosis. As a new biomarker, more research is needed for the development of a POC device testing for vitronectin.
3.3.3.3 Butyrylcholinesterase. Butyrylcholinesterase (BChE) is an enzyme that is altered in could neuroblastoma patients.302 When comparing BChE activity in MYCN amplified patients, this is around 40% lower than compared to non-MCYN amplified cases. BChE be a good biomarker to track treatments as its levels have been shown to normalise during treatment. In the context of burns and systemic inflammation, a POC testing device has been utilised in hospitals to measure BChE levels using blood samples.303,304 Thus, it is likely that such a device could be validated for neuroblastoma.
3.3.3.4 Neuropeptide Y. Neuropeptide Y (NPY) is a sympathetic neurotransmitter. NPY is expressed in neuroblastoma, with elevated levels of NPY being associated with unfavourable outcomes of neuroblastoma.305 A novel sweat-detection based POC device has been developed for NPY detection in chronic anxiety orders, but the applicability of this to neuroblastoma needs to be assessed.306
3.3.3.5 HLA-g. HLA-G is overexpressed in several tumours and is advantageous for tumour cell survival and development.307 ELISA tests have been developed for the detection of HLA-G308 which is overexpressed in neuroblastoma patients. In neuroblastoma, its levels also correlate with relapse, and thus a tool to rapidly measure this marker at POC could be used regularly by patients in remission, for earlier identification of relapse and improvement in outcomes for these patients. The use of such a device for neuroblastoma diagnosis, however, has not explored but could be a potential avenue of research for the future.
3.3.4.1 Polyamines. Polyamines are small molecules that regulate cell growth and differentiation. Thus, the polyamine pathway is implicated in cancer growth and progression, and in neuroblastoma, these molecules support processes regulated by the MYC family.309 Therefore, elevated levels of polyamines in patients could be used as a poor prognostic marker in children. Polyamines can be measured directly in blood or urine through detection of their metabolites, namely spermine, spermidine and putrescine. These molecules can be assayed in blood, saliva and urine samples,310 thus development of POC devices for their quantitation should not be difficult nor invasive. In the context of colorectal cancer, Igarashi et al. developed a multisegment injection capillary electrophoresis triple quadrupole tandem mass spectrometry (MSI-CE-MS/MS).311 This was used to detect polyamines to help differentiate between colorectal cancer, benign polyps and healthy controls. They successfully screened 359 salivary samples within 6 hours. This method proved to be sensitive and selective and shows promising applications within the field of POC screening. In polyamine synthesis, ornithine decarboxylase 1 (ODC1) is a rate-limiting enzyme involved in the conversion of ornithine to putrescine. ODC1 is also one of the transcriptional targets of MYCN, and thus high expression of ODC1 is found in MYCN-amplified neuroblastoma, linking this marker to poor outcomes in children.309 Another interesting consideration is the recent development of difluoromethylornithine (DFMO) which has been developed for inhibition of ODC1.312 Thus, a tool for the detection of ODC1 could be used not only for diagnosis, but also for monitoring of treatment efficacy.
3.3.4.2 Catecholamines. Urine catecholamines, namely dopamine, epinephrine, and norepinephrine, are important liquid biomarkers for neuroblastoma since they, and their metabolites such as VMA and HVA, are elevated in patients. While studies have suggested that elevated VMA and HVA are linked to later stage disease, previous implementation of devices to screen for this were unsuccessful.23,98,313 HVA and VMA concentrations in urine should remain below 16.8 and 13.2 mmol mol−1 creatinine in infants under 6 months of age, respectively.314 Several assay methods are currently available for their detection, although liquid chromatography couples with tandem mass spectrometry (LC-MS/MS) is the gold standard for measurement of catecholamines and their metabolites.315 Detection of these analytes is one of the first-line diagnostic tests performed when a patient presents with symptoms of neuroblastoma and could be used in the future as part of multiplexed POC devices for rapid screening and diagnosis. However, catecholamines are elevated in the majority of neuroblastoma patients, and their rise is not linked to poorer outcomes, thus limiting their use in risk stratification.
3.3.4.3 Glycosylated ferritin. Neuroblastoma cells secrete abnormal forms of ferritin (glycosylated ferritin). This biomarker is a poor prognostic marker for the disease, as levels of glycosylated ferritin are elevated mainly in stage 3 and 4S disease states.316 Levels of glycosylated ferritin can be measured in the blood using assays such as ELISA which are commercially available, and thus integrating this onto POC platforms should be possible.
3.3.4.4 Serum cholesterol. Total serum cholesterol (Tchol) in neuroblastoma patients was analysed through a retrospective study.317 It found that neuroblastoma prognosis was associated with TChol and that it was significantly increased in patients with neuroblastoma that had relapsed and patients who died from neuroblastoma. The study concluded that TChol was also a risk factor for neuroblastoma as well as a prognostic biomarker. Traditionally TChol is measured through blood tests, but POC systems have been developed, but again, need to be validated in the context of neuroblastoma screening.318 Even though Tchol is not specific to neuroblastoma, it could contribute to a POC risk stratification panel combined with other, more specific biomarkers. Handheld, POC Tchol meters are widely available and can be implemented easily into a multiplexed biosensor.
3.3.4.5 Neutrophil to lymphocyte ratio. Neutrophil to lymphocyte ratio is (NLR) is a marker of systemic inflammation for solid cancers.319 It has been shown to have prognostic capabilities in other cancers, such as oesophageal and squamous cell carcinomas and could possibly predict overall survival in paediatric solid tumours. The information to work out the NLR is part of the initial work up for diagnosis, so would not require further invasive testing, so NLR could be a potential useful prognostic biomarker in the neuroblastoma space. NLR can be calculated from complete blood counts, which is already feasible in portable haematology analysers. So, integrating these already developed technologies into a rapid tool for neuroblastoma risk stratification is practical.
3.3.4.6 HALP scores. Haemoglobin, albumin, lymphocyte, and platelet (HALP) scores have been used as a prognostic marker in other solid tumours.320 It is calculated by the product of haemoglobin (g/L), albumin (g/L), lymphocyte count (per L) and platelet count (per L). Qi et al.320 conducted a retrospective study which showed a low HALP score was associated with poor prognosis. An optimal HALP cut off score was suggested at 27.0. Although invasive, as the components of the HALP score would need to be obtained through blood tests, these tests would be required anyway for a NB diagnosis, so a HALP score would be convenient to calculate.
3.3.5.1 Ganglioside GD2. Ganglioside GD2 is a glycolipid that is expressed on the membrane of NB tumours. It is shed into circulation, with its most predominant circulating form being C18 lipoform. Research has shown that ganglioside GD2 is a specific and sensitive tumour biomarker that can distinguish between high and low risk forms of NB, showing promising qualities for a diagnostic and prognostic NB biomarker.321 Balis et al. found that GD2 concentrations were significantly higher in patients with MYCN amplified tumours. Other studies corroborate this and showed that C18 and C20 forms of GD2 were higher in metastatic disease.322 Plasma concentrations of C18 and C20 were found to decrease during treatment, but increased during relapse, showing potential of GD2 monitoring treatment and disease. Current tests to detect GD2 in plasma involve high-pressure liquid chromatography/tandem mass spectrometry (HPLC/MS/MS), which can be adapted for clinical use.321
3.3.5.2 Trk family. Tyrosine kinase receptors (Trk) play an important role in the development and clinical behaviour of NB.323 The Trk family consists of 3 Trks: Trk A, B and C. High expression of Trk A is associated with a good prognosis, with tumours displaying favourable features and is seen in tumours that spontaneously regress.323,324 On the other hand, high Trk B expression is associated with features such as MYCN amplification and unfavourable features and prognosis. The role of Trk C is less well known, but current literature shows that it is associated with positive outcomes.324 Tanaka et al.325 developed a immunohistochemical method to examine Trk A in tumours in a clinical setting. They concluded that Trk A was a strong predictor of good prognosis. This study however was performed in 1995 and little research on Trks as a biomarker has been conducted in recent times.
3.4 Challenges associated with diagnosing neuroblastoma at POC
Despite the clear advantages of POC devices for diagnosis of diseases, many issues can be faced in their development. Firstly, it is unlikely that POC technologies will completely replace current diagnostic devices, owing to the fact that they could not aid in identification of tumour location and may be of limited use for staging.326 However, they can improve time taken to diagnosis and be used as a companion to current methods.9 Developing POC devices with sufficient sensitivity to match gold-standard technologies is one of the biggest concerns; this is especially true for diseases for which the analyte may be in very low concentrations.17 For many biomarkers considered in this review, they are present in small quantities in blood and urine. One major disadvantage of POC devices is that samples should not be processed before detecting the biomarker as this will increase detection time. In infants, it is difficult to collect large enough blood samples, and urine tests rely on 24 hour collection.327 Sample enrichment or pre-concentration could be important for the detection of analytes, but this would increase detection times which should be a major consideration during development.328 Such issues can be overcome through the use of POC enhancement techniques, for example the use of fluorescent labels in LFAs or ELISA tests.329 Furthermore, since assays such as LFAs often utilise antibodies as detection reagents, cross-reactivity is a major concern. This has greater implications for childhood illnesses such as neuroblastoma, as false positive or negative results may lead to stress and anxiety for the child and their family, or even unnecessary invasive measures to investigate the child.330 Thus, the use of aptamers or methods which do not involve antibodies, such as chip technologies, could be a potential future move in the neuroblastoma field. Another major challenge would be validation of the device once it has been developed. A clinical trial would need to be undertaken to test that the device can be used routinely as a diagnostic device, and to compare its abilities to current methods.331 This would require a large sample size; however, neuroblastoma is a rare disease and recruiting enough subjects would be extremely difficult. The ability to overcome these issues and develop rapid POC devices for neuroblastoma diagnosis will be essential to the future management of this disease. The use of any developed tools for mass screening is a major concern, as it can lead to unnecessary interventions in children without hereditary germline risks. Thus, future development of diagnostic tools for neuroblastoma should focus on the differential diagnosis of high-risk disease.4. Conclusion
Neuroblastoma is a clinically and biologically heterogeneous childhood malignancy, presenting mainly in babies and infants.18 The disease is characterised by significant morbidity in high-risk patients, which is often difficult to diagnose, and early screening attempts have been unsuccessful.13,332 Current diagnostic techniques for neuroblastoma rely on several biochemical, imaging, and histochemical tests. Patients often present to the hospital due to symptoms of the disease, most commonly palpable abdominal masses.333 From here, blood and urine tests showing hormone imbalances coupled with abnormal findings on CT/X-ray are enough to strongly suggest the diagnosis of neuroblastoma, although positive biopsy results are often required to confirm this.21 Thus, diagnosing neuroblastoma is extremely invasive and time-consuming, requiring several visits and causing significant stress and long-term health effects in young children. Furthermore, the cost of diagnosing neuroblastoma patients, and subsequent treatment of high-risk patients whose disease has progressed due to late diagnosis, is high. Over the past two decades, the diagnostic landscape of neuroblastoma has evolved significantly. Technological advancements have led to the discovery of several biomarkers, including genetic, proteomic and metabolic.334 Importantly, these diagnostic tools are increasingly being designed to be portable, cost-effective and rapid. Urine-based sensors for catecholamines and blood-based detection of exosomal proteins prove this.158 Furthermore, in integration of AI and machine learning algorithms holds great potential, and will allow researchers to automate interpretations, delivering rapid results without the requirement for specific equipment or training.156 The future of neuroblastoma is likely to rest on technologies that enable risk stratification at the bedside.POC diagnostic devices have gained interest over recent years as they enable rapid diagnosis of disease at the patient's location, thus enabling more rapid implementation of interventions, saving time and money associated with long waiting times. Some POC devices such as LFAs are particularly useful since they are very cheap and rapid, and do not require trained professionals.40 In the future, POC devices can be manufactured to measure high-risk disease biomarkers such as MYCN protein and polyamine metabolites in biological samples.335 For other more generic biomarkers, such as catecholamine metabolites, this may also be useful as patients can use them at home and they can be used not only as diagnostic tools, but also for patient monitoring. Given that levels of biomarkers such as polyamine metabolites and catecholamine metabolites are known to be elevated in the blood and urine, and that this is well-documented in literature, developing and training POC devices is a logical step forward. Moreover, novel POC techniques are being developed and will continue to do so. Multiplexing is an emerging field of research which enables simultaneous biomarker measurement from a single sample at POC.336 This will ensure that for childhood diseases such as neuroblastoma, the impacts of false positive or negative results are minimised given that multiple analytes will be investigated before a conclusion is reached about the child's health. Such a device could act as a first step in patient care or as a screening tool in babies, helping to eliminate late diagnosis of this devastating disease.
Data availability
All data used in this review are available in the cited literature. No new datasets were generated.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
A. K. Y. and Y. H. thank the Engineering and Physical Sciences Research Council for a New Investigator Award (EP/T013567/1). N. J. acknowledges the Fundamental Research Funds for the Central Universities (YJ202152) and the National Natural Science Foundation of China (No. 82102182). A. K. Y. thanks the CRUK Convergence Science iPhD Award (WSCA_P84261).References
- J. I. Johnsen, C. Dyberg and M. Wickström, Neuroblastoma-A Neural Crest Derived Embryonal Malignancy, Front. Mol. Neurosci., 2019, 12, 9, DOI:10.3389/fnmol.2019.00009 , From NLM.
- E. K. Barr and M. A. Applebaum, Genetic Predisposition to Neuroblastoma, Children, 2018, 5(9), 119, DOI:10.3390/children5090119 , From NLM.
- E. Ward, C. DeSantis, A. Robbins, B. Kohler and A. Jemal, Childhood and adolescent cancer statistics, 2014, Ca-Cancer J. Clin., 2014, 64(2), 83–103 CrossRef PubMed.
- J. Becker and J. Wilting, WNT Signaling in Neuroblastoma, Cancers, 2019, 11(7), 1013, DOI:10.3390/cancers11071013 , From NLM.
- K. Nolan, C. Kattamuri, D. M. Luedeke, E. B. Angerman, S. A. Rankin, M. L. Stevens, A. M. Zorn and T. B. Thompson, Structure of neuroblastoma suppressor of tumorigenicity 1 (NBL1): insights for the functional variability across bone morphogenetic protein (BMP) antagonists, J. Biol. Chem., 2015, 290(8), 4759–4771, DOI:10.1074/jbc.M114.628412 , From NLM.
- K. C. Helming, X. Wang and C. W. M. Roberts, Vulnerabilities of mutant SWI/SNF complexes in cancer, Cancer Cell, 2014, 26(3), 309–317, DOI:10.1016/j.ccr.2014.07.018 , From NLM.
- Y. P. Mossé, M. Laudenslager, L. Longo, K. A. Cole, A. Wood, E. F. Attiyeh, M. J. Laquaglia, R. Sennett, J. E. Lynch and P. Perri, et al., Identification of ALK as a major familial neuroblastoma predisposition gene, Nature, 2008, 455(7215), 930–935, DOI:10.1038/nature07261 , From NLM.
- A. G. Knudson, Jr. and L. C. Strong, Mutation and cancer: neuroblastoma and pheochromocytoma, Am. J. Hum. Genet., 1972, 24(5), 514–532 Search PubMed , From NLM.
- K. K. Matthay, J. M. Maris, G. Schleiermacher, A. Nakagawara, C. L. Mackall, L. Diller and W. A. Weiss, Neuroblastoma, Nat. Rev. Dis. Primers, 2016, 2, 16078, DOI:10.1038/nrdp.2016.78 , From NLM.
- J. M. Maris, Y. P. Mosse, J. P. Bradfield, C. Hou, S. Monni, R. H. Scott, S. Asgharzadeh, E. F. Attiyeh, S. J. Diskin and M. Laudenslager, et al., Chromosome 6p22 locus associated with clinically aggressive neuroblastoma, N. Engl. J. Med., 2008, 358(24), 2585–2593, DOI:10.1056/NEJMoa0708698 , From NLM.
- P. Mathew, M. B. Valentine, L. C. Bowman, S. T. Rowe, M. B. Nash, V. A. Valentine, S. L. Cohn, R. P. Castleberry, G. M. Brodeur and A. T. Look, Detection of MYCN gene amplification in neuroblastoma by fluorescence in situ hybridization: a pediatric oncology group study, Neoplasia, 2001, 3(2), 105–109, DOI:10.1038/sj.neo.7900146 , From NLM.
- P. A. Marks, Thioredoxin in cancer--role of histone deacetylase inhibitors, Semin. Cancer Biol., 2006, 16(6), 436–443, DOI:10.1016/j.semcancer.2006.09.005 , From NLM.
- A. Nakagawara, Y. Li, H. Izumi, K. Muramori, H. Inada and M. Nishi, Neuroblastoma, Jpn. J. Clin. Oncol., 2018, 48(3), 214–241, DOI:10.1093/jjco/hyx176 , From NLM.
- Cancer.Net, Neuroblastoma - Childhood: Statistics, 2021, https://www.cancer.net/cancer-types/neuroblastoma-childhood/statistics, (accessed 23/03/2023) Search PubMed.
- S. L. Cohn, A. D. Pearson, W. B. London, T. Monclair, P. F. Ambros, G. M. Brodeur, A. Faldum, B. Hero, T. Iehara and D. Machin, The International Neuroblastoma Risk Group (INRG) classification system: an INRG task force report, J. Clin. Oncol., 2009, 27(2), 289 CrossRef PubMed.
- Society, A. C., Neuroblastoma Survival Rates by Risk Group, 2021, https://www.cancer.org/cancer/neuroblastoma/detection-diagnosis-staging/survival-rates.html (accessed 01/12/2023) Search PubMed.
- N. Aygun, Biological and Genetic Features of Neuroblastoma and Their Clinical Importance, Curr. Pediatr. Rev., 2018, 14(2), 73–90, DOI:10.2174/1573396314666180129101627 , From NLM.
- J. M. Maris, Recent advances in neuroblastoma, N. Engl. J. Med., 2010, 362(23), 2202–2211, DOI:10.1056/NEJMra0804577 , From NLM.
- C. B. Matthias Schell, Neuroblastoma, in Orphanet, 2003 Search PubMed.
- J. Chamberlain, Screening for Neuroblastoma: A Review of the Evidencejocelyn Chamberlain, J. Med. Screen., 1994, 1(3), 169–175 CrossRef CAS PubMed.
- C. C. Swift, M. J. Eklund, J. M. Kraveka and A. L. Alazraki, Updates in Diagnosis, Management, and Treatment of Neuroblastoma, Radiographics, 2018, 38(2), 566–580, DOI:10.1148/rg.2018170132 , From NLM.
- M. A. Musarella, H. S. Chan, G. DeBoer and B. L. Gallie, Ocular involvement in neuroblastoma: prognostic implications, Ophthalmology, 1984, 91(8), 936–940 CrossRef CAS PubMed.
- F. Berthold, D. Hunneman, D. Harms, H. Käser and J. Zieschang, Serum vanillylmandelic acid/homovanillic acid contributes to prognosis estimation in patients with localised but not with metastatic neuroblastoma, Eur. J. Cancer, 1992, 28(12), 1950–1954 CrossRef PubMed.
- K. Yamamoto, Y. Hayashi, R. Hanada, A. Kikuchi, M. Ichikawa, M. Tanimura and S. Yoshioka, Mass screening and age-specific incidence of neuroblastoma in Saitama Prefecture, Japan, J. Clin. Oncol., 1995, 13(8), 2033–2038 CrossRef CAS PubMed.
- Y. Kaneko, N. Kanda, N. Maseki, K. Nakachi, T. Takeda, I. Okabe and M. Sakurai, Current urinary mass screening for catecholamine metabolites at 6 months of age may be detecting only a small portion of high-risk neuroblastomas: a chromosome and N-myc amplification study, J. Clin. Oncol., 1990, 8(12), 2005–2013 CrossRef CAS PubMed.
- W. G. Woods, R.-N. Gao, J. J. Shuster, L. L. Robison, M. Bernstein, S. Weitzman, G. Bunin, I. Levy, J. Brossard and G. Dougherty, Screening of infants and mortality due to neuroblastoma, N. Engl. J. Med., 2002, 346(14), 1041–1046 CrossRef PubMed.
- F. H. Schilling, C. Spix, F. Berthold, R. Erttmann, N. Fehse, B. Hero, G. Klein, J. Sander, K. Schwarz and J. Treuner, Neuroblastoma screening at one year of age, N. Engl. J. Med., 2002, 346(14), 1047–1053 CrossRef PubMed.
- D. Fénart, A. Deville, M. Donzeau and J. Bruneton, Retroperitoneal neuroblastoma diagnosed in utero. Apropos of 1 case, J. Radiol., 1983, 64(5), 359–361 Search PubMed.
- L. Helson, Neuroblastoma: early diagnosis a key to successful treatment, SLACK Incorporated Thorofare, NJ, 1974, vol. 3, pp. 46–54 Search PubMed.
- Future, M. R., Global Neuroblastoma Research Report, 2021, https://www.marketresearchfuture.com/reports/neuroblastoma-market-6555#description (accessed) Search PubMed.
- Watch, M., Neuroblastoma Treatment Market Trends 2021, 2021, https://www.marketwatch.com/press-release/neuroblastoma-treatment-market-trends-2021-global-industry-share-size-industry-analysis-market-growth-analysis-segments-emerging-technologies-opportunity-and-forecast-2021-to-2027-2021-11-11 (accessed) Search PubMed.
- C. Yue, Q. Zhang, F. Sun and Q. Pan, Global, regional and national burden of neuroblastoma and other peripheral nervous system tumors, 1990 to 2021 and predictions to 2035: visualizing epidemiological characteristics based on GBD 2021, Neoplasia, 2025, 60, 101122, DOI:10.1016/j.neo.2025.101122.
- Y. Hayashi, R. Hanada and K. Yamamoto, Biology of neuroblastomas in Japan found by screening, Am. J. Pediatr. Hematol./Oncol., 1992, 14(4), 342–347 CrossRef CAS , From NLM.
- J. L. Weinstein, H. M. Katzenstein and S. L. Cohn, Advances in the diagnosis and treatment of neuroblastoma, Oncologist, 2003, 8(3), 278–292, DOI:10.1634/theoncologist.8-3-278 , From NLM.
- C. P. Price, Point of care testing, BMJ, 2001, 322(7297), 1285–1288, DOI:10.1136/bmj.322.7297.1285 , From NLM.
- B. Hayes, C. Murphy, A. Crawley and R. O'Kennedy, Developments in Point-of-Care Diagnostic Technology for Cancer Detection, Diagnostics, 2018, 8(2), 39, DOI:10.3390/diagnostics8020039 , From NLM.
- M. Rodrigues, I. Andrade and R. Cruz, Current Point-of-Care testing in cancer and future perspectives: a systematic review, Eur. J. Public Health, 2020, 30(Supplement_2), ckaa040.033 CrossRef.
- D. Crosby, S. Bhatia, K. M. Brindle, L. M. Coussens, C. Dive, M. Emberton, S. Esener, R. C. Fitzgerald, S. S. Gambhir and P. Kuhn, et al., Early detection of cancer, Science, 2022, 375(6586), eaay9040, DOI:10.1126/science.aay9040 , From NLM.
- L. Syedmoradi, M. L. Norton and K. Omidfar, Point-of-care cancer diagnostic devices: From academic research to clinical translation, Talanta, 2021, 225, 122002, DOI:10.1016/j.talanta.2020.122002 , From NLM.
- K. M. Koczula and A. Gallotta, Lateral flow assays, Essays Biochem., 2016, 60(1), 111–120 CrossRef PubMed.
- T. Mahmoudi, M. de la Guardia and B. Baradaran, Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends, TrAC, Trends Anal. Chem., 2020, 125, 115842 CrossRef CAS.
- H. N. Lee, J. Lee, Y. K. Kang, J. H. Lee, S. Yang and H. J. Chung, A lateral flow assay for nucleic acid detection based on rolling circle amplification using capture ligand-modified oligonucleotides, BioChip J., 2022, 16(4), 441–450 CrossRef CAS PubMed.
- C. Özyurt, İ. Uludağ, B. İnce and M. K. Sezgintürk, Lab-on-a-chip systems for cancer biomarker diagnosis, J. Pharm. Biomed. Anal., 2023, 226, 115266 CrossRef PubMed.
- N. C. Colon and D. H. Chung, Neuroblastoma, Adv. Pediatr., 2011, 58(1), 297–311, DOI:10.1016/j.yapd.2011.03.011 , From NLM.
- K. Kuchalska, M. Barełkowska, K. Derwich, K. Jończyk-Potoczna and A. Gotz-Więckowska, Incidence of Horner syndrome associated with neuroblastic disease, Childs Nerv. Syst., 2021, 37(4), 1243–1247 CrossRef.
- J. W. Rudolf and M. Thapa, Thoracic neuroblastoma, Radiol. Case Rep., 2011, 6(2), 440, DOI:10.2484/rcr.v6i2.440 , From NLM.
- K. Połczyńska, E. Bień, J. Stefanowicz, E. Drozyńska, A. Szołkiewicz, T. Stachowicz-Stencel, D. Sierota, B. Kaczorowska-Hać, W. Kosiak and A. Balcerska, Neurologic symptoms in the course of neuroblastoma in children. Own observations, Med. Wieku Rozwoj., 2005, 9(3 Pt 2), 477–486 Search PubMed , From NLM.
- S. G. DuBois, Y. Kalika, J. N. Lukens, G. M. Brodeur, R. C. Seeger, J. B. Atkinson, G. M. Haase, C. T. Black, C. Perez and H. Shimada, Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival, J. Pediatr. Hematol./Oncol., 1999, 21(3), 181–189 CrossRef CAS PubMed.
- D. A. Morgenstern, W. B. London, D. Stephens, S. L. Volchenboum, T. Simon, A. Nakagawara, H. Shimada, G. Schleiermacher, K. K. Matthay and S. L. Cohn, Prognostic significance of pattern and burden of metastatic disease in patients with stage 4 neuroblastoma: A study from the International Neuroblastoma Risk Group database, Eur. J. Cancer, 2016, 65, 1–10 CrossRef PubMed.
- A. F. Belgaumi, W. M. Kauffman, J. J. Jenkins, J. Cordoba, L. C. Bowman, V. M. Santana and W. L. Furman, Blindness in children with neuroblastoma, Cancer, 1997, 80(10), 1997–2004 CrossRef CAS PubMed , From NLM.
- B. Hero and G. Schleiermacher, Update on pediatric opsoclonus myoclonus syndrome, Neuropediatrics, 2013, 44(06), 324–329 CrossRef PubMed.
- E. Bluhm, D. E. McNeil, S. Cnattingius, G. Gridley, L. El Ghormli and J. F. Fraumeni, Jr., Prenatal and perinatal risk factors for neuroblastoma, Int. J. Cancer, 2008, 123(12), 2885–2890, DOI:10.1002/ijc.23847 , From NLM.
- S. Narod, C. Stiller and G. Lenoir, An estimate of the heritable fraction of childhood cancer, Br. J. Cancer, 1991, 63(6), 993–999 CrossRef CAS PubMed.
- M. Hashemi, S. Sarabandi, S. Karami, J. Śmieja, A. Moazeni-Roodi, S. Ghavami and M. J. Łos, LMO1 polymorphisms and the risk of neuroblastoma: Assessment of meta-analysis of case-control studies, J. Cell. Mol. Med., 2020, 24(2), 1160–1168, DOI:10.1111/jcmm.14836 , From NLM.
- M. N. Cook, A. F. Olshan, H. A. Guess, D. A. Savitz, C. Poole, J. Blatt, M. L. Bondy and B. H. Pollock, Maternal medication use and neuroblastoma in offspring, Am. J. Epidemiol., 2004, 159(8), 721–731 CrossRef PubMed.
- S. Kramer, E. Ward, A. T. Meadows and K. Malone, Medical and drug risk factors associated with neuroblastoma: a case-control study, J. Natl. Cancer Inst., 1987, 78(5), 797–804 CAS.
- J. Schüz, U. Kaletsch, R. Meinert, P. Kaatsch, C. Spix and J. Michaelis, Risk factors for neuroblastoma at different stages of disease. Results from a population-based case-control study in Germany, J. Clin. Epidemiol., 2001, 54(7), 702–709 CrossRef PubMed.
- J. Schüz, T. Weihkopf and P. Kaatsch, Medication use during pregnancy and the risk of childhood cancer in the offspring, Eur. J. Pediatr., 2007, 166(5), 433–441 CrossRef PubMed.
- M. R. Spitz and C. C. Johnson, Neuroblastoma and Paternal Occupation A Case-Control Analysis, Am. J. Epidemiol., 1985, 121(6), 924–929 CrossRef CAS PubMed.
- A. J. De Roos, A. F. Olshan, K. Teschke, C. Poole, D. A. Savitz, J. Blatt, M. L. Bondy and B. H. Pollock, Parental occupational exposures to chemicals and incidence of neuroblastoma in offspring, Am. J. Epidemiol., 2001, 154(2), 106–114 CrossRef CAS PubMed.
- S. E. Hamrick, A. F. Olshan, J. P. Neglia and B. H. Pollock, Association of pregnancy history and birth characteristics with neuroblastoma: a report from the Children's Cancer Group and the Pediatric Oncology Group, Paediatr. Perinat. Epidemiol., 2001, 15(4), 328–337 CrossRef CAS PubMed.
- K. Y. Urayama, J. Von Behren and P. Reynolds, Birth characteristics and risk of neuroblastoma in young children, Am. J. Epidemiol., 2007, 165(5), 486–495 CrossRef PubMed.
- E. J. Chow, D. L. Friedman and B. A. Mueller, Maternal and perinatal characteristics in relation to neuroblastoma, Cancer, 2007, 109(5), 983–992 CrossRef PubMed.
- C. C. Johnson and M. R. Spitz, Neuroblastoma: case-control analysis of birth characteristics, J. Natl. Cancer Inst., 1985, 74(4), 789–792 CAS.
- F. Menegaux, A. F. Olshan, P. J. Reitnauer, J. Blatt and S. L. Cohn, Positive association between congenital anomalies and risk of neuroblastoma, Pediatr. Blood Cancer, 2005, 45(5), 649–655 CrossRef PubMed.
- J. P. Neglia, W. A. Smithson, P. Gunderson, F. L. King, L. J. Singher and L. L. Robison, Prenatal and perinatal risk factors for neuroblastoma. A case-control study, Cancer, 1988, 61(11), 2202–2206 CrossRef CAS PubMed.
- M. Dumba, N. Jawad and K. McHugh, Neuroblastoma and nephroblastoma: a radiological review, Cancer Imaging, 2015, 15(1), 1–14 CrossRef PubMed.
- B. S. Shamsian, M. Kajizadi, N. Rezaei, N. Ghojehvand, R. Azma, M. Rouzrokh, M. Kazemi Aghdam, S. M. Mesbah, F. Ghazizadeh and M. T. Arzanian, Primary intrarenal neuroblastoma with hypertension and disseminated intravascular coagulation, Case Rep. Oncol. Med., 2013, 2013, 684939, DOI:10.1155/2013/684939 , From NLM.
- M. Fawzy, M. El-Beltagy, M. E. Shafei, M. S. Zaghloul, N. A. Kinaai, A. Refaat and S. Azmy, Intraspinal neuroblastoma: Treatment options and neurological outcome of spinal cord compression, Oncol. Lett., 2015, 9(2), 907–911, DOI:10.3892/ol.2014.2795 , From NLM.
- D. A. Morgenstern, W. B. London, D. Stephens, S. L. Volchenboum, B. Hero, A. Di Cataldo, A. Nakagawara, H. Shimada, P. F. Ambros and K. K. Matthay, et al., Metastatic neuroblastoma confined to distant lymph nodes (stage 4N) predicts outcome in patients with stage 4 disease: A study from the International Neuroblastoma Risk Group Database, J. Clin. Oncol., 2014, 32(12), 1228–1235, DOI:10.1200/jco.2013.53.6342 , From NLM.
- R. T. Peaston and C. Weinkove, Measurement of catecholamines and their metabolites, Ann. Clin. Biochem., 2004, 41(Pt 1), 17–38, DOI:10.1258/000456304322664663 , From NLM.
- N. J. Lacayo, Pediatric Neuroblastoma Workup, 2021, https://emedicine.medscape.com/article/988284-workup (accessed) Search PubMed.
- A. E. Evans and K. Hummeler, The significance of primitive cells in marrow aspirates of children with neuroblastoma, Cancer, 1973, 32(4), 906–912, DOI:10.1002/1097-0142(197310)32:4<906::aid-cncr2820320423>3.0.co;2-6 , From NLM.
- V. L. Reid, A. Mullan and L.-P. Erwig, Rapid recovery of membrane cofactor protein (MCP; CD46) associated atypical haemolytic uraemic syndrome with plasma exchange, Case Rep., 2013, 2013, bcr2013200980 Search PubMed.
- S. Nishikawa, H. Noguchi, T. Tokumitsu, A. Ohno, S. Moriguchi-Goto, K. Maekawa, Y. Asada, H. Moritake, M. Kinoshita and A. Yamada, Diagnosis of pediatric neuroblastoma by urine cytology: a case report, Diagn. Cytopathol., 2018, 46(3), 280–283 CrossRef PubMed.
- J. H. H. Alsaadi and S. Y. Yousif, Study of influence Chemotherapy on Sii, ESR and Liver Functions Tests in Children with Neuroblastoma in Southern Iraq, Lat. Am. J. Pharm., 2024, 43(2), 466–471 CAS.
- C. Wang, Y. Zhang, F. Wang, W. Lu, J. Wen, L. Zhao, X. Feng and J. Li, A case of recurrent abnormal liver function in children resulting from neuroblastoma amplified sequence gene defect, Zhonghua Ganzangbing Zazhi, 2020, 28(1), 80–82 CAS.
- M. B. McCarville, Imaging neuroblastoma: what the radiologist needs to know, Cancer Imaging, 2011, 11 Spec No A(1a), S44–S47, DOI:10.1102/1470-7330.2011.9008 , From NLM.
- G. Papaioannou and K. McHugh, Neuroblastoma in childhood: review and radiological findings, Cancer Imaging, 2005, 5(1), 116 CrossRef PubMed.
- J. Youngblood, Neuroblastoma: Sonography's Major Role in Its Diagnosis and Treatment, J. Diagn. Med. Sonogr., 2012, 28(2), 58–65 CrossRef.
- S. Acharya, S. Jayabose, S. J. Kogan, O. Tugal, D. Beneck, D. Leslie and M. Slim, Prenatally diagnosed neuroblastoma, Cancer, 1997, 80(2), 304–310 CrossRef CAS PubMed.
- T. Monclair, G. M. Brodeur, P. F. Ambros, H. J. Brisse, G. Cecchetto, K. Holmes, M. Kaneko, W. B. London, K. K. Matthay and J. G. Nuchtern, The international neuroblastoma risk group (INRG) staging system: an INRG task force report, J. Clin. Oncol., 2009, 27(2), 298 CrossRef PubMed.
- M. P. Hiorns and C. M. Owens, Radiology of neuroblastoma in children, Eur. Radiol., 2001, 11(10), 2071–2081, DOI:10.1007/s003300100931 , From NLM.
- K. Mehta, J. O. Haller and A. C. Legasto, Imaging neuroblastoma in children, Crit. Rev. Comput. Tomogr., 2003, 44(1), 47–61 CrossRef PubMed , From NLM.
- F. Sauvat, S. Sarnacki, H. Brisse, J. Medioni, H. Rubie, Y. Aigrain, F. Gauthier, G. Audry, P. Helardot and P. Landais, et al., Outcome of suprarenal localized masses diagnosed during the perinatal period: a retrospective multicenter study, Cancer, 2002, 94(9), 2474–2480, DOI:10.1002/cncr.10502 , From NLM.
- J. S. Meyer, M. P. Harty and Z. Khademian, Imaging of neuroblastoma and Wilms' tumor, Magn. Reson. Imaging Clin. N. Am., 2002, 10(2), 275–302, DOI:10.1016/s1064-9689(01)00010-1 , From NLM.
- I. Ilias, A. Sahdev, R. H. Reznek, A. B. Grossman and K. Pacak, The optimal imaging of adrenal tumours: a comparison of different methods, Endocr.-Relat. Cancer, 2007, 14(3), 587–599 Search PubMed.
- B. H. Kushner, Neuroblastoma: A Disease Requiring a Multitude of Imaging Studies*, J. Nucl. Med., 2004, 45(7), 1172–1188 Search PubMed.
- M. Rogosnitzky and S. Branch, Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms, BioMetals, 2016, 29(3), 365–376, DOI:10.1007/s10534-016-9931-7 , From NLM.
- B. Decarolis, C. Schneider, B. Hero, T. Simon, R. Volland, F. Roels, M. Dietlein, F. Berthold and M. Schmidt, Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison study, J. Clin. Oncol., 2013, 31(7), 944–951 CrossRef CAS PubMed.
- V. S. Dhull, P. Sharma, C. Patel, P. Kundu, S. Agarwala, S. Bakhshi, V. Bhatnagar, C. Bal and R. Kumar, Diagnostic value of 18F-FDG PET/CT in paediatric neuroblastoma: comparison with: 131: I-MIBG scintigraphy, Nucl. Med. Commun., 2015, 36(10), 1007–1013 CrossRef PubMed.
- D. D. Stark, A. A. Moss, R. C. Brasch, A. A. deLorimier, A. R. Albin, D. A. London and C. A. Gooding, Neuroblastoma: diagnostic imaging and staging, Radiology, 1983, 148(1), 101–105, DOI:10.1148/radiology.148.1.6856817 , From NLM.
- H. K. Hussain, J. E. Kingston, P. Domizio, A. J. Norton and R. H. Reznek, Imaging-guided core biopsy for the diagnosis of malignant tumors in pediatric patients, Am. J. Roentgenol., 2001, 176(1), 43–47 CrossRef CAS PubMed.
- T. Metz, A. Heider, R. Vellody, M. D. Jarboe, J. J. Gemmete, J. J. Grove, E. A. Smith, R. Mody, E. A. Newman and J. R. Dillman, Image-guided percutaneous core needle biopsy of soft-tissue masses in the pediatric population, Pediatr. Radiol., 2016, 46(8), 1173–1178 CrossRef PubMed.
- M. Sklair-Levy, P. D. Lebensart, Y. H. Applbaum, N. Ramu, A. Freeman, D. Gozal, E. Gross, Y. Sherman, J. Bar-Ziv and E. Libson, Percutaneous image-guided needle biopsy in children–summary of our experience with 57 children, Pediatr. Radiol., 2001, 31(10), 732–736 CrossRef CAS PubMed.
- H. Wang, F. Li, J. Liu and S. Zhang, Ultrasound-guided core needle biopsy in diagnosis of abdominal and pelvic neoplasm in pediatric patients, Pediatr. Surg. Int., 2014, 30(1), 31–37 CrossRef PubMed.
- P. Rastogi, S. Naseem, N. Varma, R. Das, J. Ahluwalia, M. U. S. Sachdeva, P. Sharma, N. Kumar and R. K. Marwaha, Bone marrow involvement in neuroblastoma: a study of hemato-morphological features, Indian J. Hematol. Blood Transfus., 2015, 31(1), 57–60 CrossRef PubMed.
- I. R. Verly, A. B. van Kuilenburg, N. G. Abeling, S. M. Goorden, M. Fiocco, F. M. Vaz, M. M. van Noesel, C. M. Zwaan, G. L. Kaspers and J. H. Merks, et al., Catecholamines profiles at diagnosis: Increased diagnostic sensitivity and correlation with biological and clinical features in neuroblastoma patients, Eur. J. Cancer, 2017, 72, 235–243, DOI:10.1016/j.ejca.2016.12.002 , From NLM.
- M. Dumba, N. Jawad and K. McHugh, Neuroblastoma and nephroblastoma: a radiological review, Cancer Imaging, 2015, 15(1), 5, DOI:10.1186/s40644-015-0040-6 , From NLM.
- M. Asim, G. Mudassir, A. A. Hashmi, M. Abid, A. K. Sheikh, H. Naveed, M. Habib, M. M. Edhi and A. Khan, Diagnostic accuracy of fine needle aspiration biopsy in pediatric small round cell tumors, BMC Res. Notes, 2018, 11(1), 1–5 CrossRef PubMed.
- E. Bombardieri, F. Giammarile, C. Aktolun, R. P. Baum, A. Bischof Delaloye, L. Maffioli, R. Moncayo, L. Mortelmans, G. Pepe and S. N. Reske, 131 I/123 I-Metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imaging, Eur. J. Nucl. Med. Mol. Imaging, 2010, 37, 2436–2446 CrossRef PubMed.
- G. Bleeker, G. A. Tytgat, J. A. Adam, H. N. Caron, L. C. Kremer, L. Hooft and E. C. van Dalen, 123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma, Cochrane Database Syst. Rev., 2015, 2015(9), Cd009263, DOI:10.1002/14651858.CD009263.pub2 , From NLM.
- W. P. Fendler, H. I. Melzer, C. Walz, D. von Schweinitz, E. Coppenrath, I. Schmid, P. Bartenstein and T. Pfluger, High 123I-MIBG uptake in neuroblastic tumours indicates unfavourable histopathology, Eur. J. Nucl. Med. Mol. Imaging, 2013, 40(11), 1701–1710, DOI:10.1007/s00259-013-2491-y , From NLM.
- S. Biasotti, A. Garaventa, G. P. Villavecchia, M. Cabria, M. Nantron and B. De Bernardi, False-negative metaiodobenzylguanidine scintigraphy at diagnosis of neuroblastoma, Med. Pediatr. Oncol., 2000, 35(2), 153–155, DOI:10.1002/1096-911x(200008)35:2<153::aid-mpo18>3.0.co;2-7 , From NLM.
- Z. Bar-Sever, L. Biassoni, B. Shulkin, G. Kong, M. S. Hofman, E. Lopci, I. Manea, J. Koziorowski, R. Castellani and A. Boubaker, et al., Guidelines on nuclear medicine imaging in neuroblastoma, Eur. J. Nucl. Med. Mol. Imaging, 2018, 45(11), 2009–2024, DOI:10.1007/s00259-018-4070-8.
- S. E. Sharp, A. T. Trout, B. D. Weiss and M. J. Gelfand, MIBG in neuroblastoma diagnostic imaging and therapy, Radiographics, 2016, 36(1), 258–278 CrossRef PubMed.
- N. Pandit-Taskar, P. Zanzonico, K. D. Staton, J. A. Carrasquillo, D. Reidy-Lagunes, S. Lyashchenko, E. Burnazi, H. Zhang, J. S. Lewis and R. Blasberg, Biodistribution and dosimetry of 18F-meta-fluorobenzylguanidine: a first-in-human PET/CT imaging study of patients with neuroendocrine malignancies, J. Nucl. Med., 2018, 59(1), 147–153 CrossRef CAS PubMed.
- C. Beijst, B. de Keizer, M. G. Lam, G. O. Janssens, G. A. Tytgat and H. W. de Jong, A phantom study: Should 124I-mIBG PET/CT replace 123I-mIBG SPECT/CT?, Med. Phys., 2017, 44(5), 1624–1631 CrossRef CAS PubMed.
- H. I. Melzer, E. Coppenrath, I. Schmid, M. H. Albert, D. von Schweinitz, C. Tudball, P. Bartenstein and T. Pfluger, 123 I-MIBG scintigraphy/SPECT versus 18 F-FDG PET in paediatric neuroblastoma, Eur. J. Nucl. Med. Mol. Imaging, 2011, 38, 1648–1658 CrossRef PubMed.
- G. M. Kramer, V. Frings, N. Hoetjes, O. S. Hoekstra, E. F. Smit, A. J. de Langen and R. Boellaard, Repeatability of quantitative whole-body 18F-FDG PET/CT uptake measures as function of uptake interval and lesion selection in non–small cell lung cancer patients, J. Nucl. Med., 2016, 57(9), 1343–1349 CrossRef CAS PubMed.
- L. K. Shankar, Standardization of PET imaging in clinical trials, PET Clin., 2008, 3(1), 1–4 CrossRef PubMed.
- N. D. Papathanasiou, M. N. Gaze, K. Sullivan, M. Aldridge, W. Waddington, A. Almuhaideb and J. B. Bomanji, 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: diagnostic comparison and survival analysis, J. Nucl. Med., 2011, 52(4), 519–525 CrossRef CAS PubMed.
- J. E. Gains, M. D. Aldridge, M. V. Mattoli, J. B. Bomanji, L. Biassoni, A. Shankar and M. N. Gaze, 68Ga-DOTATATE and 123I-mIBG as imaging biomarkers of disease localisation in metastatic neuroblastoma: implications for molecular radiotherapy, Nucl. Med. Commun., 2020, 41(11), 1169–1177 CrossRef CAS PubMed.
- J. E. Gains, J. B. Bomanji, N. L. Fersht, T. Sullivan, D. D'Souza, K. P. Sullivan, M. Aldridge, W. Waddington and M. N. Gaze, 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma, J. Nucl. Med., 2011, 52(7), 1041–1047 CrossRef PubMed.
- M. E. Mayerhoefer, A. Materka, G. Langs, I. Häggström, P. Szczypiński, P. Gibbs and G. Cook, Introduction to radiomics, J. Nucl. Med., 2020, 61(4), 488–495 CrossRef CAS PubMed.
- H. Wu, C. Wu, H. Zheng, L. Wang, W. Guan, S. Duan and D. Wang, Radiogenomics of neuroblastoma in pediatric patients: CT-based radiomics signature in predicting MYCN amplification, Eur. Radiol., 2021, 31(5), 3080–3089, DOI:10.1007/s00330-020-07246-1 , From NLM.
- X. Chen, H. Wang, K. Huang, H. Liu, H. Ding, L. Zhang, T. Zhang, W. Yu and L. He, CT-based radiomics signature with machine learning predicts MYCN amplification in pediatric abdominal neuroblastoma, Front. Oncol., 2021, 11, 687884 CrossRef.
- S. Ghosh, T. Gupta, N. Rastogi, A. Gaur, A. Misra, S. N. Tripathi, D. P. Paul, V. Tare, O. Prakash and D. Bhattu, Chemical characterization of summertime dust events at Kanpur: insight into the sources and level of mixing with anthropogenic emissions, Aerosol Air Qual. Res., 2014, 14(3), 879–891 CrossRef CAS.
- L.-D. Qian, S.-X. Zhang, S.-Q. Li, L.-J. Feng, Z.-A. Zhou, J. Liu, M.-Y. Zhang and J.-G. Yang, Predicting MYCN amplification in paediatric neuroblastoma: development and validation of a 18F-FDG PET/CT-based radiomics signature, Insights Imaging, 2023, 14(1), 205 CrossRef PubMed.
- G. Furlanetto, F. Spagnol, A. P. Alegretti, M. G. Farias, V. J. Soares, L. E. Daudt, J. F. Loss, M. L. Scroferneker and M. B. Michalowski, Flow cytometry as a diagnostic tool in neuroblastoma, J. Immunol. Methods, 2021, 498, 113135, DOI:10.1016/j.jim.2021.113135 , From NLM.
- N. Jain, S. Sattar, S. Inglott, S. Burchill, J. Fisher, A. M. Serban, R. Thomas, C. Connor, N. Ghara and T. Chowdhury, et al., Flow cytometry of bone marrow aspirates from neuroblastoma patients is a highly sensitive technique for quantification of low-level neuroblastoma, F1000Research, 2021, 10, 947, DOI:10.12688/f1000research.53133.2 , From NLM.
- J. Y. Cai, Y. J. Tang, L. M. Jiang, C. Pan, J. Chen and J. Y. Tang, Prognostic influence of minimal residual disease detected by flow cytometry and peripheral blood stem cell transplantation by CD34+ selection in childhood advanced neuroblastoma, Pediatr. Blood Cancer, 2007, 49(7), 952–957, DOI:10.1002/pbc.21253 , From NLM.
- W. Ye, J. Wang, W. Li and H. Shen, Comparative Analysis of Flow Cytometry and Cytomorphology for Neuroblastoma Cell Detection in Effusion and Bone Marrow Specimens, Fetal Pediatr. Pathol., 2019, 38(1), 1–7, DOI:10.1080/15513815.2018.1494231 , From NLM.
- A. B. Shrirao, Z. Fritz, E. M. Novik, G. M. Yarmush, R. S. Schloss, J. D. Zahn and M. L. Yarmush, Microfluidic flow cytometry: The role of microfabrication methodologies, performance and functional specification, Technology, 2018, 6(1), 1–23, DOI:10.1142/s2339547818300019 , From NLM.
- Accelix, 2025, https://www.accellix.com/ (accessed).
- J. Hu, B. Xia, X. Yuan, H. Chen, F. Ou, L. Huang, L. Xu and X. Feng, Neuroblastoma with superficial soft tissue mass as the first symptom: case reports with atypical ultrasonic image and literature review, Braz. J. Med. Biol. Res., 2023, 56, e12975 CrossRef PubMed.
- C. B. Haley and D. Mackenzie, Cat got your artery? Point of care ultrasound in the evaluation of penetrating trauma by a feline: a case report, Clin. Exp. Emerg. Med., 2022, 9(1), 63 CrossRef PubMed.
- R. S. Jamjoom, Y. Etoom, T. Solano, M.-P. Desjardins and J. W. Fischer, Emergency point-of-care ultrasound detection of cancer in the pediatric emergency department, Pediatr. Emerg. Care, 2015, 31(8), 602–604 CrossRef PubMed.
- V. A. Dinh, W. S. Dukes, J. Prigge and M. Avila, Ultrasound integration in undergraduate medical education: comparison of ultrasound proficiency between trained and untrained medical students, J. Ultrasound Med., 2015, 34(10), 1819–1824 CrossRef PubMed.
- M. Lodrini, J. Graef, T. M. Thole-Kliesch, K. Astrahantseff, A. Sprüssel, M. Grimaldi, C. Peitz, R. B. Linke, J. F. Hollander and E. Lankes, Targeted analysis of cell-free circulating tumor DNA is suitable for early relapse and actionable target detection in patients with neuroblastoma, Clin. Cancer Res., 2022, 28(9), 1809–1820 CrossRef PubMed.
- Y. Su, L. Wang, C. Jiang, Z. Yue, H. Fan, H. Hong, C. Duan, M. Jin, D. Zhang and L. Qiu, Increased plasma concentration of cell-free DNA precedes disease recurrence in children with high-risk neuroblastoma, BMC Cancer, 2020, 20, 1–7 CrossRef PubMed.
- M. Kojima, E. Hiyama, I. Fukuba, E. Yamaoka, Y. Ueda, Y. Onitake, S. Kurihara and T. Sueda, Detection of MYCN amplification using blood plasma: noninvasive therapy evaluation and prediction of prognosis in neuroblastoma, Pediatr. Surg. Int., 2013, 29(11), 1139–1145, DOI:10.1007/s00383-013-3374-9 , From NLM.
- M. Morini, D. Cangelosi, D. Segalerba, D. Marimpietri, F. Raggi, A. Castellano, D. Fruci, J. Font de Mora, A. Cañete and Y. Yáñez, Exosomal microRNAs from longitudinal liquid biopsies for the prediction of response to induction chemotherapy in high-risk neuroblastoma patients: a proof of concept SIOPEN study‖, Cancers, 2019, 11(10), 1476 CrossRef CAS PubMed.
- K. Klega, A. Imamovic-Tuco, G. Ha, A. N. Clapp, S. Meyer, A. Ward, C. Clinton, A. Nag, E. Van Allen and E. Mullen, Detection of somatic structural variants enables quantification and characterization of circulating tumor DNA in children with solid tumors, CO Precis. Oncol., 2018, 2, 1–13 CrossRef PubMed.
- M. Chennakesavalu, K. Moore, G. Chaves, S. Veeravalli, R. TerHaar, T. Wu, R. Lyu, A. Chlenski, C. He and A. Piunti, 5-hydroxymethylcytosine profiling of cell-free DNA identifies bivalent genes that are prognostic of survival in high-risk neuroblastoma, CO Precis. Oncol., 2024, 8, e2300297 CrossRef PubMed.
- I. H. Mahardika, S. Naorungroj, W. Khamcharoen, S. Kin, N. Rodthongkum, O. Chailapakul and K. Shin, Point-of-Care Testing (POCT) Devices for DNA Detection: A Comprehensive Review, Adv. NanoBiomed Res., 2023, 3(11), 2300058 CrossRef CAS.
- P. Kalligosfyri, S. Nikou, V. Bravou and D. P. Kalogianni, Liquid biopsy genotyping by a simple lateral flow strip assay with visual detection, Anal. Chim. Acta, 2021, 1163, 338470, DOI:10.1016/j.aca.2021.338470 , From NLM.
- M. Kojima, T. Harada, T. Fukazawa, S. Kurihara, R. Touge, I. Saeki, S. Takahashi and E. Hiyama, Single-cell next-generation sequencing of circulating tumor cells in patients with neuroblastoma, Cancer Sci., 2023, 114(4), 1616–1624 CrossRef CAS PubMed.
- S. Merugu, L. Chen, E. Gavens, H. Gabra, M. Brougham, G. Makin, A. Ng, D. Murphy, A. S. Gabriel and M. L. Robinson, Detection of circulating and disseminated neuroblastoma cells using the ImageStream flow cytometer for use as predictive and pharmacodynamic biomarkers, Clin. Cancer Res., 2020, 26(1), 122–134 CrossRef CAS PubMed.
- T. Kuroda, N. Morikawa, K. Matsuoka, A. Fujino, T. Honna, A. Nakagawa, M. Kumagai, H. Masaki and M. Saeki, Prognostic significance of circulating tumor cells and bone marrow micrometastasis in advanced neuroblastoma, J. Pediatr. Surg., 2008, 43(12), 2182–2185 CrossRef PubMed.
- C. Lu, J. Han, X. Sun and G. Yang, Electrochemical detection and point-of-care testing for circulating tumor cells: current techniques and future potentials, Sensors, 2020, 20(21), 6073 CrossRef CAS PubMed.
- Y. Chen, X. Chen, M. Li, P. Fan, B. Wang, S. Zhao, W. Yu, S. Zhang, Y. Tang and T. Gao, A new analytical platform for potential point-of-care testing of circulating tumor cells, Biosens. Bioelectron., 2021, 171, 112718 CrossRef CAS PubMed.
- F. Farshchi and M. Hasanzadeh, Microfluidic biosensing of circulating tumor cells (CTCs): Recent progress and challenges in efficient diagnosis of cancer, Biomed. Pharmacother., 2021, 134, 111153 CrossRef CAS PubMed.
- I. Goldstein, S. Alyas, W. Asghar and A. Ilyas, Biosensors for the isolation and detection of circulating tumor cells (CTCs) in point-of-care settings, Micromachines, 2023, 14(5), 1035 CrossRef PubMed.
- R. Yang, S. Zheng and R. Dong, Circulating tumor cells in neuroblastoma: Current status and future perspectives, Cancer Med., 2023, 12(1), 7–19 CrossRef CAS PubMed.
- N. H. Lee, M. H. Son, Y. B. Choi, E. Yi, J. W. Lee, K. H. Yoo, K. W. Sung and H. H. Koo, Clinical significance of tyrosine hydroxylase mRNA transcripts in peripheral blood at diagnosis in patients with neuroblastoma, Cancer Res. Treat., 2016, 48(4), 1399 CrossRef CAS PubMed.
- A. Marachelian, J. G. Villablanca, C. W. Liu, B. Liu, F. Goodarzian, H. A. Lai, H. Shimada, H. C. Tran, J. A. Parra and R. Gallego, Expression of five neuroblastoma genes in bone marrow or blood of patients with relapsed/refractory neuroblastoma provides a new biomarker for disease and prognosis, Clin. Cancer Res., 2017, 23(18), 5374–5383 CrossRef CAS PubMed.
- F. Zeka, A. Decock, A. Van Goethem, K. Vanderheyden, F. Demuynck, T. Lammens, H. H. Helsmoortel, J. Vermeulen, R. Noguera and A. P. Berbegall, Circulating microRNA biomarkers for metastatic disease in neuroblastoma patients, JCI Insight, 2018, 3(23), e97021 CrossRef PubMed.
- S. K. Ramraj, S. Aravindan, D. B. Somasundaram, T. S. Herman, M. Natarajan and N. Aravindan, Serum-circulating miRNAs predict neuroblastoma progression in mouse model of high-risk metastatic disease, Oncotarget, 2016, 7(14), 18605 CrossRef PubMed.
- K. B. Challagundla, P. M. Wise, P. Neviani, H. Chava, M. Murtadha, T. Xu, R. Kennedy, C. Ivan, X. Zhang and I. Vannini, Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy, J. Natl. Cancer Inst., 2015, 107(7), djv135 CrossRef PubMed.
- J. Ma, M. Xu, M. Yin, J. Hong, H. Chen, Y. Gao, C. Xie, N. Shen, S. Gu and X. Mo, Exosomal hsa-miR199a-3p promotes proliferation and migration in neuroblastoma, Front. Oncol., 2019, 9, 459 CrossRef PubMed.
- C. Wang, Y. Zhang, C. Liu, S. Gou, S. Hu and W. Guo, A portable colorimetric point-of-care testing platform for MicroRNA detection based on programmable entropy-driven dynamic DNA network modulated DNA-gold nanoparticle hybrid hydrogel film, Biosens. Bioelectron., 2023, 225, 115073 CrossRef PubMed.
- S. Erzegovesi, R. M. Martoni, M. Oliva, L. Bellodi, R. G. Grgic and A. Ogliari, Neonatal Neuroblastoma in a Full Term Saudi Girl: A Case Report, J. Neonatal. Stud., 2022, 5(1), 1 Search PubMed.
- Y. Liu, Q. Shi, Y. Su, Z. Chen and X. He, Heat shock transcription factor 1 facilitates liver cancer progression by driving super-enhancer-mediated transcription of MYCN, Cancer Med., 2024, 13(17), e70157 CrossRef PubMed.
- I. R. Verly, A. B. van Kuilenburg, N. G. Abeling, S. M. Goorden, M. Fiocco, F. M. Vaz, M. M. van Noesel, C. M. Zwaan, G. L. Kaspers and J. H. Merks, Catecholamines profiles at diagnosis: increased diagnostic sensitivity and correlation with biological and clinical features in neuroblastoma patients, Eur. J. Cancer, 2017, 72, 235–243 CrossRef PubMed.
- M. Hassani-Marand, S. Jafarinejad and M. R. Hormozi-Nezhad, An AI-Enabled multi colorimetric sensor array: Towards Rapid and Noninvasive Detection of Neuroblastoma Urinary Markers, Sens. Actuators, B, 2023, 396, 134571, DOI:10.1016/j.snb.2023.134571.
- S. S. Shetty, B. Moosa, L. Zhang, B. Alshankiti, W. Baslyman, V. Mani, N. M. Khashab and K. N. Salama, Polyoxometalate-cyclodextrin supramolecular entities for real-time in situ monitoring of dopamine released from neuroblastoma cells, Biosens. Bioelectron., 2023, 229, 115240, DOI:10.1016/j.bios.2023.115240.
- R. Abdalla, A. Mahmoud, A. Al-Alamein, M. Galal and D. Mously, Point-of-Care Sensor Using Modified Nickel-doped Zinc Oxide Nanoparticle Carbon Paste Electrode for Homovanillic Acid Cancer Biomarker Detection in Urine, J. Electrochem. Soc., 2024, 171, DOI:10.1149/1945-7111/ad8ca9.
- C. Chen, C. Hu, B. He, Y. Bai, F. He, S. Li and C. S. Tan, Functionalized GD2 electrochemical immunosensor to diagnose minimum residual disease of bone marrow in neuroblastoma effectively, Biosensors, 2023, 13(10), 920 CrossRef PubMed.
- Y.-S. Lin, Y. Chen, Y.-H. Tsai, S.-H. Tseng and K.-S. Lin, In vivo imaging of neuroblastomas using GD2-targeting graphene quantum dots, J. Pediatr. Surg., 2021, 56(7), 1227–1232 CrossRef.
- J. C. Jacobson, R. A. Clark and D. H. Chung, High-risk neuroblastoma: a surgical perspective, Children, 2023, 10(2), 388 CrossRef PubMed.
- K. K. Matthay, Neuroblastoma: a clinical challenge and biologic puzzle, Ca-Cancer J. Clin., 1995, 45(3), 179–192 CrossRef PubMed.
- C. Hesko, W. Liu, D. K. Srivastava, T. M. Brinkman, L. Diller, T. M. Gibson, K. C. Oeffinger, W. M. Leisenring, R. Howell and G. T. Armstrong, Neurocognitive outcomes in adult survivors of neuroblastoma: A report from the Childhood Cancer Survivor Study, Cancer, 2023, 129(18), 2904–2914 CrossRef PubMed.
- D. J. Zheng, K. R. Krull, Y. Chen, L. Diller, Y. Yasui, W. Leisenring, P. Brouwers, R. Howell, J. S. Lai and L. Balsamo, Long-term psychological and educational outcomes for survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study, Cancer, 2018, 124(15), 3220–3230 CrossRef PubMed.
- S. B. Whittle, V. Smith, E. Doherty, S. Zhao, S. McCarty and P. E. Zage, Overview and recent advances in the treatment of neuroblastoma, Expert Rev. Anticancer Ther., 2017, 17(4), 369–386, DOI:10.1080/14737140.2017.1285230 , From NLM.
- V. P. Tolbert and K. K. Matthay, Neuroblastoma: clinical and biological approach to risk stratification and treatment, Cell Tissue Res., 2018, 372(2), 195–209 CrossRef PubMed.
- S. Nayak, N. R. Blumenfeld, T. Laksanasopin and S. K. Sia, Point-of-care diagnostics: recent developments in a connected age, Anal. Chem., 2017, 89(1), 102–123 CrossRef PubMed.
- C. Wang, M. Liu, Z. Wang, S. Li, Y. Deng and N. He, Point-of-care diagnostics for infectious diseases: From methods to devices, Nano Today, 2021, 37, 101092 CrossRef PubMed.
- J. A. Goble and P. T. Rocafort, Point-of-care testing: future of chronic disease state management?, J. Pharm. Pract., 2017, 30(2), 229–237 CrossRef PubMed.
- N. K. Tran, S. Albahra, H. Rashidi and L. May, Innovations in infectious disease testing: leveraging COVID-19 pandemic technologies for the future, Clin. Biochem., 2023, 117, 10–15 CrossRef PubMed.
- S.-M. Yang, S. Lv, W. Zhang and Y. Cui, Microfluidic point-of-care (POC) devices in early diagnosis: A review of opportunities and challenges, Sensors, 2022, 22(4), 1620 CrossRef PubMed.
- W. Huang, L. Shenglin, Y. Dong and S. Zhang, Applications of smartphone-based near-infrared (NIR) imaging, measurement, and spectroscopy technologies to point-of-care (POC) diagnostics, J. Zhejiang Univ., Sci., B, 2021, 22(3), 171 CrossRef PubMed.
- B. Purohit, A. Kumar, K. Mahato and P. Chandra, Smartphone-assisted personalized diagnostic devices and wearable sensors, Curr. Opin. Biomed. Eng., 2020, 13, 42–50 CrossRef.
- D. Kuupiel, V. Bawontuo and T. P. Mashamba-Thompson, Improving the accessibility and efficiency of point-of-care diagnostics services in low-and middle-income countries: lean and agile supply chain management, Diagnostics, 2017, 7(4), 58 CrossRef PubMed.
- C. P. Price and L. J. Kricka, Improving healthcare accessibility through point-of-care technologies, Clin. Chem., 2007, 53(9), 1665–1675 CrossRef PubMed.
- B. Heidt, W. F. Siqueira, K. Eersels, H. Diliën, B. van Grinsven, R. T. Fujiwara and T. J. Cleij, Point of care diagnostics in resource-limited settings: A review of the present and future of PoC in its most needed environment, Biosensors, 2020, 10(10), 133 CrossRef PubMed.
- A. Sena-Torralba, R. Álvarez-Diduk, C. Parolo, A. Piper and A. Merkoçi, Toward next generation lateral flow assays: integration of nanomaterials, Chem. Rev., 2022, 122(18), 14881–14910 CrossRef PubMed.
- Y. Liu, L. Zhan, Z. Qin, J. Sackrison and J. C. Bischof, Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis, ACS Nano, 2021, 15(3), 3593–3611 CrossRef PubMed.
- S. Arshavsky-Graham and E. Segal, Lab-on-a-chip devices for point-of-care medical diagnostics, Microfluid. Biotech., 2020, 247–265 Search PubMed.
- N. Azizipour, R. Avazpour, D. H. Rosenzweig, M. Sawan and A. Ajji, Evolution of biochip technology: A review from lab-on-a-chip to organ-on-a-chip, Micromachines, 2020, 11(6), 599 CrossRef PubMed.
- W. Li, W. Luo, M. Li, L. Chen, L. Chen, H. Guan and M. Yu, The impact of recent developments in electrochemical POC sensor for blood sugar care, Front. Chem., 2021, 9, 723186 CrossRef PubMed.
- E. T. da Silva, D. E. Souto, J. T. Barragan, J. de F. Giarola, A. C. de Moraes and L. T. Kubota, Electrochemical biosensors in point-of-care devices: recent advances and future trends, ChemElectroChem, 2017, 4(4), 778–794 CrossRef.
- S. Rasheed, T. Kanwal, N. Ahmad, B. Fatima, M. Najam-ul-Haq and D. Hussain, Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics, TrAC, Trends Anal. Chem., 2024, 117640 CrossRef.
- Y.-T. Chen, Y.-C. Lee, Y.-H. Lai, J.-C. Lim, N.-T. Huang, C.-T. Lin and J.-J. Huang, Review of integrated optical biosensors for point-of-care applications, Biosensors, 2020, 10(12), 209 CrossRef PubMed.
- N. Jiang, R. Ahmed, M. Damayantharan, B. Ünal, H. Butt and A. K. Yetisen, Lateral and Vertical Flow Assays for Point-of-Care Diagnostics, Adv. Healthcare Mater., 2019, 8(14), 1900244 CrossRef PubMed.
- S. Kakkar, P. Gupta, S. P. S. Yadav, D. Raj, G. Singh, S. Chauhan, M. K. Mishra, E. Martín-Ortega, S. Chiussi and K. Kant, Lateral flow assays: Progress and evolution of recent trends in point-of-care applications, Mater. Today Bio, 2024, 101188 CrossRef PubMed.
- R. Wu, S. Zhou, T. Chen, J. Li, H. Shen, Y. Chai and L. S. Li, Quantitative and rapid detection of C-reactive protein using quantum dot-based lateral flow test strip, Anal. Chim. Acta, 2018, 1008, 1–7 CrossRef PubMed.
- Y. Wu, Y. Hu, N. Jiang, M. W. Georgi, A. K. Yetisen and M. F. Cordeiro, Dual lateral flow assay using quantum nanobeads for quantitative detection of BDNF and TNF-α in tears, Lab Chip, 2025, 25(9), 2291–2303 RSC.
- S. Gao, X. Zheng, J. Zhu, Y. Zhang, R. Zhou, T. Wang, J. Katona, D. Zhang and X. Zou, Magnetic nanoprobe-enabled lateral flow assays in the applications of food safety and in vitro diagnostic, Coord. Chem. Rev., 2025, 534, 216588 CrossRef.
- W. Ren and J. Irudayaraj, Magnetic control-enhanced lateral flow technique for ultrasensitive nucleic acid target detection, ACS Omega, 2022, 7(33), 29204–29210 CrossRef PubMed.
- R. H. Althomali, H. Uinarni, K. Gandla, A. M. Mayet, R. M. Romero-Parra, I. Cahalib, K. H. Oudaha, A. F. Almulla and Y. S. Bisht, Applications of magnetic nanomaterials in the fabrication of lateral flow assays toward increasing performance of food safety analysis: Recent advances, Food Biosci., 2023, 56, 103149 CrossRef.
- K. L. Rahn, S. Y. Osman, Q. G. Pollak and R. K. Anand, Electrokinetic focusing of SARS-CoV-2 spike protein via ion concentration polarization in a paper-based lateral flow assay, Anal. Methods, 2024, 16(1), 91–104 RSC.
- S. I. Han, Y. K. Yoo, J. Lee, C. Kim, K. Lee, T. H. Lee, H. Kim, D. S. Yoon, K. S. Hwang and R. Kwak, High-ionic-strength pre-concentration via ion concentration polarization for blood-based biofluids, Sens. Actuators, B, 2018, 268, 485–493 CrossRef.
- L. Zhan, S.-z. Guo, F. Song, Y. Gong, F. Xu, D. R. Boulware, M. C. McAlpine, W. C. Chan and J. C. Bischof, The role of nanoparticle design in determining analytical performance of lateral flow immunoassays, Nano Lett., 2017, 17(12), 7207–7212 CrossRef PubMed.
- A. Tukova, N. T. T. Nguyen, A. Garcia-Bennett, A. Rodger and Y. Wang, Plasmonic nanostars: Unique properties that distinguish them from spherical nanoparticles from a biosensing perspective, Adv. Opt. Mater., 2024, 12(28), 2401183 CrossRef.
- J. B. Shao, Z. M. Gao, W. Y. Huang and Z. B. Lu, The mechanism of epithelial-mesenchymal transition induced by TGF-β1 in neuroblastoma cells, Int. J. Oncol., 2017, 50(5), 1623–1633, DOI:10.3892/ijo.2017.3954 , From NLM.
- H. Li, X. Fu, Q. You, D. Shi, L. Su, M. Song, R. Peng, T. Fu, P. Wang and W. Tan, Multiple aptamer recognition-based quantum dot lateral flow platform: ultrasensitive point-of-care testing of respiratory infectious diseases, J. Mater. Chem. B, 2025, 13(5), 1681–1689 RSC.
- Y. Tian, L. Chen, X. Liu, Y. Chang, R. Xia, J. Zhang, Y. Kong, Y. Gong, T. Li and G. Wang, Colored Cellulose Nanoparticles with High Stability and Easily Modified Surface for Accurate and Sensitive Multiplex Lateral Flow Assay, ACS Nano, 2025, 19(4), 4704–4717 CrossRef PubMed.
- W. Jung, J. Han, J.-W. Choi and C. H. Ahn, Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies, Microelectron. Eng., 2015, 132, 46–57 CrossRef.
- V. Narayanamurthy, Z. Jeroish, K. Bhuvaneshwari, P. Bayat, R. Premkumar, F. Samsuri and M. M. Yusoff, Advances in passively driven microfluidics and lab-on-chip devices: A comprehensive literature review and patent analysis, RSC Adv., 2020, 10(20), 11652–11680 RSC.
- A. Olanrewaju, M. Beaugrand, M. Yafia and D. Juncker, Capillary microfluidics in microchannels: from microfluidic networks to capillaric circuits, Lab Chip, 2018, 18(16), 2323–2347 RSC.
- P. Azizian, J. Casals-Terré, J. Ricart and J. M. Cabot, Diffusion-free valve for preprogrammed immunoassay with capillary microfluidics, Microsyst. Nanoeng., 2023, 9(1), 91 CrossRef CAS PubMed.
- S. Moon, Extending the shelf-life of immunoassay-based microfluidic chips through freeze-drying sublimation techniques, Sensors, 2023, 23(20), 8524 CrossRef CAS PubMed.
- A. Chiadò, G. Palmara, A. Chiappone, C. Tanzanu, C. F. Pirri, I. Roppolo and F. Frascella, A modular 3D printed lab-on-a-chip for early cancer detection, Lab Chip, 2020, 20(3), 665–674 RSC.
- C. Zhu, G. Yang, H. Li, D. Du and Y. Lin, Electrochemical sensors and biosensors based on nanomaterials and nanostructures, Anal. Chem., 2015, 87(1), 230–249 CrossRef CAS PubMed.
- A. Azadbakht, M. Roushani, A. R. Abbasi and Z. Derikvand, A novel impedimetric aptasensor, based on functionalized carbon nanotubes and prussian blue as labels, Anal. Biochem., 2016, 512, 58–69 CrossRef CAS PubMed.
- M. D. Jerez-Masaquiza, L. Fernández, G. González, M. Montero-Jiménez and P. J. Espinoza-Montero, Electrochemical sensor based on prussian blue electrochemically deposited at ZrO2 doped carbon nanotubes glassy carbon modified electrode, Nanomaterials, 2020, 10(7), 1328 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, E. B. Noruzi, E. Chidar, M. Jafari, F. Davoodi, A. Kashtiaray, M. G. Gorab, S. M. Hashemi, S. Javanshir and R. A. Cohan, Applications of carbon-based conductive nanomaterials in biosensors, Chem. Eng. J., 2022, 442, 136183 CrossRef CAS.
- X. Liu, X. Mei, J. Yang and Y. Li, Hydrogel-involved colorimetric platforms based on layered double oxide nanozymes for point-of-care detection of liver-related biomarkers, ACS Appl. Mater. Interfaces, 2022, 14(5), 6985–6993 CrossRef CAS PubMed.
- K. Yan, J. Wu, W. Ji, J. Wu and J. Zhang, Integration of redox cycling in a photoelectrochemical sensing platform for tyrosinase activity evaluation, Electrochem. Commun., 2019, 108, 106555 CrossRef CAS.
- N. Tiwari, S. Chatterjee, K. Kaswan, J.-H. Chung, K.-P. Fan and Z.-H. Lin, Recent advancements in sampling, power management strategies and development in applications for non-invasive wearable electrochemical sensors, J. Electroanal. Chem., 2022, 907, 116064 CrossRef CAS.
- J. F. Diforti, T. Cunningham, E. Piccinini, W. A. Marmisollé, J. M. Piccinini and O. Azzaroni, Noninvasive and Multiplex Self-Test of Kidney Disease Biomarkers with Graphene-Based Lab-on-a-Chip (G-LOC): Toward Digital Diagnostics in the Hands of Patients, Anal. Chem., 2024, 96(15), 5832–5842 CrossRef CAS PubMed.
- S. Jeong, S.-H. Park, S. Lee, H. Cho, K.-y. Lee, B. K. Ju, Y. J. Lee and S. H. Lee, Electrochemical biosensor based on gold nanoparticles/laser induced graphene for diagnosis of Parkinson's disease by detecting phosphorylated α-synuclein in human blood, Chem. Eng. J., 2025, 509, 161329 CrossRef CAS.
- S. M. Borisov and O. S. Wolfbeis, Optical biosensors, Chem. Rev., 2008, 108(2), 423–461 CrossRef PubMed.
- A. B. González-Guerrero, J. Maldonado, S. Herranz and L. M. Lechuga, Trends in photonic lab-on-chip interferometric biosensors for point-of-care diagnostics, Anal. Methods, 2016, 8(48), 8380–8394 RSC.
- C. Serafinelli, A. Fantoni, E. C. Alegria and M. Vieira, Recent progresses in plasmonic biosensors for point-of-care (POC) devices: A critical review, Chemosensors, 2023, 11(5), 303 CrossRef.
- X. Liu, W. Wu, D. Cui, X. Chen and W. Li, Functional micro−/nanomaterials for multiplexed biodetection, Adv. Mater., 2021, 33(30), 2004734 CrossRef PubMed.
- A. S. Manna, S. Ghosh, T. Ghosh, N. Karchaudhuri, S. Das, A. Roy and D. K. Maiti, Smart Luminescent Materials for Emerging Sensors: Fundamentals and Advances, Chem. – Asian J., 2025, 20(6), e202401328 CrossRef PubMed.
- Z. Li, M. Guo and W. Zhong, Multiplex Detection of Biomarkers Empowered by Nanomaterials, Precis. Chem., 2025, 3(6), 297–318 CrossRef PubMed.
- L. Kong, W. Li, T. Zhang, H. Ma, Y. Cao, K. Wang, Y. Zhou, A. Shamim, L. Zheng and X. Wang, Wireless technologies in flexible and wearable sensing: from materials design, system integration to applications, Adv. Mater., 2024, 36(27), 2400333 CrossRef PubMed.
- S. Ye, C. Xu, H. Li, S. Feng, Y. Wang and F. Gao, Enhancing lateral flow immunoassay performance for cardiac troponin I detection with pore-size tailored silica nanoparticles and smartphone-based “AdaptiScan” analysis, Front. Bioeng. Biotechnol., 2025, 13, 1568719 CrossRef PubMed.
- D. V. Baker, J. Bernal-Escalante, C. Traaseth, Y. Wang, M. V. Tran, S. Keenan and W. R. Algar, Smartphones as a platform for molecular analysis: concepts, methods, devices and future potential, Lab Chip, 2025, 25(5), 884–955 RSC.
- Y. Xia, J. Hu, S. Zhao, L. Tao, Z. Li, T. Yue and J. Kong, Build-in sensors and analysis algorithms aided smartphone-based sensors for point-of-care tests, Biosens. Bioelectron.: X, 2022, 11, 100195 Search PubMed.
- Y. Zhang, Y. Hu, Y. Montelongo, M. Hsu, J. Blyth, N. Jiang and A. K. Yetisen, A conformable holographic sensing bandage for wound monitoring, Adv. Funct. Mater., 2024, 34(16), 2308490 CrossRef.
- A. Sajeevan, R. A. Sukumaran, L. R. Panicker and Y. G. Kotagiri, Trends in ready-to-use portable electrochemical sensing devices for healthcare diagnosis, Microchim. Acta, 2025, 192(2), 80 CrossRef PubMed.
- A. Firoozbakhtian, M. Hosseini, M. N. Sheikholeslami, F. Salehnia, G. Xu, H. Rabbani and E. Sobhanie, Detection of COVID-19: a smartphone-based machine-learning-assisted ECL immunoassay approach with the ability of RT-PCR CT value prediction, Anal. Chem., 2022, 94(47), 16361–16368 CrossRef PubMed.
- S. Bhatt, S. Kumar, M. K. Gupta, S. K. Datta and S. K. Dubey, Colorimetry-based and smartphone-assisted machine-learning model for quantification of urinary albumin, Meas. Sci. Technol., 2023, 35(1), 015030 CrossRef.
- K. E. McCracken and J.-Y. Yoon, Recent approaches for optical smartphone sensing in resource-limited settings: a brief review, Anal. Methods, 2016, 8(36), 6591–6601 RSC.
- L. Yan, Y. Bai, J. Zhou, Y. He, C. Shen, J. Huang and P. Chen, Enzyme-free nucleic acid and luminescent material cascade amplifications enable urinary dual miRNAs assay for non-invasive bladder cancer diagnosis, Sens. Actuators, B, 2023, 396, 134627 CrossRef CAS.
- A. Reda, S. A. El-Safty, M. M. Selim and M. A. Shenashen, Optical glucose biosensor built-in disposable strips and wearable electronic devices, Biosens. Bioelectron., 2021, 185, 113237 CrossRef CAS PubMed.
- J. Liu, Z. Geng, Z. Fan, J. Liu and H. Chen, Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018), Biosens. Bioelectron., 2019, 132, 17–37 CrossRef CAS PubMed.
- A. Jędrzak, M. Kuznowicz, T. Rębiś and T. Jesionowski, Portable glucose biosensor based on polynorepinephrine@ magnetite nanomaterial integrated with a smartphone analyzer for point-of-care application, Bioelectrochemistry, 2022, 145, 108071 CrossRef PubMed.
- A. Sampagar, S. Keerthana, M. C. Dias, N. A. Reddy and N. Patil, A Study of Factors Influencing Delayed Diagnosis in Pediatric Cancers: A Step Towards Better Outcomes-A Cross-sectional Study, J. Pediatr. Hematol./Oncol., 2023, 45(6), 327–332, DOI:10.1097/mph.0000000000002664 , From NLM.
- A. Pulumati, A. Pulumati, B. S. Dwarakanath, A. Verma and R. V. L. Papineni, Technological advancements in cancer diagnostics: Improvements and limitations, Cancer Rep., 2023, 6(2), e1764, DOI:10.1002/cnr2.1764 , From NLM.
- A. Marusyk and K. Polyak, Tumor heterogeneity: causes and consequences, Biochim. Biophys. Acta, 2010, 1805(1), 105–117, DOI:10.1016/j.bbcan.2009.11.002 , From NLM.
- P. Bhardwaj, B. Arora, S. Saxena, S. Singh, P. Palkar, J. S. Goda and R. Banerjee, Paper-based point of care diagnostics for cancer biomarkers, Sens. Diagn., 2024, 3(4), 504–535 RSC.
- B. Asci Erkocyigit, O. Ozufuklar, A. Yardim, E. Guler Celik and S. Timur, Biomarker detection in early diagnosis of cancer: recent achievements in point-of-care devices based on paper microfluidics, Biosensors, 2023, 13(3), 387 CrossRef CAS PubMed.
- T.-H. Ulep, R. Zenhausern, A. Gonzales, D. S. Knoff, P. A. L. Diaz, J. E. Castro and J.-Y. Yoon, Smartphone based on-chip fluorescence imaging and capillary flow velocity measurement for detecting ROR1+ cancer cells from buffy coat blood samples on dual-layer paper microfluidic chip, Biosens. Bioelectron., 2020, 153, 112042 CrossRef CAS PubMed.
- T. Mo, X. Liu, Y. Luo, L. Zhong, Z. Zhang, T. Li, L. Gan, X. Liu, L. Li and H. Wang, et al., Aptamer-based biosensors and application in tumor theranostics, Cancer Sci., 2022, 113(1), 7–16, DOI:10.1111/cas.15194 , From NLM.
- H. Wei, Y. Peng, Z. Bai, Z. Rong and S. Wang, Duplex-specific nuclease signal amplification-based fluorescent lateral flow assay for the point-of-care detection of microRNAs, Analyst, 2021, 146(2), 558–564, 10.1039/d0an01673j , From NLM.
- M. Adampourezare, G. Dehghan, M. Hasanzadeh and M. A. Hosseinpoure Feizi, Application of lateral flow and microfluidic bio-assay and biosensing towards identification of DNA-methylation and cancer detection: Recent progress and challenges in biomedicine, Biomed. Pharmacother., 2021, 141, 111845, DOI:10.1016/j.biopha.2021.111845 , From NLM.
- J. F. Edd, A. Mishra, T. D. Dubash, S. Herrera, R. Mohammad, E. K. Williams, X. Hong, B. R. Mutlu, J. R. Walsh and F. M. de Carvalho, Microfluidic concentration and separation of circulating tumor cell clusters from large blood volumes, Lab Chip, 2020, 20(3), 558–567 RSC.
- S. H. Au, J. Edd, A. E. Stoddard, K. H. Wong, F. Fachin, S. Maheswaran, D. A. Haber, S. L. Stott, R. Kapur and M. Toner, Microfluidic isolation of circulating tumor cell clusters by size and asymmetry, Sci. Rep., 2017, 7(1), 2433 CrossRef PubMed.
- R. Miano, G. O. Mele, S. Germani, P. Bove, S. Sansalone, P. F. Pugliese and F. Micali, Evaluation of a new, rapid, qualitative, one-step PSA Test for prostate cancer screening: the PSA RapidScreen test, Prostate Cancer Prostatic Dis., 2005, 8(3), 219–223, DOI:10.1038/sj.pcan.4500802 , From NLM.
- P. Moonen, L. Kiemeney and J. Witjes, Urinary NMP22® BladderChek® test in the diagnosis of superficial bladder cancer, Eur. Urol., 2005, 48(6), 951–956 CrossRef CAS PubMed.
- L. Huang, S. Tian, W. Zhao, K. Liu, X. Ma and J. Guo, Multiplexed detection of biomarkers in lateral-flow immunoassays, Analyst, 2020, 145(8), 2828–2840, 10.1039/c9an02485a , From NLM.
- W.-Q. Ye, Y.-X. Wei, Y.-Z. Zhang, C.-G. Yang and Z.-R. Xu, Multiplexed detection of micro-RNAs based on microfluidic multi-color fluorescence droplets, Anal. Bioanal. Chem., 2020, 412(3), 647–655, DOI:10.1007/s00216-019-02266-3.
- D. Chatterjee, D. S. Mansfield and A. T. Woolley, Microfluidic devices for label-free and non-instrumented quantitation of unamplified nucleic acids by flow distance measurement, Anal. Methods, 2014, 6(20), 8173–8179 RSC.
- Y. Song, Y. Zhang, P. E. Bernard, J. M. Reuben, N. T. Ueno, R. B. Arlinghaus, Y. Zu and L. Qin, Multiplexed volumetric bar-chart chip for point-of-care diagnostics, Nat. Commun., 2012, 3(1), 1–9 Search PubMed.
- M. Calderón-Santiago, F. Priego-Capote, N. Turck, X. Robin, B. Jurado-Gámez, J. C. Sanchez and M. D. Luque de Castro, Human sweat metabolomics for lung cancer screening, Anal. Bioanal. Chem., 2015, 407, 5381–5392 CrossRef PubMed.
- L. Dizdar, G. Fluegen, G. van Dalum, E. Honisch, R. P. Neves, D. Niederacher, H. Neubauer, T. Fehm, A. Rehders and A. Krieg, et al., Detection of circulating tumor cells in colorectal cancer patients using the GILUPI CellCollector: results from a prospective, single-center study, Mol. Oncol., 2019, 13(7), 1548–1558, DOI:10.1002/1878-0261.12507 , From NLM.
- C.-H. Lai, W.-S. Tsai, M.-H. Yang, T.-Y. Chou and Y.-C. Chang, A two-dimensional immunomagnetic nano-net for the efficient isolation of circulating tumor cells in whole blood, Nanoscale, 2019, 11(44), 21119–21127 RSC.
- S. Park and Y. J. Lee, AFM-based dual nano-mechanical phenotypes for cancer metastasis, J. Biol. Phys., 2014, 40, 413–419 CrossRef PubMed.
- K. R. Khondakar, M. S. Anwar, H. Mazumdar and A. Kaushik, Perspective of point-of-care sensing systems in cancer management, Mater. Adv., 2023, 4(21), 4991–5002 RSC.
- E. Poon, T. Liang, Y. Jamin, S. Walz, C. Kwok, A. Hakkert, K. Barker, Z. Urban, K. Thway and R. Zeid, Orally bioavailable CDK9/2 inhibitor shows mechanism-based therapeutic potential in MYCN-driven neuroblastoma, J. Clin. Invest., 2020, 130(11), 5875–5892 CrossRef PubMed.
- D. Hanahan and R. A. Weinberg, Hallmarks of cancer: the next generation, Cell, 2011, 144(5), 646–674, DOI:10.1016/j.cell.2011.02.013 , From NLM.
- A. Passaro, M. Al Bakir, E. G. Hamilton, M. Diehn, F. André, S. Roy-Chowdhuri, G. Mountzios, I. I. Wistuba, C. Swanton and S. Peters, Cancer biomarkers: Emerging trends and clinical implications for personalized treatment, Cell, 2024, 187(7), 1617–1635, DOI:10.1016/j.cell.2024.02.041 , From NLM.
- D. A. Giljohann and C. A. Mirkin, Drivers of biodiagnostic development, Nature, 2009, 462(7272), 461–464 CrossRef PubMed.
- V. Combaret, M. D. Hogarty, W. B. London, P. McGrady, I. Iacono, S. Brejon, K. Swerts, R. Noguera, N. Gross and R. Rousseau, Influence of neuroblastoma stage on serum-based detection of MYCN amplification, Pediatr. Blood Cancer, 2009, 53(3), 329–331 CrossRef PubMed.
- X. Wang, L. Wang, Y. Su, Z. Yue, T. Xing, W. Zhao, Q. Zhao, C. Duan, C. Huang and D. Zhang, Plasma cell-free DNA quantification is highly correlated to tumor burden in children with neuroblastoma, Cancer Med., 2018, 7(7), 3022–3030 CrossRef CAS PubMed.
- N. Van Roy, M. Van Der Linden, B. Menten, A. Dheedene, C. Vandeputte, J. Van Dorpe, G. Laureys, M. Renard, T. Sante and T. Lammens, Shallow whole genome sequencing on circulating cell-free DNA allows reliable noninvasive copy-number profiling in neuroblastoma patients, Clin. Cancer Res., 2017, 23(20), 6305–6314 CrossRef CAS PubMed.
- Y. Su, L. Wang, X. Wang, Z. Yue, T. Xing, W. Zhao, Q. Zhao, C. Duan, C. Huang and Y. Han, Dynamic alterations of plasma cell free DNA in response to chemotherapy in children with neuroblastoma, Cancer Med., 2019, 8(4), 1558–1566 CrossRef CAS PubMed.
- S. Yagyu, T. Iehara, T. Gotoh, M. Miyachi, Y. Katsumi, K. Kikuchi, K. Tsuchiya, S. Osone, H. Kuroda and T. Sugimoto, Preoperative analysis of 11q loss using circulating tumor-released DNA in serum: a novel diagnostic tool for therapy stratification of neuroblastoma, Cancer Lett., 2011, 309(2), 185–189 CrossRef CAS PubMed.
- S. L. George, E. Izquierdo, J. Campbell, E. Koutroumanidou, P. Proszek, S. Jamal, D. Hughes, L. Yuan, L. V. Marshall and F. Carceller, A tailored molecular profiling programme for children with cancer to identify clinically actionable genetic alterations, Eur. J. Cancer, 2019, 121, 224–235 CrossRef CAS PubMed.
- R. Stankunaite, S. L. George, L. Gallagher, S. Jamal, R. Shaikh, L. Yuan, D. Hughes, P. Z. Proszek, P. Carter and G. Pietka, Circulating tumour DNA sequencing to determine therapeutic response and identify tumour heterogeneity in patients with paediatric solid tumours, Eur. J. Cancer, 2022, 162, 209–220 CrossRef CAS PubMed.
- S. M. Tolaney, M. Toi, P. Neven, J. Sohn, E.-M. Grischke, A. Llombart-Cussac, H. Soliman, L. M. Litchfield, S. Wijayawardana and T. Forrester, Clinical significance of PIK3CA and ESR1 mutations in ctDNA and FFPE samples from the MONARCH 2 study of abemaciclib plus fulvestrant, Clin. Cancer Res., 2019, 28(8), 1500–1506 CrossRef PubMed.
- C. Trager, P. Kogner, M. Lindskog, F. Ponthan, A. Kullman and B. Kågedal, Quantitative analysis of tyrosine hydroxylase mRNA for sensitive detection of neuroblastoma cells in blood and bone marrow, Clin. Chem., 2003, 49(1), 104–112 CrossRef CAS PubMed.
- V. F. Viprey, W. M. Gregory, M. V. Corrias, A. Tchirkov, K. Swerts, A. Vicha, S. Dallorso, P. Brock, R. Luksch and D. Valteau-Couanet, Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: a European HR-NBL1/SIOPEN study, J. Clin. Oncol., 2019, 32(10), 1074–1083 CrossRef PubMed.
- S. A. Burchill, I. J. Lewis, K. R. Abrams, R. Riley, J. Imeson, A. D. Pearson, R. Pinkerton and P. Selby, Circulating neuroblastoma cells detected by reverse transcriptase polymerase chain reaction for tyrosine hydroxylase mRNA are an independent poor prognostic indicator in stage 4 neuroblastoma in children over 1 year, J. Clin. Oncol., 2001, 19(6), 1795–1801 CrossRef CAS PubMed.
- J. Otte, C. Dyberg, A. Pepich and J. I. Johnsen, MYCN function in neuroblastoma development, Front. Oncol., 2021, 3210 Search PubMed.
- M. Schwab, J. Ellison, M. Busch, W. Rosenau, H. E. Varmus and J. M. Bishop, Enhanced expression of the human gene N-myc consequent to amplification of DNA may contribute to malignant progression of neuroblastoma, Proc. Natl. Acad. Sci. U. S. A., 1984, 81(15), 4940–4944 CrossRef CAS PubMed.
- D. S. Rickman, J. H. Schulte and M. Eilers, The expanding world of N-MYC–driven tumors, Cancer Discovery, 2018, 8(2), 150–163 CrossRef CAS PubMed.
- M. Huang and W. A. Weiss, Neuroblastoma and MYCN, Cold Spring Harbor Perspect. Med., 2013, 3(10), a014415 Search PubMed.
- M. V. Ruiz-Pérez, A. B. Henley and M. Arsenian-Henriksson, The MYCN Protein in Health and Disease, Genes, 2017, 8(4), 113, DOI:10.3390/genes8040113 , From NLM.
- J. R. Smith, L. Moreno, S. P. Heaton, L. Chesler, A. D. Pearson and M. D. Garrett, Novel pharmacodynamic biomarkers for MYCN protein and PI3K/AKT/mTOR pathway signaling in children with neuroblastoma, Mol. Oncol., 2016, 10(4), 538–552, DOI:10.1016/j.molonc.2015.11.005 , From NLM.
- R. M. Trigg and S. D. Turner, ALK in Neuroblastoma: Biological and Therapeutic Implications, Cancers, 2018, 10(4), 113, DOI:10.3390/cancers10040113 , From NLM PubMed-not-MEDLINE.
- T. Mok, S. Peters, D. R. Camidge, J. Noe, S. Gadgeel, S. I. Ou, D. W. Kim, K. Konopa, E. Pozzi and T. Liu, et al., Outcomes According to ALK Status Determined by Central Immunohistochemistry or Fluorescence In Situ Hybridization in Patients With ALK-Positive NSCLC Enrolled in the Phase 3 ALEX Study, J. Thorac. Oncol., 2021, 16(2), 259–268, DOI:10.1016/j.jtho.2020.10.007 , From NLM Medline.
- V. Combaret, I. Iacono, A. Bellini, S. Brejon, V. Bernard, A. Marabelle, C. Coze, G. Pierron, E. Lapouble and G. Schleiermacher, et al., Detection of tumor ALK status in neuroblastoma patients using peripheral blood, Cancer Med., 2015, 4(4), 540–550, DOI:10.1002/cam4.414 , From NLM Medline.
- G. Gonzales-Cespedes and S. Navarro, High-risk neuroblastoma: ATRX and TERT as prognostic markers and therapeutic targets. Review and update on the topic, Rev. Esp. Patol., 2025, 58(1), 100790, DOI:10.1016/j.patol.2024.100790 , From NLM Medline.
- M. Ognibene, S. Parodi, L. Amoroso, F. Zara and A. Pezzolo, Overexpression of H2AFX gene in neuroblastoma is associated with worse prognosis, Pediatr. Blood Cancer, 2024, 71(9), e31146, DOI:10.1002/pbc.31146 , From NLM Medline.
- K. Otake, K. Uchida, S. Ide, Y. Kobayashi, I. Kobayashi and M. Kusunoki, Identification of DDX39A as a Potential Biomarker for Unfavorable Neuroblastoma Using a Proteomic Approach, Pediatr. Blood Cancer, 2016, 63(2), 221–227, DOI:10.1002/pbc.25778 , From NLM Medline.
- I. Sultan and A. Tbakhi, BCL11A gene over-expression in high risk neuroblastoma, Cancer Genet., 2020, 244, 30–31, DOI:10.1016/j.cancergen.2020.02.003 , From NLM Medline.
- X. Chen, Y. Xue, J. Feng, Q. Tian, Y. Zhang and Q. Wang, Identification S100A9 as a potential biomarker in neuroblastoma, Mol. Biol. Rep., 2021, 48(12), 7743–7753, DOI:10.1007/s11033-021-06783-2 , From NLM Medline.
- N. Andreeva, N. Usman and A. Druy, MicroRNAs as prospective biomarkers, therapeutic targets and pharmaceuticals in neuroblastoma, Mol. Biol. Rep., 2023, 50(2), 1895–1912, DOI:10.1007/s11033-022-08137-y , From NLM Medline.
- M. Mohammadi, M. Goodarzi, M. R. Jaafari, H. R. Mirzaei and H. Mirzaei, Circulating microRNA: a new candidate for diagnostic biomarker in neuroblastoma, Cancer Gene Ther., 2016, 23(11), 371–372, DOI:10.1038/cgt.2016.45 , From NLM Medline.
- G. Moro, C. D. Fratte, N. Normanno, F. Polo and S. Cinti, Point-of-Care Testing for the Detection of MicroRNAs: Towards Liquid Biopsy on a Chip, Angew. Chem., Int. Ed., 2023, 62(51), e202309135, DOI:10.1002/anie.202309135 , From NLM Medline.
- P. Zeltzer, A. Parma, A. Dalton, S. Siegel, P. Marangos, H. Sather, D. Hammond and R. Seeger, Raised neuron-specific enolase in serum of children with metastatic neuroblastoma: A report from the Children's Cancer Study Group, Lancet, 1983, 322(8346), 361–363 CrossRef PubMed.
- A. Abbasoglu, F. Sarialioglu, N. Yazici, N. Bayraktar, A. Haberal and A. Erbay, Serum neuron-specific enolase levels in preterm and term newborns and in infants 1–3 months of age, Pediatr. Neonatol., 2015, 56(2), 114–119 CrossRef PubMed.
- P. M. Zeltzer, P. J. Marangos, A. E. Evans and S. L. Schneider, Serum neuron-specific enolase in children with neuroblastoma. Crelationship to stage and disease course, Cancer, 1986, 57(6), 1230–1234 CrossRef PubMed.
- Y. Fan, J. Liu, Y. Wang, J. Luo, H. Xu, S. Xu and X. Cai, A wireless point-of-care testing system for the detection of neuron-specific enolase with microfluidic paper-based analytical devices, Biosens. Bioelectron., 2017, 95, 60–66, DOI:10.1016/j.bios.2017.04.003 , From NLM Medline.
- D. Thompson, K. T. Vo, W. B. London, M. Fischer, P. F. Ambros, A. Nakagawara, G. M. Brodeur, K. K. Matthay and S. G. DuBois, Identification of patient subgroups with markedly disparate rates of MYCN amplification in neuroblastoma: a report from the International Neuroblastoma Risk Group project, Cancer, 2016, 122(6), 935–945 CrossRef PubMed.
- G. Cangemi, G. Reggiardo, S. Barco, L. Barbagallo, M. Conte, P. D'Angelo, M. Bianchi, C. Favre, B. Galleni and G. Melioli, et al., Prognostic value of ferritin, neuron-specific enolase, lactate dehydrogenase, and urinary and plasmatic catecholamine metabolites in children with neuroblastoma, Onco Targets Ther, 2012, 5, 417–423, DOI:10.2147/ott.S36366 , From NLM.
- D. A. Morgenstern, U. Pötschger, L. Moreno, V. Papadakis, C. Owens, S. Ash, C. Pasqualini, R. Luksch, A. Garaventa and A. Canete, Risk stratification of high-risk metastatic neuroblastoma: a report from the HR-NBL-1/SIOPEN study, Pediatr. Blood Cancer, 2018, 65(11), e27363 CrossRef PubMed.
- Center, U. o. R. M., Lactate Dehydrogenase (Blood), https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=lactic_acid_dehydrogenase_blood#:~:text=The%20normal%20range%20for%20total,60%20to%20170%20units%2FL (accessed) Search PubMed.
- V. Moroz, D. Machin, B. Hero, R. Ladenstein, F. Berthold, P. Kao, Y. Obeng, A. D. J. Pearson, S. L. Cohn and W. B. London, The prognostic strength of serum LDH and serum ferritin in children with neuroblastoma: A report from the International Neuroblastoma Risk Group (INRG) project, Pediatr. Blood Cancer, 2020, 67(8), e28359, DOI:10.1002/pbc.28359 , From NLM.
- A. Farhana and S. Lappin, Biochemistry, Lactate Dehydrogenase, StatPearls, 2022, https://www.ncbi.nlm.nih.gov/books/NBK557536/ (accessed) Search PubMed.
- F. Boomsma, U. M. Bhaggoe, A. J. Man in 't Veld and M. A. Schalekamp, Sensitivity and specificity of a new ELISA method for determination of chromogranin A in the diagnosis of pheochromocytoma and neuroblastoma, Clin. Chim. Acta, 1995, 239(1), 57–63, DOI:10.1016/0009-8981(95)06100-r , From NLM.
- R. J. Hsiao, R. C. Seeger, A. L. Yu and D. T. O'Connor, Chromogranin A in children with neuroblastoma. Serum concentration parallels disease stage and predicts survival, J. Clin. Invest., 1990, 85(5), 1555–1559, DOI:10.1172/jci114604 , From NLM.
- M. Morini, F. Raggi, M. Bartolucci, A. Petretto, M. Ardito, C. Rossi, D. Segalerba, A. Garaventa, A. Eva and D. Cangelosi, et al., Plasma-Derived Exosome Proteins as Novel Diagnostic and Prognostic Biomarkers in Neuroblastoma Patients, Cells, 2023, 12(21), 2516, DOI:10.3390/cells12212516 , From NLM Medline.
- M. Oliveira-Rodriguez, S. Lopez-Cobo, H. T. Reyburn, A. Costa-Garcia, S. Lopez-Martin, M. Yanez-Mo, E. Cernuda-Morollon, A. Paschen, M. Vales-Gomez and M. C. Blanco-Lopez, Development of a rapid lateral flow immunoassay test for detection of exosomes previously enriched from cell culture medium and body fluids, J. Extracell. Vesicles, 2016, 5, 31803, DOI:10.3402/jev.v5.31803 , From NLM PubMed-not-MEDLINE.
- A. Lopez-Carrasco, I. Vieco-Marti, S. Granados-Aparici, D. Acevedo-Leon, N. Estan-Capell, R. Portugal, J. Huerta-Aragones, A. Canete, S. Navarro and R. Noguera, Vitronectin Levels in the Plasma of Neuroblastoma Patients and Culture Media of 3D Models: A Prognostic Circulating Biomarker?, Int. J. Mol. Sci., 2024, 25(16), 8733, DOI:10.3390/ijms25168733 , From NLM Medline.
- D. W. Coulter, A. D. Boettner, Z. P. Kortylewicz, S. P. Enke, J. A. Luther, V. Verma and J. Baranowska-Kortylewicz, Butyrylcholinesterase as a Blood Biomarker in Neuroblastoma, J. Pediatr. Hematol./Oncol., 2017, 39(4), 272–281, DOI:10.1097/MPH.0000000000000828 , From NLM Medline.
- K. Schmidt, A. R. Zivkovic, M. Thiele, J. Horter, T. Brenner, M. A. Weigand, S. Kleinschmidt and S. Hofer, Point-of-care measured serum cholinesterase activity predicts patient outcome following severe burns, Burns, 2021, 47(4), 863–872, DOI:10.1016/j.burns.2020.10.027 , From NLM Medline.
- A. R. Zivkovic, K. Schmidt, A. Sigl, S. O. Decker, T. Brenner and S. Hofer, Reduced serum butyrylcholinesterase activity indicates severe systemic inflammation in critically ill patients, Mediators Inflammation, 2015, 2015, 274607, DOI:10.1155/2015/274607 , From NLM Medline.
- S. Galli, A. Naranjo, C. Van Ryn, J. U. Tilan, E. Trinh, C. Yang, J. Tsuei, S. H. Hong, H. Wang and E. Izycka-Swieszewska, et al., Neuropeptide Y as a Biomarker and Therapeutic Target for Neuroblastoma, Am. J. Pathol., 2016, 186(11), 3040–3053, DOI:10.1016/j.ajpath.2016.07.019 , From NLM Medline.
- N. K. Mintah Churcher, S. Upasham, P. Rice, S. Bhadsavle and S. Prasad, Development of a flexible, sweat-based neuropeptide Y detection platform, RSC Adv., 2020, 10(39), 23173–23186, 10.1039/d0ra03729j , From NLM PubMed-not-MEDLINE.
- M. Loustau, F. Anna, R. Dréan, M. Lecomte, P. Langlade-Demoyen and J. Caumartin, HLA-G neo-expression on tumors, Front. Immunol., 2020, 11, 1685 CrossRef PubMed.
- F. Morandi, I. Levreri, P. Bocca, B. Galleni, L. Raffaghello, S. Ferrone, I. Prigione and V. Pistoia, Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release, Cancer Res., 2007, 67(13), 6433–6441 CrossRef PubMed.
- L. D. Gamble, S. Purgato, J. Murray, L. Xiao, D. M. T. Yu, K. M. Hanssen, F. M. Giorgi, D. R. Carter, A. J. Gifford and E. Valli, et al., Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma, Sci. Transl. Med., 2019, 11(477), 1099, DOI:10.1126/scitranslmed.aau1099 , From NLM.
- B. Lu, L. Wang, X. Ran, H. Tang and D. Cao, Recent Advances in Fluorescent Methods for Polyamine Detection and the Polyamine Suppressing Strategy in Tumor Treatment, Biosensors, 2022, 12(8), 633 CrossRef PubMed.
- K. Igarashi, S. Ota, M. Kaneko, A. Hirayama, M. Enomoto, K. Katumata, M. Sugimoto and T. Soga, High-throughput screening of salivary polyamine markers for discrimination of colorectal cancer by multisegment injection capillary electrophoresis tandem mass spectrometry, J. Chromatogr. A, 2021, 1652, 462355, DOI:10.1016/j.chroma.2021.462355 , From NLM Medline.
- G. L. S. Sholler, W. Ferguson, G. Bergendahl, J. P. Bond, K. Neville, D. Eslin, V. Brown, W. Roberts, R. K. Wada and J. Oesterheld, et al., Maintenance DFMO Increases Survival in High Risk Neuroblastoma, Sci. Rep., 2018, 8(1), 14445, DOI:10.1038/s41598-018-32659-w.
- E. Zambrano and M. Reyes-Múgica, Hormonal activity may predict aggressive behavior in neuroblastoma, Pediatr. Dev. Pathol., 2002, 5(2), 190–199 CrossRef PubMed.
- NHS, VMA & HVA, https://www.gloshospitals.nhs.uk/our-services/services-we-offer/pathology/tests-and-investigations/vma-hva/ (accessed) Search PubMed.
- Y. Shen and L. Cheng, Biochemical Diagnosis of Pheochromocytoma and Paraganglioma, Codon Publications, 2019, DOI:10.15586/paraganglioma.2019.ch2.
- H.-W. L. Hann, A. E. Evans, S. E. Siegel, K. Y. Wong, H. Sather, A. Dalton, D. Hammond and R. C. Seeger, Prognostic importance of serum ferritin in patients with Stages III and IV neuroblastoma: the Childrens Cancer Study Group experience, Cancer Res., 1985, 45(6), 2843–2848 Search PubMed.
- J. Min, Y. Wu, S. Huang, Y. Li, X. Lv, R. Tang, H. Zhao and J. Wang, Serum cholesterol level as a predictive biomarker for prognosis of Neuroblastoma, BMC Pediatr., 2024, 24(1), 205, DOI:10.1186/s12887-024-04700-7 , From NLM Medline.
- A. Pluddemann, M. Thompson, C. P. Price, J. Wolstenholme and C. Heneghan, Point-of-care testing for the analysis of lipid panels: primary care diagnostic technology update, Br. J. Gen. Pract., 2012, 62(596), e224–e226, DOI:10.3399/bjgp12X630241 , From NLM Medline.
- C. Qi, L. Wang and G. Duan, Preoperative neutrophil-to-lymphocyte ratio (NLR) as a prognostic biomarker for patients with high-risk neuroblastoma, Asian J. Surg., 2023, 46(6), 2474–2475, DOI:10.1016/j.asjsur.2022.12.069 , From NLM Medline.
- C. Qi, L. Wang and G. Duan, Nutritional and inflammatory immune-based hemoglobin, albumin, lymphocyte, and platelet (HALP) score as a prognostic biomarker in patients with high-risk neuroblastoma, Asian J. Surg., 2023, 46(9), 4068–4069 CrossRef PubMed.
- F. M. Balis, C. M. Busch, A. V. Desai, E. Hibbitts, A. Naranjo, R. Bagatell, M. Irwin and E. Fox, The ganglioside GD2 as a circulating tumor biomarker for neuroblastoma, Pediatr. Blood Cancer, 2020, 67(1), e28031 CrossRef PubMed.
- M. Morini, S. Barco, M. Ardito, A. Cafaro, F. Pigliasco, L. Rossi, M. Fragola, D. Segalerba, M. Conte and A. Garaventa, Detection of plasma circulating GD2 ganglioside in patients with neuroblastoma and age-matched healthy children. Diagnostic and prognostic evaluation, Oncologist, 2025, 30(2), oyaf008 CrossRef PubMed.
- A. Eggert, N. Ikegaki, X.-g. Liu and G. Brodeur, Prognostic and biological role of neurotrophin-receptor TrkA and TrkB in neuroblastoma, Klin. Padiatr., 2000, 212(04), 200–205 CrossRef PubMed.
- S. Maher, K. Wynne, V. Zhernovkov and M. Halasz, A temporal (phospho-) proteomic dataset of neurotrophic receptor tyrosine kinase signalling in neuroblastoma, Sci. Data, 2024, 11(1), 1111 CrossRef PubMed.
- T. Tanaka, E. Hiyama, T. Sugimoto, T. Sawada, M. Tanabe and N. Ida, trk A gene expression in neuroblastoma. The clinical significance of an immunohistochemical study, Cancer, 1995, 76(6), 1086–1095 CrossRef PubMed.
- K. Haney, P. Tandon, R. Divi, M. R. Ossandon, H. Baker and P. C. Pearlman, The Role of Affordable, Point-of-Care Technologies for Cancer Care in Low- and Middle-Income Countries: A Review and Commentary, IEEE J. Transl. Eng. Health Med., 2017, 5, 2800514, DOI:10.1109/jtehm.2017.2761764 , From NLM.
- H. Hjelmgren, B. M. Ygge, B. Nordlund and N. Andersson, Nurses' experiences of blood sample collection from children: a qualitative study from Swedish paediatric hospital care, BMC Nurs., 2022, 21(1), 62, DOI:10.1186/s12912-022-00840-2 , From NLM.
- D. J. Harney and M. Larance, The Small-Protein Enrichment Assay (SPEA) for Analysis of Low Abundance Peptide Hormones in Plasma, Methods Mol. Biol., 2023, 2628, 265–276, DOI:10.1007/978-1-0716-2978-9_17 , From NLM.
- K. Omidfar, F. Riahi and S. Kashanian, Lateral Flow Assay: A Summary of Recent Progress for Improving Assay Performance, Biosensors, 2023, 13(9), 837, DOI:10.3390/bios13090837 , From NLM.
- G. Dobrovoljski, R. Kerbl, C. Strobl, W. Schwinger, H. J. Dornbusch and H. Lackner, False-positive results in neuroblastoma screening: the parents' view, J. Pediatr. Hematol./Oncol., 2003, 25(1), 14–18, DOI:10.1097/00043426-200301000-00005 , From NLM.
- B. H. Chew, Planning and Conducting Clinical Research: The Whole Process, Cureus, 2019, 11(2), e4112, DOI:10.7759/cureus.4112 , From NLM.
- K. K. Matthay, Stage 4S neuroblastoma: what makes it special?, J. Clin. Oncol., 1998, 16(6), 2003–2006 CrossRef PubMed.
- C. M. Chu, D. D. Rasalkar, Y. J. Hu, F. W. Cheng, C. K. Li and W. C. Chu, Clinical presentations and imaging findings of neuroblastoma beyond abdominal mass and a review of imaging algorithm, Br. J. Radiol., 2011, 84(997), 81–91, DOI:10.1259/bjr/31861984 , From NLM.
- F. Shawraba, H. Hammoud, Y. Mrad, Z. Saker, Y. Fares, H. Harati, H. F. Bahmad and S. Nabha, Biomarkers in Neuroblastoma: An Insight into Their Potential Diagnostic and Prognostic Utilities, Curr. Treat. Options Oncol., 2021, 22(11), 102, DOI:10.1007/s11864-021-00898-1 , From NLM.
- H. S. Chan, B. L. Gallie, G. DeBoer, G. Haddad, N. Ikegaki, J. Dimitroulakos, H. Yeger and V. Ling, MYCN protein expression as a predictor of neuroblastoma prognosis, Clin. Cancer Res., 1997, 3(10), 1699–1706 CAS , From NLM.
- L. Anfossi, F. Di Nardo, S. Cavalera, C. Giovannoli and C. Baggiani, Multiplex lateral flow immunoassay: an overview of strategies towards high-throughput point-of-need testing, Biosensors, 2019, 9(1), 2 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |