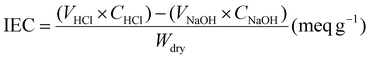Open Access Article
Open Access ArticleModification strategies for cellulose-based anion exchange membranes†
Chen
Cheng‡
a,
Siyuan
Zhu‡
ab,
Chichong
Lu
 *c and
Zhichuan J.
Xu
*c and
Zhichuan J.
Xu
 *a
*a
aSchool of Materials Science and Engineering, Nanyang Technological University, Singapore. E-mail: xuzc@ntu.edu.sg
bSchool of Energy Storage Science and Engineering, North China University of Technology, Beijing 100048, China
cDepartment of Chemistry, College of Light Industry Science and Engineering, Beijing Technology and Business University, Beijing 100048, China. E-mail: luchichong@btbu.edu.cn
First published on 11th June 2025
Abstract
Sustainable biomass materials are at the forefront of green technologies across energy conversion, water purification and chemical processes. These materials, which are derived from renewable resources, present distinct advantages of cost-effectiveness, tunable properties and biodegradability with advances in nanotechnology and molecular simulations. Cellulose is the most plentiful biomass material and has garnered significant attention for its excellent mechanical strength, chemical stability, and biocompatibility. Compared with traditional petroleum-based polymers, the affordability and reduced carbon footprint of cellulose resonate better with the demand of anion exchange membrane (AEM) development. In this review, we focus on the progress of cellulose-based AEMs and discuss whether they have the potential to be disruptive cross-disciplinary materials. From the perspective of the cellulose molecular structure, we expound on its distinctive advantages and the inherent challenges encountered in AEM applications. To address these challenges, improved strategies including chemical modification, cross-linking and compounding of cellulose, along with membrane-forming methods, are discussed. Moreover, we emphasize the importance of strategic modifications and rational composite design for optimizing the performance of cellulose-based AEMs. This facilitates the phasing out of petroleum-based materials with renewable alternatives.
Green foundation1. This review focuses on the advancement of utilizing eco-friendly and extensively sourced cellulose materials in anion exchange membranes. A detailed examination and discussion regarding the key properties in current applications are carried out, and all the existing optimization strategies are recapitulated.2. Owing to its distinctive structure and abundant reserves, cellulose has increasingly become the focus of researchers. They are exploring its applications as anion exchange membranes across multiple fields. The low-cost nature and renewability of cellulose make it a potential substitute for traditional petroleum-based polymer materials. 3. The insights presented in this review offer directions and systematic approaches for the future development of advanced techniques related to cellulose modification and membrane formation. This aims to facilitate the green substitution of traditional materials by cellulose. |
1. Introduction
Cellulose materials represent the bulk of terrestrial biomass and could be repurposed as promising alternatives to fossil carbon. They exhibit green and sustainable characteristics throughout their life cycle, aligning with the principles of a circular economy. This is increasingly significant as the focus on sustainable solutions continues to grow.1,2 The exploration of biomass materials has unveiled their transformative potential in key areas such as energy conversion,3,4 water purification,5–7 and chemical processes.8 In particular, their role in energy conversion as direct and indirect renewable energy sources, as well as electrode and electrolyte materials, offers an alternative to traditional materials that are both expensive and inefficient. In the development and application of renewable energy, anion exchange membranes (AEMs) are critical as ion-selective conductive channels in energy conversion technologies, such as water electrolysis and fuel cells.9–13 Owing to the superior reaction stability and catalytic activity exhibited by nonprecious metal catalysts under alkaline conditions, AEM-based systems offer a much more cost-effective alternative to precious metal catalyst systems. Therefore, finding an appropriate AEM material that combines superior performance, cost-effectiveness, and eco-friendliness has long been a pivotal challenge in AEM applications.The structure of AEMs is usually composed of a strong polymer backbone and functional anion exchange groups.14 The polymer backbone provides the necessary mechanical strength, whereas the anion exchange groups are responsible for conducting hydroxide ions. Currently, the most widely used polymers include polyaryl ether materials such as polysulfone,15 polyether ketone16 and polyphenylene ether,17 which are widely used because of their high stability and mechanical strength. However, these artificial polymers are derived from petroleum-based compounds, which are nonrenewable resources, and their manufacturing processes are typically associated with high energy consumption and greenhouse gas emissions. The complex synthesis procedures and high production costs further limit their ability to meet the requirements of a sustainable circular economy. As a result, the use of biomass materials to replace artificial polymers in the preparation of AEMs plays a vital role in supporting the environmentally friendly advancement of this technology.18
Cellulose, the most abundant natural polymer in the world, is characterized by its broad availability, renewability, low cost, and environmental friendliness, making it highly valuable for applications in biomass materials. Owing to its unique molecular structure and abundant active functional groups, cellulose has excellent mechanical strength and outstanding potential for chemical modification.19 The inherent biodegradability and low environmental impact of cellulose make it an ideal choice for substituting petroleum-based materials, facilitating carbon footprint reduction and driving the sustainable development of the renewable energy sector.
As depicted in Fig. S1,† although research on cellulose-based anion exchange membranes (AEMs) originated relatively early, it garnered limited attention during its initial stages, with most early investigations focused on biological applications. However, over the past five years, propelled by the rise of renewable energy technologies and advancements in related preparation processes, there has been a marked surge in publications addressing cellulose-based AEMs, which are now predominantly centered on energy and chemical engineering-related fields. This shift reflects the growing recognition among researchers of the potential of cellulose-based AEMs in applications such as sustainable energy conversion and storage systems, aligning with the increasing demand for environmentally friendly and efficient materials in addressing global energy challenges.
In this review, from the perspective of the molecular structure of cellulose, we thoroughly discuss the merits and limitations of using cellulose as a fundamental material for AEMs. By incorporating the latest research advancements, we discuss modification approaches that target the key performance challenges faced by cellulose-based AEMs. Finally, we propose a range of future research recommendations to support the expanded utilization of cellulose in the development of AEM materials.
2. Structure of cellulose
As a natural polysaccharide widely distributed in the earth's biosphere, cellulose is considered as one of the most abundant organic compounds.20 It is widely distributed in the cell walls of plants and plays a role in supporting and protecting plant cells. As shown in Fig. 1, the cellulose fibers within the cell wall follow a hierarchical structure, progressing from cellulose molecular chains to microfibrils, and then to macrofibrils, ultimately forming cellulose fibers. The fundamental cellulose molecular chain is a linear polymer of repeated β-D-glucose units connected through β-1,4-glycosidic bonds.21 The unique structure of cellulose endows it with high mechanical stability and specific physicochemical properties. In each glucose unit, there is a primary hydroxyl group (–OH) at the C2 and C3 positions, and a secondary –OH at the C6 position. At each end of the cellulose molecular chain, there is a reducing hemiacetal –OH and a nonreducing terminal –OH, both of which are critical in regulating the degree of degradation and polymerization degree of cellulose. Owing to its abundant –OH, cellulose is highly hydrophilic and reactive, making it capable of reacting with a wide range of chemical reagents to produce various functional derivatives.22,23 For example, through esterification or etherification reactions, cellulose can be modified into cellulose ethers24 or cellulose esters,25,26 which have broad applications in the fields of personal care, pharmaceuticals, and industrial materials.27Through intra- and intermolecular hydrogen bonding interactions, cellulose molecular chains establish highly ordered crystalline regions and relatively disordered amorphous regions.28 The semi-crystalline structure of cellulose endows it with outstanding mechanical properties and thermal stability. The crystalline regions, owing to their tightly packed molecular chains, confer strong mechanical properties and chemical resistance, restrict the absorption of water and solvents, and reduce swelling of the material. Amorphous regions endow cellulose with moderate flexibility and hygroscopic properties, enabling it to maintain a certain level of ductility in specific environments.29
Additionally, different morphologies of cellulose affect their application forms.30 Cellulose fibers generally have a high aspect ratio, enabling them to form stable three-dimensional network structures through physical stacking and intermolecular hydrogen bonding. In particular, for microfibrillated cellulose and nanocellulose, the micro-nano scale fibers expose abundant hydroxyl groups, endowing them with better film-forming properties and higher mechanical strength. Membranes prepared from these materials are widely used in various fields.31–34 However, cellulose nanocrystals have a higher degree of degradation during preparation, which leads to a short rod-like shape of cellulose nanocrystals and makes them difficult to form films directly. Therefore, they are often used as functional additives.
3. Key properties of cellulose-based AEMs
The design and optimization of AEMs require attention to critical performance indicators, such as alkaline stability, ionic conductivity, mechanical strength, ion exchange capacity (IEC), and degree of swelling.35,36 Alkaline stability is essential for ensuring the long-term chemical stability of AEMs in applications such as water electrolysis and fuel cells, making it one of the most critical and challenging criteria. Ionic conductivity reflects the ability of a membrane to conduct anions (e.g., OH−) and the performance sets the upper limit for the overall efficiency and operational lifetime of the electrochemical device system. On the one hand, ionic conductivity is affected by the ion exchange capacity (IEC), which denotes the number of exchangeable anion sites in the AEM. Theoretically, higher IEC should enhance ionic conductivity. On the other hand, ionic conductivity also depends on AEM structure and water content. Excessive IEC may cause increased swelling, compromising dimensional stability and mechanical integrity, thereby destabilizing ion conduction channels and potentially leading to deformation or even rupture of the AEM. High ionic conductivity ensures efficient ion transport, while metrics such as ionic conductivity, mechanical stability, chemical resistance, and crossover suppression contribute to the long-term operation and durability of the device. These properties are intricately linked and do not have a simple positive correlation. Consequently, the design of cellulose-based AEMs requires striking an effective balance among these properties based on the specific application requirements of the membrane to meet diverse functional demands.3.1 Alkaline stability
AEMs are generally operated under alkaline conditions, resulting in a high concentration of hydroxide ions (OH−) within the membrane. Generally, cellulose maintains good stability in alkaline solutions. In the actual production of cellulose nanofibrils, cellulose raw material is sometimes alkali-treated with a 20 wt% (≈6 M) sodium hydroxide solution to increase the thermal stability of the cellulose fibers. However, the minimum service life of AEMs is no less than several hundred hours. During prolonged immersion, the alkaline solution gradually penetrates the amorphous regions of the cellulose molecular chains, leading to significant swelling and possible degradation of cellulose-based AEMs.Alkaline degradation: in Fig. 2, it can be seen that OH− can nucleophilically attack the β-1,4-glycosidic bonds within the cellulose chains, leading to bond cleavage and a reduction in molecular weight. This is similar to the alkaline degradation mechanism observed in AEMs with an aryl ether backbone. However, such degradation mechanisms typically occur in strongly alkaline solutions at temperatures above 140 °C, and thus are not the primary cause of alkaline degradation in cellulose-based AEMs.37
Peeling and ending reactions: in alkaline media, the reducing glucose end group of cellulose undergoes multiple isomerization processes, resulting in the migration of the carbonyl group along the carbon chain. Upon reaching certain positions, the carbonyl group undergoes elimination reactions, causing glucose units to be released from the molecular chain one by one. This depolymerization process, known as the peeling reaction, is a critical factor behind the low alkaline stability of cellulose. The key reaction steps in this reaction are shown in Fig. 3.
 | ||
| Fig. 3 Various reaction mechanisms involved in the peeling and chemical stopping reactions of cellulose. | ||
In Fig. 4, it can be seen that the peeling reaction begins with keto–enol tautomerization of the reducing glucose end group (2), yielding an enediol that deprotonates to form an enolate (3), which in turn promotes β-alkoxycarbonyl elimination at C4 (4). After the elimination reaction at C4, a hexose monomer unit detaches from the cellulose molecular chain, allowing the next glucose end group to continue the reaction (5). If the elimination reaction does not take place at C4, the hexose monomer unit will remain attached to the cellulose chain, resulting in the termination of depolymerization, which is called the chemical stopping reaction. After each elimination reaction, a diketone intermediate forms, which then rearranges through a benzoic acid mechanism to yield the final product. The two most common final products produced by the peeling reaction and the chemical stopping reaction are the two diastereomers isosaccharinic acid (ISA) and metasaccharinic acid (MSA), respectively.38
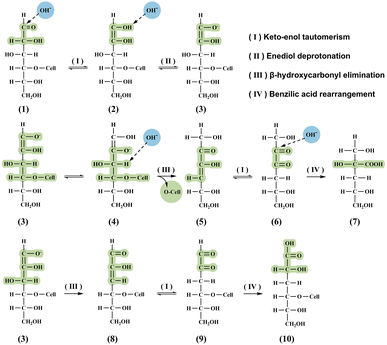 | ||
| Fig. 4 Cellulose reactions and the main reaction products in alkaline media: (second row) peeling reaction path and (third row) chemical stopping reaction; ISA (7) and MSA (10). | ||
Consequently, stabilizing the reducing end group to reduce chain depolymerization is essential for enhancing the alkaline stability of cellulose-based AEMs.
3.2 Ionic conductivity
The ionic conductivity of AEMs is closely related to the transport characteristics of anions.39 In general, anion transport mechanisms in AEMs are similar to the proton transport mechanisms in PEMs, which are based on two types: the vehicular mechanism and the Grotthuss mechanism.40,41 As shown in Fig. 5, in the vehicular mechanism, hydrated hydroxide ions are transported within the membrane under the influence of factors such as the electric field, pressure, and concentration, primarily through diffusion and convection mechanisms. In the Grotthuss mechanism, hydroxide ions are transported through the hydrogen bond network by forming and breaking covalent bonds with H2O molecules. Under low hydration conditions, anion transport in AEMs is predominantly driven by the vehicular mechanism, with hydroxide ions migrating slowly through the bulk movement of H2O molecules. In contrast, under high hydration conditions, the stable and highly interconnected hydrogen bond network within the membrane supports the efficient function of the Grotthuss mechanism., allowing hydroxide ions to be transported rapidly via a hopping process. Thus, the degree of hydration of AEMs is crucially important for their ionic conductivity.The cellulose molecular chain is rich in hydrophilic polar –O which establish stable hydrogen bond networks with H2O molecules, providing a uniform hydration environment that favors the efficient conduction of hydroxide ions. Nevertheless, –OH ions do not possess ion-exchange functionality, making them unable to selectively adsorb or transport hydroxide ions, leading to low hydroxide ion mobility even with a high degree of membrane hydration. To improve the overall ionic conductivity of cellulose-based AEMs, it is necessary to employ a grafting modification to replace part of –OH on the cellulose molecular chain with ion-exchange functional groups.
3.3 IEC, mechanical strength and swelling ratio
The IEC corresponds to the number of counterion equivalents in a specified quantity of a material. Acid–base titration is a commonly used IEC test method in AEMs and the calculation is based on the following formula:42where VHCl and CHCl represent the volume and concentration of the initial HCl solution, respectively; VNaOH and CNaOH represent the volume and concentration of the NaOH solution consumed for titration; and the dry weight of AEM is Wdry. The IEC value is directly proportional to the quantity of ion exchange groups in the membrane. Thus, the grafting of ion exchange groups onto the –OH of cellulose-based molecular chains can effectively increase the IEC of AEMs, thereby improving their ionic conductivity. However, extensive modification of –OH results in the degradation of two other properties.
One issue is that the mechanical strength of the membrane can be adversely affected. In cellulose-based AEMs, the mechanical strength not only is derived from the three-dimensional interwoven structure created by the physical entanglement of cellulose fibers but is also more significantly attributed to the intramolecular and intermolecular hydrogen bonding interactions formed by the abundant –OH.43 The other issue is the increased swelling ratio of the AEM. Effective hydration is one of the key conditions for the rapid migration of hydroxide ions, so most ion exchange groups exhibit excellent hydrophilicity. The substantial introduction of hydrophilic groups increases the swelling ratio of the AEM, increasing the migration length for hydroxide ions and diluting the density of ion exchange groups, ultimately resulting in reduced ionic conductivity. Therefore, in the design of cellulose-based AEMs, it is necessary to harmonize the relationships among the IEC, mechanical strength, and swelling ratio, ensuring high mechanical strength and low swelling while striving to maximize IEC.
4. Improvement strategies
The unique molecular structure and physicochemical properties of cellulose reveal that, although the abundant –OH provide cellulose-based AEMs with good mechanical strength and hydrophilicity, cellulose alone struggles to achieve the long-term alkaline stability which is in demand in practical AEM applications. Moreover, owing to the lack of ion exchange groups, cellulose-based AEMs are unable to efficiently transport anions, even under highly hydrated conditions. Thus, chemical modification, cross-linking, and inorganic doping are necessary to tailor cellulose for AEM applications.4.1 Functionalization with anion exchange groups
The ionic conductivity of AEMs can be effectively improved by introducing the corresponding anion exchange groups such as quaternary amine groups,44 quaternary phosphine functional groups,45,46 imidazolium groups,47 guanidine functional groups48 and pyridinium groups.49 Quaternary amine groups stand out because of their efficient anion exchange capacity, excellent chemical stability and relatively simple synthesis process, making them among the most widely used anion exchange groups. Unlike petroleum-based AEMs, which rely on chloromethylation reactions during quaternization, the plentiful –OH in cellulose allows for the use of a gentler quaternization process, avoiding the use of hazardous chloromethylating agents and minimizing the risk of cellulose degradation under strong acidic conditions. In Table 1, it can be seen that quaternary ammonium functionalization serves as an example to outline the advantages and disadvantages of using nucleophilic substitution reactions, ring-opening addition reactions and silane coupling reactions to modify the –OH in cellulose.| Grafting method | Reaction process | Advantages | Disadvantages |
|---|---|---|---|
| Nucleophilic substitution reactions | Tosylation | –OTs is an excellent leaving group; gentle reaction conditions | The reaction is complex and generates acidic by-products. Particularly, TsCl is a corrosive lachrymatory agent that produces toxic and corrosive fumes when heated |
| The –OH ions react with p-toluenesulfonyl chloride (TsCl) to form a p-toluenesulfonate ester (–OTs), which later leaves through nucleophilic substitution reactions | |||
| Alkylation reaction | Simple reaction steps; suitable for a variety of quaternary ammonium compounds | The reaction suffers from low efficiency, and alkyl halides generally exhibit high volatility and toxicity. Furthermore, hydrogen halides generated from the reaction may corrode equipment, necessitating additional neutralization steps, which increases the cost of waste liquid treatment | |
| The –OH ions react with alkyl halides carrying quaternary amine groups | |||
| Silane coupling reactions | Hydrolysis of silanizing reagents produces silanol groups (–Si–OH) that combine with the –OH on cellulose, forming Si–O–C bonds and yielding stable silanized products | Helps to form organic/inorganic composite materials and microphase separation structures; the Si–O–C bonds have high chemical stability | The hydrolysis and coupling reactions of silanes are difficult to precisely control, and their large molecular structures simultaneously create steric hindrance, which restricts the grafting density. Furthermore, silanized reagents containing quaternary ammonium groups typically require multistep synthesis, and the high cost of raw materials limits their large-scale application |
| Ring-opening addition reactions | The –OH ions act as nucleophiles to attack the epoxy group, causing it to open and form an ether bond (–O–), thereby grafting the quaternary ammonium group onto the cellulose34 | Easy to control the length of the grafted side chain; helps to form a microphase separation structure | The reaction is contingent upon the activity of epoxy reagents and is prone to side reactions. Furthermore, some epoxy reagents (such as epichlorohydrin) are highly toxic and irritating, necessitating strict safety precautions during their production and use |
On the cellulose molecular chain, the oxygen atom in –OH has two lone pairs of electrons, endowing it with significant nucleophilicity. As shown in Fig. 6, during the tosylation reaction, a highly reactive group of –OTs is initially introduced to enable its replacement in the next step by nucleophilic reagents carrying quaternary ammonium groups. Schmitt et al. used TsCl and cellulose to synthesize cellulose p-toluenesulfonate, and then used diazabicyclo [2.2.2] octane (DABCO) to replace –OTs to introduce a quaternary ammonium group.50 In the alkylation reaction, the –OH attacks the C on the alkyl halide, which can directly introduce the quaternary ammonium compound on the cellulose. Ho et al. successfully obtained cationized nanofibrillated cellulose containing trimethylammonium groups by reacting chlorocholine chloride (ClChCl) with cellulose.51 Kandil et al. first carried out chlorination treatment on cellulose powder using thionyl chloride (SOCl2) to prepare deoxy chlorinated cellulose, and then conducted a quaternization reaction with dimethylamine to prepare quaternized dimethylamine cellulose (QDC).52
 | ||
| Fig. 6 Reaction process of several methods of grafting quaternary amine groups onto cellulose molecular chains. | ||
However, in nucleophilic substitution, the introduced anion exchange groups are close to the cellulose backbone, which leads to steric hindrance and thus reduces the degree of substitution. In the case of the reaction between cellulose p-toluenesulfonate and DABCO, steric hindrance was found to inhibit the complete replacement of –OTs with the tertiary amines, even when DABCO was used in excess. On the other hand, oversubstitution leads to decreased mechanical strength of the membrane. Quaternary ammonium-grafted DABCO-cellulose is slightly water-soluble and cannot establish a stable membrane structure without cross-linking agents, which limits its practicality.
When the –OH groups on cellulose act as nucleophiles and attack quaternary ammonium-containing epoxide compounds, long alkyl chains can be grafted onto the cellulose via the addition of ring opening. Yunphuttha et al. added glycidyltrimethyl ammonium chloride (GAC) to a sodium hydroxide solution containing cellulose, leveraging the high reactivity of the epoxide ring to produce quaternized cellulose with long side chains.53 This strategy positions the hydrophilic anion exchange groups away from the cellulose backbone, reducing the risk of backbone degradation. Additionally, it facilitates the formation of a hydrophilic/hydrophobic microphase separation structure within the membrane, enhancing the ionic conductivity while maintaining a low swelling ratio.
Coupling hydroxyl groups with quaternary ammonium-functionalized silanes is another commonly used strategy for the introduction of quaternary ammonium groups. Cao et al. used Si–OH groups formed from the hydrolysis of n-trimethoxysilylpropyl-n,n,n-trimethylammonium chloride (TPC), which reacts with the hydroxyl groups on cellulose to form Si–O–C bonds, ultimately resulting in stable quaternized cellulose.54 The silane coupling reaction also facilitates the formation of a microphase separation structure within the membrane. Interactions among multiple siloxane bonds form physical crosslinks, decreasing the swelling ratio and enhancing the mechanical robustness of the membrane.
Based on the above grafting methods, although nucleophilic substitution reactions feature mild reaction conditions and easy process control, they involve the use of toxic reagents (such as p-toluenesulfonyl chloride and quaternary ammonium halides), generate numerous by-products, and pose a high risk of pollution. Silane coupling reactions yield products with high stability and aqueous-phase reactions can reduce organic reagent pollution. However, silane synthesis is complex and energy-intensive. Ring-opening addition reactions offer high efficiency and no halogen pollution, from the perspectives of green chemistry and industrial production; ring-opening addition is more likely to meet industrial requirements compared to the other two methods. However, it is also necessary to optimize the synthetic routes to prepare greener epoxy reagents.
In addition to traditional chemical synthesis, researchers often use new grafting methods. Qian et al. grafted the monomer glycidyl methacrylate containing active epoxy groups onto the surface of commercial RC membranes via surface-initiated atom transfer radical polymerization (SI-ATRP), and then derivatized it with diethylamine to produce high-capacity AEMs.55 This method achieves precise control of the polymer molecular weight and structure through controllable free radical generation and chain growth processes,56,57 have high grafting efficiency and reduces the use of organic solvents. However, decomposition products of some initiators and quaternary ammonium monomers are toxic, necessitating strict control of the reaction process and the establishment of a sound recycling system. Samaniego et al. used gamma radiation to graft vinylbenzyl chloride on the surface of a cellulose acetate membrane, and further immersed it in trimethylamine to introduce quaternary amine groups. This method reduces the use of chemical reagents, and the ionic conductivity of the prepared B25-AEM can be similar to that of the commercial AEM Tokuyama A201 at a lower IEC.58 A decrease in swelling helps maintain the structural stability and mechanical strength of the membrane, thereby reducing the cost of membrane assembly. However, the high energy consumption of radiation sources and the high cost of equipment restrict the industrial scale-up of this process.
4.2 Cross-linking
Although anion exchange group functionalization effectively enhances the IEC of cellulose-based AEMs, the substantial introduction of hydrophilic groups increases the swelling ratio, which increases the risk of damage to the mechanical strength and structural stability of AEMs. It also increases the risk of the cellulose backbone and anion exchange groups being degraded through nucleophilic attack by OH−. In Fig. 7, it can be seen that compared with other anion exchange groups, quaternary amine groups have better alkali stability, but their high hydration capacity still makes them vulnerable to OH− attack and degradation reactions: (1) α-nucleophilic substitution, (2) the Hofmann elimination and (3) formation of N-ylide intermediates.59 Fortunately, the abundant hydroxyl groups in cellulose increase its ability to undergo physical and chemical cross-linking with various materials. In particular, for cellulose at the nano-micro scale, through the synergistic effect of fiber entanglement and crosslinking agents, it promotes the construction of a three-dimensional network structure.60,61 Through cross-linking agents, cellulose can form a stable network structure with other polymers that are also rich in –OH groups, which can not only effectively improve the mechanical strength of the membrane, but also reduce the swelling rate, protect the anion exchange groups in the membrane, and improve the alkali stability of the membrane.62,63Hren et al. grafted poly (diallyl dimethylammonium chloride) (PDDA) onto cellulose to obtain quaternary ammonium cellulose, which was added to a chitosan solution and cast into a membrane.64 The mechanical strength and thermal stability of the composite membrane are improved by the hydrogen bonding and electrostatic interactions formed between quaternary ammonium cellulose and chitosan. However, such physical cross-linking is not stable under high hydration conditions, resulting in an elevated swelling ratio. Additionally, the interactions between molecules are highly dependent on noncovalent bonds, which may diminish the ion exchange capacity of the composite membrane. Thus, chemical cross-linking is widely adopted to enhance the diverse properties of cellulose-based AEMs.
As shown in Fig. 8(a), PDDA and bacterial cellulose (BC) fibers form a stable crosslinked network through glutaraldehyde, capturing the quaternary ammonium groups within the polymer framework of BC and thereby protecting the cationic groups. When BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDDA = 1
PDDA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, the material exhibited the best tensile strength and the highest ion conductivity.65 Functionalized cellulose can also be added as a reinforcing agent to the polymer matrix. By cross-linking quaternized cellulose (QC) with the polysulfone (PSF) backbone with dibromobutane, QC effectively enhanced the mechanical properties of the QPSF matrix (4 times under wet conditions). The stable cross-linking network endows the composite with excellent anti-swelling ability. Even if the water absorption rate reached 80.47%, the swelling rate of the membrane was less than 20%. Compared with the noncrosslinked QC/QPSF membrane which disintegrated within just 5 h of immersion, the crosslinked QC/QPSF membrane had good structural stability even in strongly polar N-methylpyrrolidone solution at 80 °C.66 Cellulose acetate (CA) is a membrane material widely used for industrial water purification and gas separation.67 Zhao et al. introduced an anionic isocyanate crosslinker with carboxyl groups to crosslink CA and quaternized cellulose nanocrystals (QNC). Thus, the CMQ membrane was prepared with mixed-charge zwitterionic properties for drinking water purification. The rigid QNC not only increased the tensile strength of the CA membrane by 2.2 times, but the mixed-charge zwitterionic properties formed with the crosslinker endowed the CMQ membrane with excellent hydrophilicity and antifouling properties, effectively enhancing its stability.68
0.5, the material exhibited the best tensile strength and the highest ion conductivity.65 Functionalized cellulose can also be added as a reinforcing agent to the polymer matrix. By cross-linking quaternized cellulose (QC) with the polysulfone (PSF) backbone with dibromobutane, QC effectively enhanced the mechanical properties of the QPSF matrix (4 times under wet conditions). The stable cross-linking network endows the composite with excellent anti-swelling ability. Even if the water absorption rate reached 80.47%, the swelling rate of the membrane was less than 20%. Compared with the noncrosslinked QC/QPSF membrane which disintegrated within just 5 h of immersion, the crosslinked QC/QPSF membrane had good structural stability even in strongly polar N-methylpyrrolidone solution at 80 °C.66 Cellulose acetate (CA) is a membrane material widely used for industrial water purification and gas separation.67 Zhao et al. introduced an anionic isocyanate crosslinker with carboxyl groups to crosslink CA and quaternized cellulose nanocrystals (QNC). Thus, the CMQ membrane was prepared with mixed-charge zwitterionic properties for drinking water purification. The rigid QNC not only increased the tensile strength of the CA membrane by 2.2 times, but the mixed-charge zwitterionic properties formed with the crosslinker endowed the CMQ membrane with excellent hydrophilicity and antifouling properties, effectively enhancing its stability.68
 | ||
| Fig. 8 (a) PDDA and BC matrix membranes form a network structure through a cross-linking agent glutaraldehyde, reproduced from ref. 65 with permission from Elsevier; (b) transport mechanisms proposed for hydroxide ions in QPVA/QNC composite AEMs based on (a) Grotthuss hopping and (b) vehicular mechanisms, reproduced from ref. 53 with permission from Springer Nature; and (c) the ion transport process and cross-linked network in the PBC/QPPO membranes, reproduced from ref. 69 with permission from American Chemical Society. | ||
Additionally, the structural design of crosslinking networks can further enhance the stability of the membrane. Samaniego et al. immersed an electrospun cellulose diacetate (CDA) mat into a chitosan solution and used glutaraldehyde to crosslink the chitosan component, forming a semi-interpenetrating network (semi-IPN) membrane named CDA-CS. Compared with the uncross-linked CDA membrane, the tensile strength of CDA-CS increased by 95%, and its thermal stability was significantly enhanced.62
Cross-linking between cellulose and polymers not only enhances the strength of the membrane but also reduces the crystallinity of other polymers through intermolecular interactions, thereby facilitating the migration of OH– within the membrane. Viravathana et al. blended QNC to blend with quaternized polyvinyl alcohol (PVA) at an addition amount of 0–25%, and used glutaraldehyde cross-linking to form a composite AEM. By offering additional anion exchange sites and engaging in hydrogen bonding with PVA, QNC reduces the crystallinity of polymers, creating a larger proportion of amorphous regions that enhance OH− transport. As shown in Fig. 8(b), the introduction of the QNC helps form a continuous ion conduction network in the membrane, and significantly improves the conduction efficiency of OH− through the Grotthuss and carrier mechanisms. Moreover, due to the presence of the QNC, the probability of the Hofmann elimination of hexadecyltrimethyl ammonium bromide in AEM is reduced, thereby improving the alkaline stability.53
It should be noted that although cross-linking is beneficial for improving the mechanical strength and dimensional stability of the membrane, excessive cross-linking will make the membrane denser at the microscopic level, limiting the migration of anions and simultaneously increasing the brittleness of the membrane. When the QNC content is increased to 25%, the interactions between PVA and QNC were intensified, thereby resulting in a reduction in the matrix hydrophilicity. Moreover, as a consequence of the aggregation of cationic groups, the membrane exhibits a significant decrease in both ion exchange capacity (IEC) and ionic conductivity. Therefore, reasonable control over the cross-linking degree is essential for balancing various performance characteristics of cellulose-based AEM.
Furthermore, the choice of the cross-linking agent also affects the performance of cellulose-based AEMs. Bifunctional crosslinkers, which are capable of both cross-linking and introducing anion exchange groups, present significant application potential as alternatives to traditional toxic crosslinkers such as glutaraldehyde. Wang et al. functionalized bacterial cellulose-based membranes (PBC) with dopamine hydrochloride, poly(ethylenimine) (BPEI) and glycidyl trimethylammonium chloride (GTA) to prepare a new PBC/quaternized poly (phenylene oxide) (QPPO) composite membrane using Br-PPO as a crosslinker. Compared with the tensile strength of the pure QPPO membrane, which was only 7.14 MPa, the introduction of PBC increased the tensile strength to 53.24 MPa, which was able to hang a weight 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times greater than its own weight (0.021 g). In addition, as shown in Fig. 8(c), BPEI in the crosslinked structure reduced the attack of hydroxide on the ether bonds of the PPO main chain and the quaternary amine groups of the side chains through steric hindrance, increasing the hydroxide ion conductivity of the pure QPPO membrane at 80 °C by 135.93%, and after being immersed in 1 M NaOH at 60 °C for 192 hours, the AEM still maintained 85.9% of its initial hydroxide ion conductivity.69 However, this approach involves the use of bromine-containing crosslinkers, which does not comply with the principles of inherently safer design in green chemistry. Therefore, the development of halogen-free crosslinkers and milder crosslinking reactions is necessary.
000 times greater than its own weight (0.021 g). In addition, as shown in Fig. 8(c), BPEI in the crosslinked structure reduced the attack of hydroxide on the ether bonds of the PPO main chain and the quaternary amine groups of the side chains through steric hindrance, increasing the hydroxide ion conductivity of the pure QPPO membrane at 80 °C by 135.93%, and after being immersed in 1 M NaOH at 60 °C for 192 hours, the AEM still maintained 85.9% of its initial hydroxide ion conductivity.69 However, this approach involves the use of bromine-containing crosslinkers, which does not comply with the principles of inherently safer design in green chemistry. Therefore, the development of halogen-free crosslinkers and milder crosslinking reactions is necessary.
4.3 Organic/inorganic composite AEMs
Researchers have reported that during the preparation of PEMs, dispersing inorganic nanofillers within the polymer matrix can effectively enhance the mechanical strength and thermal stability of the membrane. Additionally, these fillers can create effective pathways for proton transport, such as hydration channels, thereby improving proton conductivity within the membrane and facilitating proton transport under varying humidity and temperature conditions.70,71 Similarly, in cellulose-based AEMs, the introduction of inorganic fillers can significantly improve various properties of composite AEMs. There are two primary methods for introducing inorganic fillers: (1) in situ formation of dispersed inorganic particles within the matrix via the sol–gel technology and (2) the organic and inorganic materials were mixed prior to casting.72 The former method is commonly used for hygroscopic oxides such as titanium dioxide and silicon dioxide. Inorganic particle precursors dissolve in water to form a sol, which is uniformly distributed within the cellulose matrix at the nanoscale, and upon curing, a stable organic/inorganic hybrid membrane is formed. As shown in Fig. 9(a), titanium dioxide (TiO2) is synthesized in situ within BC via the sol–gel method to produce TiO2@BC AEMs, which react with trimethoxysilylpropyl chloride ammonium to graft quaternary ammonium groups. The doping of TiO2 effectively enhances the mechanical properties, dimensional stability, and alkaline resistance of the membrane.73 However, organic–inorganic hybrid AEMs prepared by the sol–gel method are typically porous and require integration with other flexible polymers to form dense membranes. Cao et al. used the inorganic precursor tetraethyl silicate (TEOS) to induce silica mineralization on the surface of BC to obtain SiO2@BC via hydrolysis and condensation reactions.74 Then, unsaturated double bonds were introduced with 3-(trimethoxysilyl) propyl methacrylate (MPS) to SiO2@BC, and finally, the cationic monomer vinylbenzyltrimethylammonium chloride (VBTAC) was used for in situ filling and cross-linking polymerization to prepare the MSiO2@BC-PVD AEM. Owing to the synergistic reinforcement effect of the inorganic SiO2 coated three-dimensional fiber network, the tensile strength of MSiO2@BC-PVD was 95% greater than that of the pure BC/PVD membrane.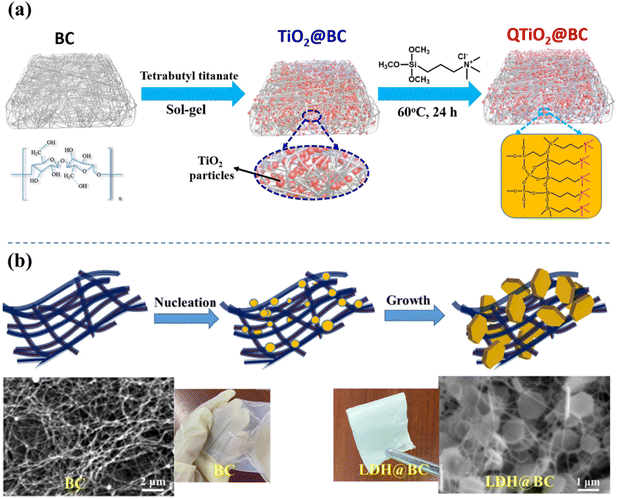 | ||
| Fig. 9 (a) Preparation process of the QTiO2@BC porous substrate by the sol–gel method, reproduced from ref. 73 with permission from Elsevier. (b) Schematic illustration of the preparation of LDH@BC, reproduced from ref. 76 with permission from Elsevier. | ||
In addition to the sol–gel method, inorganic and organic materials are often mixed and then cast into a membrane. Layered double hydroxides (LDHs) are a type of anionic clay composed of positively charged brucite-like layers and interlayer regions filled with charge-balancing anions and solvated molecules. Owing to their intrinsic hydroxide ion conductivity, LDHs are widely employed to increase the ionic conductivity of composite materials.75 In Fig. 9(b), it can be seen that Ni et al. employed BC as a template to develop a 3D nanostructured LDH, which was then embedded in a quaternized CS matrix to prepare a composite AEM. The BC fiber network facilitates the uniform distribution of LDH nanosheets by regulating their dynamic growth process, exposing a greater number of anion exchange sites to support rapid OH− conduction, leading to a 52.5% higher ionic conductivity than that of the QCS/BC. Owing to its synergistic reinforcement effect, the LDH@BC network enhanced the mechanical properties of the composite membrane. For the following reasons: (1) lamellar inorganic LDH nanosheets themselves have ultrahigh strength; (2) the as-prepared LDH@BC, which consists of 2D LDH nanosheets anchored on 3D nanofibers, can play a synergistic role; (3) the hydrogen bond interaction between LDH and the QCS polymer matrix can partly inhibit the migration of QCS molecular chains when the composites are subjected to external force; and the tensile strengths of the QCS/0.25-LDH@BC composite membrane were ∼83.7% and ∼250% greater than that of the QCS/BC and pure QCS membranes, respectively.76
When comparing the two methods, the sol–gel approach enables the regulation of nanoparticle size and reduces agglomeration. Stable interfacial bonding endows it with higher strength. However, its reaction conditions are highly sensitive, and the prolonged gelation time diminishes production efficiency. Additionally, the synthesis of some precursors relies on the petrochemical industry. Thus, the blending method is more suitable for large-scale industrial production. Although nanoparticles are prone to agglomeration during the addition process, this phenomenon can be mitigated by using surface modifiers to promote dispersion. Meanwhile, the blending process is simpler and allows for the introduction of various fillers.
5. Conclusion and outlook
Numerous studies have demonstrated the potential of cellulose as a material for anion exchange membranes, highlighting its advantages such as abundant availability, environmental friendliness, and excellent mechanical strength. The primary long-term challenges for cellulose-based AEMs are their inadequate anion exchange capacity and their chemical instability in strongly alkaline environments. To address these limitations and increase the practical application value of cellulose-based AEMs, various modification techniques such as anion exchange group functionalization, cross-linking, and inorganic doping have been explored. While significant progress has been made, many issues remain to be resolved (Fig. 10).1. In the functionalization of cellulose with anion exchange groups, precisely controlling the density of cationic groups via conventional chemical synthesis methods remains a challenge. This often leads to the aggregation of cationic groups, thereby preventing the formation of continuous hydroxide ion transport pathways. This necessitates the development of advanced and controllable anion functionalization strategies. (1) Through a synergistic strategy of living polymerization and click chemistry, the limitations of traditional random grafting can be overcome to achieve controllable distribution of cationic groups on cellulose molecular chains. Directional functionalization techniques enable precise regulation of grafting sites and densities, and the combination with microwave solid-phase synthesis suppresses side reactions, laying the foundation for constructing a homogenized ion transport network. (2) Attention should be given to developing novel preparation processes to replace traditional toxic solvent-based and high-temperature/high-pressure processes to strike a balance between performance and sustainability. For example, both achieving milder reaction conditions and source reduction of waste and preserving the functionalization efficiency of traditional methods should be considered.
2. The design of multiscale crosslinked networks with ion-conductive channels in cellulose-based AEMs requires simultaneous coordination of IEC, mechanical robustness, and swelling resistance. (1) Incorporating dynamic covalent bonds (e.g., Schiff base or disulfide linkages) to establish adaptive networks capable of autonomously modulating mechanical stress and swelling behaviour; (2) engineering well-defined hydrophilic/hydrophobic microdomains through synergistic integration of nano-structural modulation and controlled phase separation, thereby enabling continuous ion transport pathways; and (3) synergistically combining gradient crosslinking architectures with reaction-induced phase separation techniques to optimize pore interconnectivity and directional ion mobility.
3. To achieve full-life cycle sustainable management of cellulose-based AEMs, integrating green chemistry and industrial ecological design concepts is essential to reduce overall production costs and environmental impacts. (1) Intelligent deconstruction–regeneration technologies, such as ionic liquid-mediated selective decrosslinking processes, should be developed to efficiently and nondestructively separate cellulose matrices from functional groups under mild conditions. This enables the targeted conversion of waste membranes into reconfigurable monomer feedstocks, supporting closed-loop material flow. (2) Raw material traceability models should be established based on fast-growing forests/agricultural waste to enable quality certification and circulation tracking of recycled materials, ensuring consistency in quality control at industrial scales. (3) To support the United Nations Sustainable Development Goals (SDGs), it is essential to develop a unified quantitative framework that systematically integrates atomic economy and biodegradability indicators under the guidance of the twelve principles of green membrane materials and manufacturing processes. This will facilitate the sustainable commercialization of bio-based materials.
To this end, future research should focus on advancing precise and controllable functionalization strategies, multi-scale network structure design, and green circular manufacturing processes to further enhance their overall performance and promote the widespread application of cellulose-based AEMs in the energy, environmental, and chemical industries. The development of cellulose-based AEMs faces multiple bottlenecks, such as difficulties in balancing material performance and cost, poor stability of large-scale processes, and a lack of green production standards. However, with the continuous emergence of new materials and technologies, the potential of cellulose-based AEMs can be gradually harnessed, accelerating the transformation towards green manufacturing and large-scale application.
Conflicts of interest
The authors declare no competing financial or non-financial interests.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This research was supported by the National Research Foundation, Singapore, and A*STAR (Agency for Science, Technology and Research) under its LCER Phase 2 Programme Hydrogen & Emerging Technologies FI, Directed Hydrogen Programme (Award No. U2305D4003). We thank the support from the Youth Research Special Project of NCUT.References
- H. Kargarzadeh, M. Mariano, D. Gopakumar, I. Ahmad, S. Thomas, A. Dufresne, J. Huang and N. Lin, Cellulose, 2018, 25(4), 2151–2189 CrossRef CAS.
- M. Muddasar, A. Beaucamp, M. Culebras and M. N. Collins, Int. J. Biol. Macromol., 2022, 219, 788–803 CrossRef CAS PubMed.
- S. A. Muhmed, N. A. M. Nor, J. Jaafar, A. F. Ismail, M. H. D. Othman, M. A. Rahman, F. Aziz and N. Yusof, Energy, Ecol. Environ., 2019, 5(2), 85–107 CrossRef.
- A. J. Samaniego and R. Espiritu, Green Chem. Lett. Rev., 2022, 15(1), 253–275 CrossRef CAS.
- A. Mautner, K.-Y. Lee, T. Tammelin, A. P. Mathew, A. J. Nedoma, K. Li and A. Bismarck, React. Funct. Polym., 2015, 86, 209–214 CrossRef CAS.
- A. W. Carpenter, C.-F. de Lannoy and M. R. Wiesner, Environ. Sci. Technol., 2015, 49(9), 5277–5287 CrossRef CAS PubMed.
- Z. Karim, D. Georgouvelas, A. Svedberg, S. Monti and A. P. Mathew, Sep. Purif. Technol., 2022, 289, 120745 CrossRef CAS.
- S. Rajesh, C. Crandall, S. Schneiderman and T. J. Menkhaus, ACS Appl. Nano Mater., 2018, 1(7), 3321–3330 CrossRef CAS.
- J. R. Varcoe, P. Atanassov, D. R. Dekel, A. M. Herring, M. A. Hickner, P. A. Kohl, A. R. Kucernak, W. E. Mustain, K. Nijmeijer, K. Scott, T. Xu and L. Zhuang, Energy Environ. Sci., 2014, 7(10), 3135–3191 RSC.
- D. A. Salvatore, C. M. Gabardo, A. Reyes, C. P. O'Brien, S. Holdcroft, P. Pintauro, B. Bahar, M. Hickner, C. Bae, D. Sinton, E. H. Sargent and C. P. Berlinguette, Nat. Energy, 2021, 6(4), 339–348 CrossRef CAS.
- C. Santoro, A. Lavacchi, P. Mustarelli, V. Di Noto, L. Elbaz, D. R. Dekel and F. Jaouen, ChemSusChem, 2022, 15(8), e202200027 CrossRef CAS PubMed.
- T. B. Ferriday and P. H. Middleton, Int. J. Hydrogen Energy, 2021, 46(35), 18489–18510 CrossRef CAS.
- S. Thangarasu and T. H. Oh, Polymers, 2022, 14(23), 5248 CrossRef CAS PubMed.
- R. Yu, H. Yang, X. Yu, J. Cheng, Y. Tan and X. Wang, Int. J. Hydrogen Energy, 2024, 50, 582–604 CrossRef CAS.
- T. Huang, G. He, J. Xue, O. Otoo, X. He, H. Jiang, J. Zhang, Y. Yin, Z. Jiang, J. C. Douglin, D. R. Dekel and M. D. Guiver, J. Membr. Sci., 2020, 597, 117769 CrossRef CAS.
- M. Kumari, J. C. Douglin and D. R. Dekel, J. Membr. Sci., 2021, 626, 119167 CrossRef CAS.
- J. Xue, X. Liu, J. Zhang, Y. Yin and M. D. Guiver, J. Membr. Sci., 2020, 595, 117507 CrossRef.
- A. Sueszer, Preparation of anion exchange membranes from cellulosic sheets, U.S. Patent, 3,714,010, 1973 Search PubMed.
- D. Bekchanov, M. Mukhamediev, D. Eshtursunov, P. Lieberzeit and X. Su, Polym. Adv. Technol., 2023, 35(1), e6207 CrossRef.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44(22), 3358–3393 CrossRef CAS PubMed.
- G. B. Pedersen, L. Blaschek, K. E. H. Frandsen, L. C. Noack and S. Persson, Mol. Plant, 2023, 16(1), 206–231 CrossRef CAS PubMed.
- F. Rol, M. N. Belgacem, A. Gandini and J. Bras, Prog. Polym. Sci., 2019, 88, 241–264 CrossRef CAS.
- S. Kalia, S. Boufi, A. Celli and S. Kango, Colloid Polym. Sci., 2013, 292(1), 5–31 CrossRef.
- H. Y. Choi, J. H. Bae, Y. Hasegawa, S. An, I. S. Kim, H. Lee and M. Kim, Carbohydr. Polym., 2020, 234, 115881 CrossRef CAS PubMed.
- J. You, X. Zhang, Q. Mi, J. Zhang, J. Wu and J. Zhang, Cellulose, 2022, 29(18), 9583–9596 CrossRef CAS.
- G. J. Irvine, S. Rajesh, M. Georgiadis and W. A. Phillip, Environ. Sci. Technol., 2013, 47(23), 13745–13753 CrossRef CAS PubMed.
- R. J. Hickey and A. E. Pelling, Front. Bioeng. Biotechnol., 2019, 7, 45 CrossRef PubMed.
- M. Wohlert, T. Benselfelt, L. Wågberg, I. Furó, L. A. Berglund and J. Wohlert, Cellulose, 2021, 29(1), 1–23 CrossRef.
- S. Wang, A. Lu and L. Zhang, Prog. Polym. Sci., 2016, 53, 169–206 CrossRef CAS.
- R. Höfer, Nano-sized Materials in Paper and Food Packaging Barrier Coating, 2024 Search PubMed.
- Y. Matsuo, T. Kimura, H. Koyanagi, N. Netsu, F. Komatsu, T. Nagata, S. Nishitsuji, J. Matsui and A. Masuhara, Green Chem., 2025, 27(13), 3532–3541 RSC.
- M. H. Abdellah, C. Oviedo and G. Szekely, J. Membr. Sci., 2023, 687, 122040 CrossRef CAS.
- D. T. Nayak, V. K. Raja, G. Arthanareeswaran, T. D. Khoa and W. Taweepreda, RSC Sustainability, 2025, 3(4), 1966–1981 RSC.
- D. G. Oldal, F. Topuz, T. Holtzl and G. Szekely, ACS Sustainable Chem. Eng., 2023, 11(3), 994–1005 CrossRef CAS.
- W. You, K. J. T. Noonan and G. W. Coates, Prog. Polym. Sci., 2020, 100, 101177 CrossRef CAS.
- G. Sriram, K. Dhanabalan, K. V. Ajeya, K. Aruchamy, Y. C. Ching, T. H. Oh, H.-Y. Jung and M. Kurkuri, J. Mater. Chem. A, 2023, 11(39), 20886–21008 RSC.
- T. Hosoya, M. Bacher, A. Potthast, T. Elder and T. Rosenau, Cellulose, 2018, 25(7), 3797–3814 CrossRef CAS.
- D. J. Mozdyniewicz, K. Nieminen and H. Sixta, Cellulose, 2013, 20(3), 1437–1451 CrossRef CAS.
- R. Vera, L. Gelde, E. Anticó, M. V. Martínez de Yuso, J. Benavente and C. Fontàs, J. Membr. Sci., 2017, 529, 87–94 CrossRef CAS.
- G. Merle, M. Wessling and K. Nijmeijer, J. Membr. Sci., 2011, 377(1–2), 1–35 CrossRef CAS.
- V. Elumalai and D. Sangeetha, J. Power Sources, 2019, 412, 586–596 CrossRef CAS.
- F. Karas, J. Hnát, M. Paidar, J. Schauer and K. Bouzek, Int. J. Hydrogen Energy, 2014, 39(10), 5054–5062 CAS.
- C. Shao, K. Shi, Q. Hua, L. Zhang, Y. Dai, W. You, Y. Liu, C. Li and C. Zhang, J. Mol. Model., 2018, 24(6), 1–11 CrossRef CAS PubMed.
- X. Chu, Y. Shi, L. Liu, Y. Huang and N. Li, J. Mater. Chem. A, 2019, 7(13), 7717–7727 CAS.
- K. M. Meek, J. R. Nykaza and Y. A. Elabd, Macromolecules, 2016, 49(9), 3382–3394 CAS.
- X. Cheng, J. Wang, Y. Liao, C. Li and Z. Wei, ACS Appl. Mater. Interfaces, 2018, 10(28), 23774–23782 CAS.
- K. M. Hugar, H. A. t. Kostalik and G. W. Coates, J. Am. Chem. Soc., 2015, 137(27), 8730–8737 CAS.
- X. Gong, X. Yan, T. Li, X. Wu, W. Chen, S. Huang, Y. Wu, D. Zhen and G. He, J. Membr. Sci., 2017, 523, 216–224 CAS.
- Z. Sun, B. Lin and F. Yan, ChemSusChem, 2018, 11(1), 58–70 CAS.
- F. Schmitt, R. Granet, C. Sarrazin, G. Mackenzie and P. Krausz, Carbohydr. Polym., 2011, 86(1), 362–366 CAS.
- T. T. T. Ho, T. Zimmermann, R. Hauert and W. Caseri, Cellulose, 2011, 18(6), 1391–1406 CAS.
- U. Kandil, E. Taha, E. Mahmoud, M. Mahmoud and M. Taha, Egypt. J. Chem., 2022, 65(10), 47–56 Search PubMed.
- C. Yunphuttha, S. Midpanon, D. W. M. Marr and P. Viravathana, Cellulose, 2024, 31(3), 1569–1601 CAS.
- M. Cao, S. Nie, J. Wang, Q. Zhang, Z. Xu, C. Gong and H. Liu, J. Nat. Fibers, 2023, 20(1), 2181272 CrossRef.
- X. Qian, H. Fan, C. Wang and Y. Wei, Appl. Surf. Sci., 2013, 271, 240–247 CAS.
- E. Jabiyev, M. H. M. Pour and C. J. Porter, J. Membr. Sci., 2025, 726, 124049 CrossRef CAS.
- S. C. Schmal, R. Dosi, A. Fessler, C. Kwiatkowski, A. Sahu and J. C. Poler, Cellulose, 2023, 30(17), 11055–11069 CrossRef CAS.
- A. J. Samaniego, A. K. Arabelo, M. Sarker, F. Mojica, J. Madrid, P. Y. A. Chuang, J. Ocon and R. Espiritu, J. Appl. Polym. Sci., 2020, 138(10), 49947 CrossRef.
- J. Thomas, M. E. Thomas, S. Thomas, A. Schechter and F. Grynszpan, Mater. Today Chem., 2024, 35, 101866 CrossRef CAS.
- H. Yang, J. Edberg, V. Gueskine, M. Vagin, M. G. Say, J. Erlandsson, L. Wågberg, I. Engquist and M. Berggren, Carbohydr. Polym., 2022, 278, 118938 CrossRef CAS PubMed.
- H. Yang, V. Gueskine, M. Berggren and I. Engquist, ACS Appl. Energy Mater., 2022, 5(12), 15740–15748 CrossRef CAS.
- A. J. Samaniego, M. Sarker, Z. Najafianashrafi, P.-Y. A. Chuang, M. Vasquez, J. D. Ocon, C. Xu and R. Espiritu, ACS Appl. Polym. Mater., 2024, 6(15), 9047–9058 CrossRef CAS.
- C. Wang, Z. Zhou, T. Zhou, X. Luo, A. Gao, Y. Zhou, X. Yang, Z. Li, Q. Yang and J. Qiao, Adv. Funct. Mater., 2024, 34(51), 2410009 CrossRef CAS.
- M. Hren, M. Roschger, V. Hacker, B. Genorio, D. Fakin and S. Gorgieva, Int. J. Biol. Macromol., 2023, 253(Pt 8), 127693 CrossRef CAS PubMed.
- Q. Zou, X. Guo, L. Gao, F. Hong and J. Qiao, Sep. Purif. Technol., 2021, 272, 118910 CrossRef CAS.
- G. Das, B. J. Park and H. H. Yoon, J. Mater. Chem. A, 2016, 4(40), 15554–15564 RSC.
- F. Hassan Hassan Abdellatif, J. Babin, C. Arnal-Herault, L. David and A. Jonquieres, Carbohydr. Polym., 2018, 196, 176–186 CrossRef CAS PubMed.
- X. Zhao, P. Zhao, D. Cao, J. Xiang, K. Li, J. Liu, Y. Su and G. Liu, J. Membr. Sci., 2025, 718, 123680 CrossRef CAS.
- F. Wang, T. Qu, H. Yang, H. Yang, Y. Ou, Q. Zhang, F. Cheng, F. Hu, H. Liu, Z. Xu and C. Gong, ACS Appl. Mater. Interfaces, 2024, 16(2), 2751–2762 CrossRef CAS PubMed.
- A. Pokprasert and S. Chirachanchai, Electrochim. Acta, 2023, 441, 141764 CrossRef CAS.
- R. T. Liu, Z. L. Xu, F. M. Li, F. Y. Chen, J. Y. Yu, Y. Yan, Y. Chen and B. Y. Xia, Chem. Soc. Rev., 2023, 52(16), 5652–5683 CAS.
- F. A. Doobi and F. Q. Mir, Results Surf. Interface., 2024, 15, 100218 Search PubMed.
- Z. Yu, W.-C. Tsen, T. Qu, F. Cheng, F. Hu, H. Liu, S. Wen and C. Gong, J. Mater. Res. Technol., 2023, 23, 6187–6199 CrossRef CAS.
- M. Cao, J. Chu, X. Fan, F. Wang, J. Wang, F. Cheng, Z. Xu, F. Hu, H. Liu and C. Gong, J. Membr. Sci., 2023, 675, 121558 CAS.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112(7), 4124–4155 CAS.
- J. Ni, J. Wang, S. Zhao, F. Zhong, T. Qu, F. Hu, H. Liu, C. Gong and S. Wen, Appl. Clay Sci., 2022, 217, 106391 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00844a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |