Design and synthesis of RNA-responsive o-phenanthroline Eu(III) complexes as probes for STED super-resolution dual-targeted bioimaging†
Hao
Yuan‡
a,
Tao
Wang‡
b,
Tong
Zhu
b,
Zhihui
Feng
b,
Fei
Wang
b,
Yupeng
Tian
 b,
Liulin
Xiong
a and
Xiaohe
Tian
b,
Liulin
Xiong
a and
Xiaohe
Tian
 *acd
*acd
aDepartment of Orthopaedic Surgery, The Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China. E-mail: xiaohe.t@wchscu.cn
bInstitue of Neuroscience, Kunming Medical University, Kunming, Yunnan, China
cDepartment of Radiology and National Clinical Research Center for Geriatrics, Huaxi MR Research Center (HMMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, West China Hospital, Sichuan University, Chengdu, China
dCollege of Chemistry and Chemical Engineering, Key Laboratory of Functional Inorganic Material Chemistry of Anhui Province, Anhui University, Hefei 230601, PR China
First published on 3rd July 2023
Abstract
Interactions between lipid droplets (LDs) and mitochondria play a critical role in beta-oxidation and the storage of fatty acids (FAs). These interactions can be influenced by adverse external conditions such as nutritional deficiencies. However, studying these interactions using two different fluorescent dyes often yields low signal-to-noise ratios due to overlapping spectra, photobleaching, and weak penetration. These limitations hinder in-depth research into the interactions between LDs and mitochondria. To address this issue, we developed a series of rare earth complexes named Eu(TTA)3-L1–3. By leveraging its property of changing fluorescence from a ‘turn-off’ to a ‘turn-on’ state upon interacting with RNA in a hydrophilic environment, Eu(TTA)3-L3 has been successfully applied to monitor changes in LDs and mitochondria during oleic acid treatment and starvation-induced autophagy under two-photon conditions. This research provides an effective tool for the comprehensive study of the interaction between LDs and mitochondria. Furthermore, it broadens the perspective for the design of dual-targeting single fluorescent probes.
Introduction
Mitochondria and lipid droplets (LDs), as the primary organelles involved in energy metabolism and storage in cells, play an essential role in the beta-oxidation and storage of fatty acids (FAs).1,2 LDs are monolayer membrane-structured organelles with a hydrophobic core where free fatty acids are stored as triacylglycerols.3 This storage is crucial for normal cellular activities, as excess free fatty acids can initiate free radical chain reactions, generating reactive oxygen species (ROS) with toxic effects on biomolecules, biofilms, cells, and tissues.4Mitochondria are double-membrane organelles that house various enzymes essential for the oxidation and decomposition of fat, sugar, protein, and other substances ingested by the cell. These processes produce a large amount of ATP, providing energy for various cellular activities.5,6 LDs, in concert with mitochondria, participate in many complex and vital cellular activities, such as the β-oxidation of FAs and their storage.2,7
When cells take in excess FAs, the free FAs in the cytoplasm are converted into triglycerides with glycerol, a process energized by mitochondria, and then stored in the hydrophobic core of LDs.8 Additionally, under nutrient-deficient conditions, lipase hydrolyzes triglycerides to produce FAs that enter the mitochondria, where FA β-oxidation in the mitochondrial matrix supplies most of the energy for the vital activities of starving cells.9
In these processes, mitochondria can be categorized based on their interaction with LDs: those in direct contact with LDs are termed peridroplet mitochondria (PDM), while those not in contact with LDs are classified as cytoplasmic mitochondria (CM).10 PDM and CM differ significantly in composition and function. PDM have a stronger capacity for pyruvate oxidation and ATP synthesis, providing energy for LD expansion. Conversely, CM have a stronger FA oxidation capacity, promoting LD consumption.11,12
Therefore, developing a fluorescent probe capable of monitoring the interaction between LDs and mitochondria is of significant importance for in-depth studies of FA oxidation, autophagy, the process of PDM generation and development, and related biomedical fields.
While fluorescent probes for LDs or mitochondria have been extensively reported, fluorescent probes capable of targeting both LDs and mitochondria are seldom described. These dual-targeting probes often suffer from poor penetration ability and weak resistance to photobleaching, resulting in a low signal-to-noise ratio.13–15 LDs, organelles with a monolayer membrane structure and a neutral hydrophobic core, are typically targeted using neutral lipophilic fluorescent probes.16 Mitochondria, characterized by a highly negative membrane potential, are commonly targeted by fluorescent probes carrying cationic groups.17 However, this targeting is limited by the membrane potential dependence.16–18 These two properties are difficult to reconcile, posing a significant challenge to the design of dual-targeting fluorescent probes for mitochondria and LDs.19,20
Inspired by these challenges, we designed and synthesized a series of rare earth complexes named Eu(TTA)3-L1–3, one of them being capable of RNA recognition for dual targeting of LDs and mitochondria (Scheme 1). This molecule exhibits a large Stokes shift and strong luminescence in a lipophilic environment, but in a hydrophilic environment, the fluorescence is ‘turned off’ due to the ACQ effect. However, o-phenanthroline can be inserted into the groove structure of RNA in mitochondria due to its excellent planarity. This insertion reduces intermolecular collisions and intramolecular twisting of the complexes, allowing the complexes’ fluorescence to be ‘turned on’. Using this ‘turn-off–turn-on’ approach, we achieved non-membrane potential-dependent two-photon single fluorescent probe imaging of LDs and mitochondria.
Thanks to the excellent optical properties of this probe, we successfully revealed the interaction of LDs with mitochondria, noting a significant increase in the number of PMDs during oleic acid treatment and starvation-induced autophagy. This work provides a powerful tool for studying the interaction of LDs with mitochondria, facilitating research in related biomedical fields such as FA oxidation, FA storage, and cellular autophagy.
Experimental sections
For more experimental details, please refer to the ESI.†Results and discussion
Synthesis and characterization of Eu(TTA)3-L1–3
With the design considerations outlined above, we obtained the ligand anion group [Eu(TTA)3]- by reacting the β-diketone ligand HTTA with rare earth ions. We then used amino-modified o-phenanthroline as the second ligand to successfully prepare a series of Eu(TTA)3-L1–3 rare earth complexes. The synthetic procedures are shown in Fig. S1.† We also obtained a crystal structure of the Eu(TTA)3-L2 molecule, with the crystal data being presented in Tables S1–S3.† The series of molecules were fully characterized by MALDI-TOF, 1H NMR, and 13C NMR, with the analytical data being provided in Fig. S2–S10.†Photophysical properties of Eu(TTA)3-L1–3
To study the effects of different polar solvents on the UV and fluorescence spectra of Eu(TTA)3-L1–3, we selected seven different solvents (water, dimethyl sulfoxide, acetonitrile, ethanol, ethyl acetate, tetrahydrofuran, and benzene) from highest to lowest polarity to dissolve Eu(TTA)3-L1–3. The absorption and fluorescence spectra of Eu(TTA)3-L1–3 in these solvents were then measured using UV-vis spectrophotometry and fluorescence spectrophotometry, respectively. The results, shown in Fig. 1a–c, reveal that the absorption peaks of the UV-vis spectra for all three complexes of Eu(TTA)3-L1–3 are located around 345 nm, with the peaks of the UV-vis absorption spectra of Eu(TTA)3-L1–3 increasing as the solvent polarity decreases (Fig. 1d). | ||
| Fig. 1 (a–c) UV-vis absorption spectra of Eu(TTA)3-L1–3 in different polar solvents. (d) UV-vis absorption spectrum of Eu(TTA)3-L1–3 in DMSO (c = 1.0 × 10–5 mol L−1). | ||
The fluorescence emission spectra, obtained by exciting Eu(TTA)3-L1–3 in different polar solvents under 345 nm excitation light, were all located around a 614 nm peak (Fig. 2a–c), with Stokes shifts up to 269 nm. This can effectively reduce background interference in further bioimaging applications. While the emission peaks appeared around 614 nm in different solvents, the intensity varied significantly. The emission intensity of the three Eu(III) complexes was the highest in acetonitrile solution, while it was the weakest in water. Due to the good planarity of the ligands HTTA and o-phenanthroline, the ligand-to-ligand gap in the complex is large. Small solvent molecules can enter this gap, causing an increase in the distance between the ligand and the central metal Eu(III). This results in a decrease in the efficiency of energy transfer from the ligand to the central metal ion and a decrease in fluorescence emission. The emission peaks of Eu(TTA)3-L1–3 in DMSO also varied significantly, with Eu(TTA)3-L3 (with a double amino group attached) showing the strongest fluorescence emission intensity and Eu(TTA)3-L1 (without an amino group attached) showing the weakest (Fig. 2d). This is because the introduction of amino groups enhances the electron-donating ability of the complex molecules, leading to increased intramolecular electron mobility, which is conducive to the enhanced fluorescence emission of the complexes.
 | ||
| Fig. 2 (a–c) One-photon fluorescence spectra of Eu(TTA)3-L1–3 in different solvents; (d) one-photon fluorescence spectra of Eu(TTA)3-L1–3 in DMSO (c = 1.0 × 10–5 mol L−1). | ||
Given the excellent intramolecular charge mobility, we separately excited these three complexes at optimal excitation wavelengths in two-photon and three-photon modes. Eu(TTA)3-L1–3 exhibited a high absorption cross section in these two modes (Fig. S11†), indicating that this series of Eu(III) complexes holds promise for fluorescence bioimaging applications with nonlinear optical properties.
In vitro response of Eu(TTA)3-L1–3
We measured the fluorescence intensity of Eu (TTA)3-L1–3 at its optimal excitation wavelength in a PBS solution, adding different common biomolecules. The results, shown in Fig. S12,† indicate a significant enhancement in fluorescence intensity in solutions with added RNA and liposomes. No significant changes were observed with the addition of other common biomolecules, suggesting that Eu(TTA)3-L1–3 can specifically respond to both RNA and liposomes. In RNA titration tests, the fluorescence intensity of Eu(TTA)3-L1–3 enhanced 5–7-fold with the addition of RNA (Fig. 3a–c).21 This can be attributed to two main factors: firstly, intramolecular torsion-induced charge transfer in Eu(TTA)3-L1–3 causes nonradiative transitions, leading to fluorescence quenching of the complexes. Adding RNA effectively limits intramolecular torsion, reducing the energy consumed by nonradiative transitions, thus enhancing the fluorescence emission of the complexes. Secondly, the larger polarity of water molecules significantly increases the dipole moment of the excited states of the complex molecules, promoting intramolecular charge transfer and thereby causing fluorescence quenching of the complexes in an aqueous environment. | ||
| Fig. 3 (a–c) The fluorescence enhancements of Eu(TTA)3L1–3(10 μM) upon the addition of RNA(0–50 mM). (d–f) 1H NMR spectra of Eu(TTA)3L1–3 (10 μM) treated with RNA(50 mM) in D2O. | ||
We further conducted the NMR titration of Eu(TTA)3-L1–3 in D2O, comparing the NMR hydrogen spectra with and without the addition of RNA (Fig. 3d–f). The figure shows that the peaks for the hydrogens in the benzene ring (Eu(TTA)3-L1:a, b; Eu(TTA)3-L2:a, b; Eu(TTA)3-L3:a) of the o-phenanthroline in the Eu(TTA)3-L1–3 molecule significantly shifted to a higher field after the addition of RNA. This indicates the π–π stacking interaction between the benzene ring in the o-phenanthroline and the RNA fragment. The hydrogen peaks (Eu(TTA)3-L2:c; Eu(TTA)3-L3:b) for the amino group (–NH2) in o-phenanthroline significantly diminished after the addition of RNA. This suggests that the amino group in the o-phenanthroline in the Eu(TTA)3-L2–3 molecule also interacts with RNA.22 Furthermore, it was verified that Eu(TTA)3-L3 can bind to RNA without causing significant damage during the dropwise addition of Eu(TTA)3-L3 to the RNA solution, as determined by circular bispectroscopy (Fig. S14†).
Simultaneous visualization of LDs and mitochondria in living cells
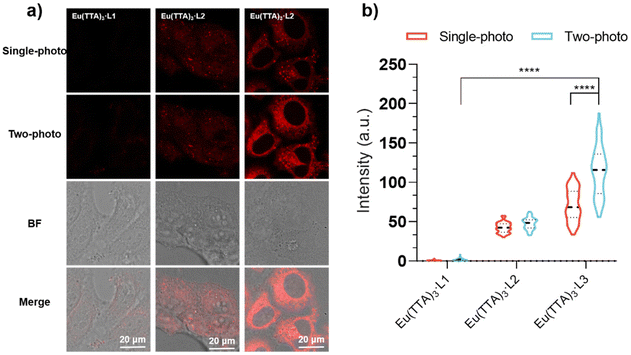
We introduced Eu(TTA)3-L3 into living Hep G2 cells and observed cellular imaging under optimal excitation for both single- and two-photon modes. As depicted in Fig. 4a and b, within Hep G2 cells, Eu(TTA)3-L3 displays the strongest fluorescence intensity under two-photon excitation. The incorporation of the amino group (–NH2) not only bolsters the biocompatibility of the complex but also enhances the molecule's planarity, facilitating the efficiency of intramolecular electron flow and energy transfer.23 In both the single-photon and two-photon fluorescence images of Eu(TTA)3-L3 (hereafter Eu3), we noted filamentous structures resembling mitochondria and several spherical structures. These spherical structures overlap with bright or dark dots in the bright field, attributable to the varying refractive capabilities of lipid droplets to light, which typically appear as small bright or dark dots in the bright field.24For concurrent co-localization tests, we employed Nile red, a commercial fluorescent dye for lipid droplets,24,25 and Mito-Tracker Deep Red (MTDR), a commercial fluorescent dye for mitochondria.26 As illustrated in Fig. 5, the green fluorescence channel of Eu3 aligns well with the blue fluorescence channel of Nile red and the red fluorescence channel of MTDR. The co-localization coefficients of Eu3 with lipid droplets and mitochondria were 0.8 and 0.84, respectively, with notable disparities in fluorescence intensity between these two regions. The average fluorescence intensity (AFI) of the lipid droplet region was 244, whereas the AFI of the mitochondrial region was 117 (Fig. 5a and b). This presents an opportunity for the identification and differentiation of lipid droplets from mitochondria using a single fluorescent probe.
Simultaneous visualization of lipid droplets and mitochondria in cells under two-photon excitation and STED
Imaging of fixed Hep G2 cells with different RNA concentrations revealed that Eu(TTA)3-L3 could respond to mitochondrial RNA in cells (Fig. S15†). We further observed the fixed Hep G2 cells under two-photon excitation, noting that the mitochondria exhibited a distinct rod shape and strong fluorescence intensity in the absence of RNAse. In contrast, the rod structure of the mitochondria was undetectable, and the fluorescence intensity significantly diminished upon the addition of RNAse (Fig. 6a and b).27 The introduction of RNAse disrupts the RNA structure, thereby extinguishing the fluorescence of Eu3, which had previously been capable of inserting into the RNA groove structure.We also assessed the photostability of Eu3, finding that the fluorescence intensity of Eu3 was maintained at about 80% of its initial value following 10 minutes of continuous irradiation under optimal excitation (Fig. S16†).28 This result suggests that Eu3 is suitable for Stimulated Emission Depletion Microscopy (STED). We imaged a single cell under both normal confocal and STED conditions. As depicted in Fig. 6c and d, in STED imaging, the fluorescence intensity distribution within the delineated area is presented as multiple peaks, while the normal confocal image displays a single peak at the same location. Moreover, in the STED image, the mitochondrial region appears in blue with weak fluorescence intensity, while the lipid droplet (LD) region is shown in yellow with strong fluorescence intensity, effectively distinguishing LDs from mitochondria.29 These experiments demonstrate that we have successfully synthesized a two-photon fluorescent probe capable of targeting both LDs and mitochondria with distinct fluorescence intensities.
Changes of LDs and mitochondria during consumption and expansion of LDs
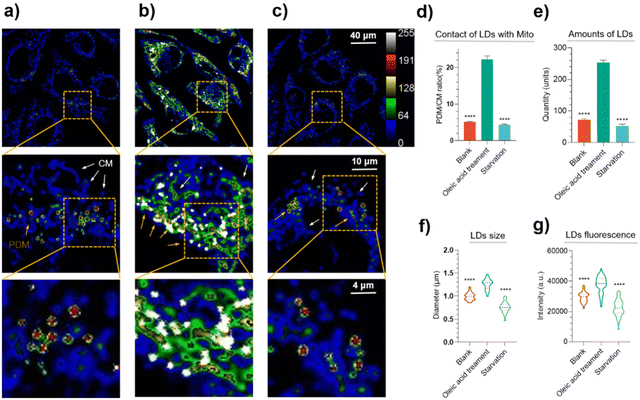
Oleic acid is the most prevalent naturally occurring unsaturated fatty acid (FA), although high concentrations are toxic to cells.30 In contrast, the process of starvation-induced autophagy utilizes FA stored in lipid droplets to provide energy to cells.31,32 We therefore treated Hep G2 cells with oleic acid and subjected them to starvation, respectively, and observed the changes in lipid droplets and mitochondria during lipid droplet consumption and expansion under two-photon excitation. The results are shown in Fig. 7.Eu3 simultaneously visualized lipid droplets and mitochondria in Hep G2 cells, with a 6–7-fold difference in the fluorescence intensity in both organelles (Fig. 7a–c). Proximity to mitochondria (PDM) and contact mitochondria (CM) could be distinguished based on the contact of lipid droplets with mitochondria (please refer to the ESI† for methodology). PDM have a high capacity for pyruvate oxidation and ATP synthesis. CM have a high capacity for fatty acid oxidation. An important role of PDM is to provide ATP for TAG synthesis and lipid droplet expansion. Although increasing the PDM content may not be a good way to increase fat consumption, it may be a way to prevent lipodystrophy or lipotoxicity by anchoring free fatty acids in lipid droplets. Nonetheless, after the cells were treated with oleic acid, it was clearly observed that the number of lipid droplets increased and the fluorescence intensity was enhanced (Fig. 7e and g, entirety of the image analyses). Furthermore, local magnification revealed an enlarged diameter of lipid droplets compared to the control group (Fig. 7f) and increased contact with mitochondria (Fig. 7d). This is due to an excess of oleic acid being stored intracellularly in lipid droplets as triacylglycerol, which stimulates intracellular lipid droplet expansion. The increased PDM can provide more energy for triacylglycerol synthesis and the lipid droplet expansion process.33
As shown in Fig. 7c, the fluorescence intensity at the lipid droplet site decreased after the cells were subjected to starvation. Local magnification also revealed that the diameter of the lipid droplets became smaller (Fig. 7f), indicative of lipid droplet consumption. This is due to the main source of energy for cells in a starved state being the β-oxidation of FA in the mitochondria.13,34 Notably, most of the consumed lipid droplets remained in close contact with the mitochondria (Fig. 7c), and this close contact facilitates the transfer of FA from the lipid droplets to the mitochondria.35
Conclusions
In summary, we have rationally designed and synthesized three single-molecule fluorescent probes based on o-phenanthroline rare-earth (Eu) complexes, which target both lipid droplets (LDs) and mitochondria. Among these, Eu3 exhibits superior RNA recognition ability and high fluorescence quantum yield. Its multiphoton properties and targeting capability open the possibility of observing the interaction of LDs with mitochondria under two-photon excitation. Utilizing the fluorescence ‘turn-off–turn-on’ property of this probe following interaction with RNA in a water-soluble environment, we successfully observed changes in LDs and mitochondria during the expansion and consumption of LDs under two-photon excitation. This revealed increased LD–mitochondria contact during both oleic acid treatment and starvation-induced autophagy. This study demonstrated an increase in the number of LDs and proximity to mitochondria (PDM), as well as changes in the LD diameter size during oleic acid treatment and starvation-induced autophagy. This research provides a new tool for studying fatty acid oxidation, metabolism, cellular autophagy, and related medical fields. The design of this new series of RNA-responsive o-phenanthroline rare-earth (Eu) probes, distinct from traditional cation-targeted probes, broadens the concept of designing dual-targeted single fluorescent probes for LDs and mitochondria.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (32171001). We also thank The Collaborative Innovation Center of Tissue Damage Repair and Regeneration Medicine of Zunyi Medical University and the Zunyi Medical University future clinical doctor talent training plan.References
- Y. Tamura, S. Kawano and T. Endo, Lipid homeostasis in mitochondria, Biol. Chem., 2020, 401, 821–833 CrossRef CAS PubMed.
- M. Veliova, A. Petcherski and M. Liesa, et al., The biology of lipid droplet-bound mitochondria, Semin. Cell Dev. Biol., 2020, 108, 55–64 CrossRef CAS PubMed.
- A. S. Mutlu, J. Duffy and M. C. Wang, Lipid metabolism and lipid signals in aging and longevity, Dev. Cell, 2021, 56, 1394–1407 CrossRef CAS PubMed.
- E. M. Storck, C. Özbalci and U. S. Eggert, Lipid Cell Biology: A Focus on Lipids in Cell Division, Annu. Rev. Biochem., 2018, 87, 839–869 CrossRef CAS PubMed.
- R. Vidhya, L. Tommy Jr. and Y. Hirabayashi, et al., Pleiotropic Mitochondria: The Influence of Mitochondria on Neuronal Development and Disease, J. Neurosci., 2019, 39, 8200 CrossRef PubMed.
- A. Bindoli, Lipid peroxidation in mitochondria, Free Radicals Biol. Med., 1988, 5, 247–261 CrossRef CAS PubMed.
- C. A. C. Freyre, P. C. Rauher and C. S. Ejsing, et al., MIGA2 Links Mitochondria, the ER, and Lipid Droplets and Promotes De Novo Lipogenesis in Adipocytes, Mol. Cell, 2019, 76, 811–825 CrossRef CAS PubMed.
- Y. Yao, L. Ding and X. Huang, Diverse Functions of Lipids and Lipid Metabolism in Development, Small Methods, 2020, 4, 1900564 CrossRef CAS.
- T. Klecker, R. J. Braun and B. Westermann, Lipid Droplets Guard Mitochondria during Autophagy, Dev. Cell, 2017, 42, 1–2 CrossRef CAS PubMed.
- L. Cui and P. Liu, Two Types of Contact Between Lipid Droplets and Mitochondria, Front. Cell Dev. Biol., 2020, 8, 618322 CrossRef PubMed.
- I. Y. Benador, M. Veliova and K. Mahdaviani, et al., Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion, Cell Metab., 2018, 27, 869–885 CrossRef CAS PubMed.
- P.-C. Liao, E. J. Yang and T. Borgman, et al., Touch and Go: Membrane Contact Sites Between Lipid Droplets and Other Organelles, Front. Cell Dev. Biol., 2022, 10, 852021 CrossRef PubMed.
- M. Tian, E. Ge and B. Dong, et al., Intramolecular Spirocyclization Enables Design of a Single Fluorescent Probe for Monitoring the Interplay between Mitochondria and Lipid Droplets, Anal. Chem., 2021, 93, 3602–3610 CrossRef CAS PubMed.
- I. Y. Benador, M. Veliova and M. Liesa, et al., Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization, Cell Metab., 2019, 29, 827–835 CrossRef CAS PubMed.
- M.-Y. Yang, J. Hong and Y. Zhang, et al., Bio-compatibility and cytotoxicity studies of water-soluble CuInS2-ZnS-AFP fluorescence probe in liver cancer cells, Hepatobiliary Pancreatic Dis. Int., 2016, 15, 406–411 CrossRef PubMed.
- P. Gao, W. Pan and N. Li, et al., Fluorescent probes for organelle-targeted bioactive species imaging, Chem. Sci., 2019, 10, 6035–6071 RSC.
- H. Chen, Z. Yu and S. Ren, et al., Fluorescent Probes Design Strategies for Imaging Mitochondria and Lysosomes, Front. Pharmacol., 2022, 13, 915609 CrossRef CAS PubMed.
- N.-E. Choi, J.-Y. Lee and E.-C. Park, et al., Recent Advances in Organelle-Targeted Fluorescent Probes [J/OL], Molecules, 2021, 26(1), 217 CrossRef CAS PubMed.
- P. Benčić, L. Mandić and I. Džeba, et al., Application of 4-amino-N-adamantylphthalimide solvatochromic dye for fluorescence microscopy in selective visualization of lipid droplets and mitochondria, Sens. Actuators, B, 2019, 286, 52–61 CrossRef.
- Q. Bai, C. Yang and M. Yang, et al., pH-Dominated Selective Imaging of Lipid Droplets and Mitochondria via a Polarity-Reversible Ratiometric Fluorescent Probe, Anal. Chem., 2022, 94, 2901–2911 CrossRef CAS PubMed.
- X. Liu, Y. Wang and C. Y. Effah, et al., Endocytosis and intracellular RNAs imaging of nanomaterials-based fluorescence probes, Talanta, 2022, 243, 123377 CrossRef CAS PubMed.
- J. Liu, S. Zhang and C. Zhang, et al., A water-soluble two-photon ratiometric triarylboron probe with nucleolar targeting by preferential RNA binding, Chem. Commun., 2017, 53, 11476–11479 RSC.
- K. Binnemans, Interpretation of europium(III) spectra, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- Y. Zhao, W. Shi and X. Li, et al., Recent advances in fluorescent probes for lipid droplets, Chem. Commun., 2022, 58, 1495–1509 RSC.
- X. Zheng, W. Zhu and F. Ni, et al., Simultaneous dual-colour tracking lipid droplets and lysosomes dynamics using a fluorescent probe, Chem. Sci., 2019, 10, 2342–2348 RSC.
- S. Samanta, Y. He and A. Sharma, et al., Fluorescent Probes for Nanoscopic Imaging of Mitochondria, Chem, 2019, 5, 1697–1726 CAS.
- C. Jiang, S. Li and C. Liu, et al., A near-infrared cationic fluorescent probe based on thieno[3,2-b]thiophene for RNA imaging and long-term cellular tracing, Sens. Actuators, B, 2023, 378, 133102 CrossRef CAS.
- Y. Li, M. Zhang and X. Chen, et al., TICT based fluorescent probe with excellent photostability for real-time and long-term imaging of lipid droplets, Tetrahedron Lett., 2019, 60, 1880–1884 CrossRef CAS.
- M. Pribasnig, B. Kien and L. Pusch, et al., Extended-resolution imaging of the interaction of lipid droplets and mitochondria, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2018, 1863, 1285–1296 CrossRef CAS PubMed.
- S. Li, W. Zhuang and J. Chen, et al., Turn-on fluorescent probe for lipid droplet specific imaging of fatty liver and atherosclerosis, J. Mater. Chem. B, 2021, 9, 4050–4055 RSC.
- Y. Ogasawara, T. Tsuji and T. Fujimoto, Multifarious roles of lipid droplets in autophagy – Target, product, and what else?, Semin. Cell Dev. Biol., 2020, 108, 47–54 CrossRef CAS PubMed.
- L. Cui, M. A. Hammad and S. Zhang, et al., Correction to: Lipid droplets and mitochondria are anchored during brown adipocyte differentiation, Protein Cell, 2021, 12, 746 CrossRef PubMed.
- N. K. Talari, U. Mattam and N. K. Meher, et al., Lipid-droplet associated mitochondria promote fatty-acid oxidation through a distinct bioenergetic pattern in male Wistar rats, Nat. Commun., 2023, 14, 766 CrossRef CAS PubMed.
- A. D. Barbosa and S. Siniossoglou, Function of lipid droplet-organelle interactions in lipid homeostasis, Biochim. Biophys. Acta, Mol. Cell Res., 2017, 1864, 1459–1468 CrossRef CAS PubMed.
- J. Pu, C. W. Ha and S. Zhang, et al., Interactomic study on interaction between lipid droplets and mitochondria, Protein Cell, 2011, 2, 487–496 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi01044a |
| ‡ These authors contribute equally to this work. |
| This journal is © the Partner Organisations 2023 |