Design, fabrication and anti-aging behavior of a multifunctional inorganic–organic hybrid stabilizer derived from co-intercalated layered double hydroxides for polypropylene†
Qian
Zhang
a,
Yixuan
Guo
a,
Adam A.
Marek
b,
Vincent
Verney
b,
Fabrice
Leroux
 *b,
Pinggui
Tang
*b,
Pinggui
Tang
 a,
Dianqing
Li
a,
Dianqing
Li
 a and
Yongjun
Feng
a and
Yongjun
Feng
 *a
*a
aState Key Laboratory of Chemical Resource Engineering, Beijing Engineering Center for Hierarchical Catalysts, Beijing University of Chemical Technology, No. 15 Beisanhuan East Road, Beijing 100029, China. E-mail: yjfeng@mail.buct.edu.cn
bUniversite Clermont Auvergne, Institut de Chimie de Clermont-Ferrand ICCF, UMR-CNRS 6296, F 63171 Aubière, France. E-mail: fabrice.leroux@uca.fr
First published on 29th July 2019
Abstract
It is of great and increasing interest to develop multifunctional additives to enhance the anti-aging performance of polypropylene (PP) and then enlarge its application fields. Here, a hindered amine light stabilizer (H for HALS) and a hindered phenolic antioxidant (D for DBHP) with low molecular weight were co-intercalated into layered double hydroxides (LDHs) through a coprecipitation method to produce a series of inorganic–organic hybrid materials Zn2Al-HxDy-LDH, adjusting the anion ratio (x + y = 1 and x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 0
y = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Subsequently, hybrid material LDH fillers (HALS-LDH, DBHP-LDH, HxDy-LDH, and (H-LDH)x(D-LDH)y), used as anti-aging agents, were successively dispersed into PP by two methods: solvent mixing/casting and extrusion mixing. The corresponding crystalline structure, morphological, rheological properties and thermal and light oxidative degradation behaviour were carefully investigated using various techniques such as XRD, TEM and rheology. The results showed that the LDH fillers were well dispersed into PP without any influence on its crystallization behavior and provided a chain extension effect on PP, and this is even more pronounced for HxDy-LDH. The thermal stability of HxDy-LDH/PP composites (x
2). Subsequently, hybrid material LDH fillers (HALS-LDH, DBHP-LDH, HxDy-LDH, and (H-LDH)x(D-LDH)y), used as anti-aging agents, were successively dispersed into PP by two methods: solvent mixing/casting and extrusion mixing. The corresponding crystalline structure, morphological, rheological properties and thermal and light oxidative degradation behaviour were carefully investigated using various techniques such as XRD, TEM and rheology. The results showed that the LDH fillers were well dispersed into PP without any influence on its crystallization behavior and provided a chain extension effect on PP, and this is even more pronounced for HxDy-LDH. The thermal stability of HxDy-LDH/PP composites (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 1
y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) is significantly improved, compared with that of other composites. Moreover, through the accelerated aging test, the co-intercalated hybrid material HxDy-LDH significantly inhibited the oxidative degradation of PP (thermal-aging and light-aging). The concomitant presence of HALS/DBHP co-intercalated within the LDH structure strongly improved the anti-aging performance of PP. Therefore, such a co-intercalated I/O hybrid adaptive system as a multifunctional additive agent opens the pathway for potential future research of high-performance PP associated with hybrid fillers.
2) is significantly improved, compared with that of other composites. Moreover, through the accelerated aging test, the co-intercalated hybrid material HxDy-LDH significantly inhibited the oxidative degradation of PP (thermal-aging and light-aging). The concomitant presence of HALS/DBHP co-intercalated within the LDH structure strongly improved the anti-aging performance of PP. Therefore, such a co-intercalated I/O hybrid adaptive system as a multifunctional additive agent opens the pathway for potential future research of high-performance PP associated with hybrid fillers.
1. Introduction
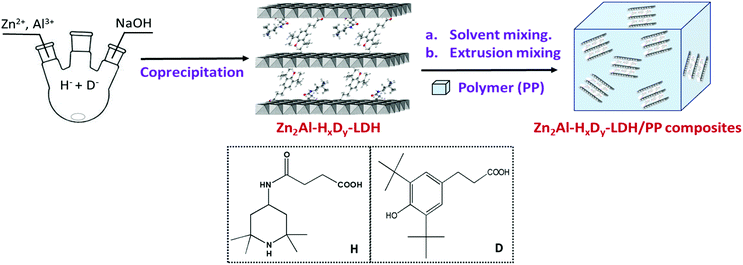
Polypropylene (PP) as one kind of thermoplastic polymer has been developed to be the second-most widely produced commodity plastic and therefore is subject to increasing requirements and technical demands in various fields, particularly in packaging, labeling, and the automobile industry. In 2017, the global production was ca. 70 million tons.1 However, owing to the α-H bonding in its molecular structure, PP suffers from serious thermal- and photo-degradation at environmental temperatures beyond 100 °C and under UV exposure.2 Therefore, various kinds of functional additives are added to PP during the melting process to enhance its application performance as, for example, light stabilizers, antioxidants, antistatic agents and fire retardants.3–5 Among them, hindered amine light stabilizers (HALSs) and hindered phenolic antioxidants (HPAs) have shown outstanding performance and have evidently been largely used in the industry, containing 2,2,6,6-tetramethyl piperidine and 2,6-di-tert-butylphenol functional groups, respectively.6,7 The active site in the HALS and HPA structure is –NH– and –OH, respectively, which captures the peroxyl radicals ROO˙ produced during the oxidative degradation of PP, and thereby terminates oxidation chains.8,9 Particularly, the combination of HALSs and HPAs in PP exhibits the positive synergistic antiaging effect.10,11 However, two challenges remain for making full applicative use of the synergistic effect to produce high-performance PP by the incorporation of multicomponent additives: (1) to lower as much as possible the high migration of organic anti-aging additives because of high compatibility between the additives and PP12 and (2) to increase significantly the low dispersion of two compounds in PP.13High migration of the additives from PP during its service life does not only directly result in the fast loss of application performance but also lead to the ingress of leached additives out of the polymer that may threaten the safety in terms of environment and health concerns. To date, two typical approaches have been developed to overcome this issue: (1) increasing the corresponding molecular weight14 and (2) immobilizing the PP chains on inorganic particles such as SiO2, CNTs, and GO.15–17 Undoubtedly, these two solutions do increase the synthesis cost of the additives making them unfeasible at large scale for PP. Besides, two components are more difficult to be highly dispersed in PP, compared to a single component. It is possible to simultaneously fix both the above issues by co-intercalation of HALS and HPA active species into the interlayer region of single-layered double hydroxides (LDHs) to develop single-composition multifunctional anti-aging additives.
Layered double hydroxides (LDHs), as one important inorganic host material, have attracted increasing attention for various kinds of functional materials by intercalation and surface immobilization in polymers,18,19 catalysis,20 environments,21 electrochemistry,22 biology, etc.23 More recently, some interesting multifunctional additives have been explored by the co-intercalation of different active species into the interlayer region of LDHs, and they exhibited more excellent application performance related to the corresponding active species themselves due to the synergistic effect between the active species and the LDH host.24,25 In the intercalated LDH, the interaction between the host sheet and the guest species effectively anchors the active species and then hinders their migration out of the LDH vessel.26 Additionally the interface effects between the inorganic LDH host sheet and the PP chains reduce the motion of the intercalated LDH.27 Besides, two active species with adjustable ratios are intercalated into the interlayer region of the LDH to produce novel intercalated additives in a single composition; this has never been reported so far, and it is observed here to favorably enhance the dispersion of the additives in PP.
In this work, we designed and fabricated a series of novel inorganic/organic (I/O) hybrid materials Zn2Al-HxDy-LDH (x + y = 1 and x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 0
y = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) by co-intercalating the low molecular weight HALS and the HPA as the active species into LDHs,28,29 by preparing a series of LDH/PP composites by both solvent or extrusion mixing using Zn2Al-HxDy-LDH as the anti-aging nanofiller, and carefully investigating the oxidative degradation behavior (thermo- and light-oxidative) of LDH/PP composites as well as the structure–performance relationship. Scheme 1 describes the synthesis process of Zn2Al-HxDy-LDH and Zn2Al-HxDy-LDH/PP composites, where H represents the hindered amine light stabilizer such as 4-oxo-4-((2,2,6,6-tetramethylpiperidin-4-yl)amino)butanoic acid (HALS) and D represents the hindered phenolic antioxidant such as 3-(3,5-ditertbutyl-4-hydroxyphenyl) propionic acid (DBHP).
2) by co-intercalating the low molecular weight HALS and the HPA as the active species into LDHs,28,29 by preparing a series of LDH/PP composites by both solvent or extrusion mixing using Zn2Al-HxDy-LDH as the anti-aging nanofiller, and carefully investigating the oxidative degradation behavior (thermo- and light-oxidative) of LDH/PP composites as well as the structure–performance relationship. Scheme 1 describes the synthesis process of Zn2Al-HxDy-LDH and Zn2Al-HxDy-LDH/PP composites, where H represents the hindered amine light stabilizer such as 4-oxo-4-((2,2,6,6-tetramethylpiperidin-4-yl)amino)butanoic acid (HALS) and D represents the hindered phenolic antioxidant such as 3-(3,5-ditertbutyl-4-hydroxyphenyl) propionic acid (DBHP).
2. Experimental section
2.1. Chemicals
The reagent grade materials, e.g., Zn(NO3)2·6H2O, Al(NO3)3·9H2O, NaOH, xylene, hexane, ethanol and acetone, all commercially available, were of A.R. grade and used as received. Polypropylene (Type: PP1300, melting point: 164–170 °C; melting index: 1.5 g per 10 min; density: 0.91 g cm−3) was provided by Sinopec Beijing Yanshan Company. Deionized water was used in all the experiments. The detailed synthesis of the HALS and DBHP were individually reported in our previous work: the HALS was prepared by the addition reaction of tetramethylpiperidinamine and succinic anhydride28 and the DBHP was obtained by the hydrolytic reaction of Irganox 1010.292.2. Synthesis of Zn2Al-HxDy-LDH nanofillers
A series of the HALS (H) and DBHP (D) co-intercalated Zn2Al-HxDy-LDHs (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 0
y = 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were prepared using the one-step coprecipitation method. Typically, for Zn2Al-H1D1-LDH, the HALS (0.4557 g, 1.8 mmol) and the DBHP (0.4948 g, 1.8 mmol) were solubilized in 50 mL of water containing 3.6 mmol NaOH; 0.5295 g (1.8 mmol) of Zn(NO3)2·6H2O and 0.3339 g (0.9 mmol) of Al(NO3)3·9H2O were dissolved in 50 mL H2O to form a metal-salt solution. At room temperature, salt solution and 0.5 M NaOH solution were simultaneously added dropwise to the prepared HALS/DBHP solution under vigorous stirring and a nitrogen atmosphere. The pH was maintained constant at 9.5 (±0.1) in the reaction system. The resulting white precipitate was aged for another 4 h, afterwards centrifuged and washed twice with distilled water. Part of the Zn2Al-H1D1-LDH slurry was dried at 40 °C to get the resulting powder for structural analysis. The remaining slurry was washed with an EtOH/H2O mixture (50/50, V/V) and acetone, which was subjected to surface modification. The slurry and powder of LDHs were used directly to prepare the LDH/PP composites. In a similar procedure, Zn2Al-H2D1-LDH, Zn2Al-H1D2-LDH, Zn2Al-HALS-LDH and Zn2Al-DBHP-LDH were also synthesized.
2) were prepared using the one-step coprecipitation method. Typically, for Zn2Al-H1D1-LDH, the HALS (0.4557 g, 1.8 mmol) and the DBHP (0.4948 g, 1.8 mmol) were solubilized in 50 mL of water containing 3.6 mmol NaOH; 0.5295 g (1.8 mmol) of Zn(NO3)2·6H2O and 0.3339 g (0.9 mmol) of Al(NO3)3·9H2O were dissolved in 50 mL H2O to form a metal-salt solution. At room temperature, salt solution and 0.5 M NaOH solution were simultaneously added dropwise to the prepared HALS/DBHP solution under vigorous stirring and a nitrogen atmosphere. The pH was maintained constant at 9.5 (±0.1) in the reaction system. The resulting white precipitate was aged for another 4 h, afterwards centrifuged and washed twice with distilled water. Part of the Zn2Al-H1D1-LDH slurry was dried at 40 °C to get the resulting powder for structural analysis. The remaining slurry was washed with an EtOH/H2O mixture (50/50, V/V) and acetone, which was subjected to surface modification. The slurry and powder of LDHs were used directly to prepare the LDH/PP composites. In a similar procedure, Zn2Al-H2D1-LDH, Zn2Al-H1D2-LDH, Zn2Al-HALS-LDH and Zn2Al-DBHP-LDH were also synthesized.
2.3. Preparation of LDH/PP composites
LDH/PP composites were prepared by two methods: solvent mixing and extrusion mixing.30,31 The weight percentage of LDHs in PP was confirmed to be 4.0 wt%. In the solvent mixing method, for example, 20.00 g PP and the fixed amount of LDH slurry (according to the solid content) were dispersed in 70 mL xylene, then transferred to a round-bottomed glass reactor (500 mL capacity). Under vigorous stirring, the system was heated (up to 140 °C) in a water-cooled condenser. After 3 hours, the mixture was poured into 50 mL hexane immediately and cooled to room temperature. The product was placed in a fume hood and finally dried. In the extrusion mixing method, for instance, 5.00 g PP and the corresponding LDH powder were mixed and melt-processed in a twin screwed extruder equipment Haake Minilab Micro Compounder (at 180 °C, 100 rpm for 5 min). For comparison, (HALS-LDH)x(DBHP-LDH)y/PP (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 1
y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) composites with direct mixing of two LDH fillers (HALS-LDH, DBHP-LDH) were carried out, as well as without any additive (PP) defined as reference composite. For further analysis, LDH/PP composites were pressed into polymer films (a thickness of ∼0.1 mm) between Teflon sheets at 170 °C. Finally, PP composites using a LDH filler in 4.0 wt% loading HALS-LDH/PP, DBHP-LDH/PP, H1D1-LDH/PP, H2D1-LDH/PP, H1D2-LDH/PP, (H-LDH)1(D-LDH)1/PP, (H-LDH)2(D-LDH)1/PP, (H-LDH)1(D-LDH)2/PP, HALS/PP and DBHP/PP films were individually prepared by the two described methods.
2) composites with direct mixing of two LDH fillers (HALS-LDH, DBHP-LDH) were carried out, as well as without any additive (PP) defined as reference composite. For further analysis, LDH/PP composites were pressed into polymer films (a thickness of ∼0.1 mm) between Teflon sheets at 170 °C. Finally, PP composites using a LDH filler in 4.0 wt% loading HALS-LDH/PP, DBHP-LDH/PP, H1D1-LDH/PP, H2D1-LDH/PP, H1D2-LDH/PP, (H-LDH)1(D-LDH)1/PP, (H-LDH)2(D-LDH)1/PP, (H-LDH)1(D-LDH)2/PP, HALS/PP and DBHP/PP films were individually prepared by the two described methods.
2.4. Characterization
XRD measurements of LDH and LDH/PP composites were recorded using a powder X-ray diffractometer (Philipps X-Pert Pro) using a Cu Kα source (λ = 0.154 nm) from 2.0 to 70.0/2θ with a step size of 0.03° and a step counting time of 10 s. Small Angle X-ray Scattering (SAXS) experiments were performed using Empyrean Panalytical equipment with ScatterX78 using a θ/θ goniometer and Cu anode (45 kV and 40 mA). An elliptic W/Si focusing X-ray mirror for Cu radiation was used, and a divergence fixed slit of 1/32°. The distance from the incident beam to the sample was 140 mm. The GaliPIX3D detector was placed at a distance of 240 mm from the sample. SAXS curves were recorded in a continuous scan mode. The background (no sample) was removed in each case. Fourier transform infrared (FT-IR) spectra were recorded using a Thermo Scientific Nicolet 6700 FT-IR spectrometer using thin KBr pellets in the range of 4000–400 cm−1 in a transmission mode. Thermogravimetric analysis (TGA) curves were examined using PerkinElmer TGA4000 apparatus in an air atmosphere (a gas flow of 30 mL min−1) in the range of 30–700 °C with a heating rate of 10 °C min−1. Solid-state 13C NMR (I = 1/2) experiments were carried out using a 300 Bruker spectrometer at 75.47 MHz, under magic angle spinning (MAS) conditions at 10 kHz, using a 4 mm diameter size zirconia rotor. 13C spectra recorded by the proton enhanced cross-polarization method (CP) were referenced to the carbonyl of glycine calibrated at 176.03 ppm. The rheological properties of LDH/PP composites were recorded at 180 °C using a rotational spectrometer (ARES, TA, USA), with a parallel plate geometry with an 8 mm diameter and a gap distance of 1 mm. All the measurements were certainly within the linear viscoelastic region. The dynamic stress against oscillatory shearing frequency was from 0.1 to 100 rad s−1. As a function of the frequencies, G′ (storage modulus), G′′ (loss modulus) and the ratio of G′′ to G′ (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) were monitored automatically. Then, the Cole–Cole plot curves containing storage viscosity (η′ = G′′/ω) and loss viscosity (η′′ = G′/ω) were compiled for discussion. Newtonian zero-shear viscosity η′0 of PP was obtained by extrapolating the semi-circular depression consistent with η′0 = K·Mwa.
δ) were monitored automatically. Then, the Cole–Cole plot curves containing storage viscosity (η′ = G′′/ω) and loss viscosity (η′′ = G′/ω) were compiled for discussion. Newtonian zero-shear viscosity η′0 of PP was obtained by extrapolating the semi-circular depression consistent with η′0 = K·Mwa.
2.5. Thermal properties of LDH/PP composites
The thermal properties of LDH/PP composites were analyzed by two methods: thermogravimetric analysis (TGA) and accelerated thermal-aging. The LDH/PP composites with different mass loadings in terms of LDH nanofillers were analyzed under a heating condition of 10 °C min−1 from 25 °C to 600 °C. Thermal-aging experiments on LDH/PP composites were conducted with a constant temperature of 150 °C, and the thermal aging behavior of LDH/PP composites was recorded using the FT-IR spectra every 80 min. As the aging time increased, the increasing area of the IR absorption band (1810–1660 cm−1) was used to evaluate the thermal-aging degree.322.6. Light-aging test of LDH/PP composites
The light-aging test of LDH/PP composites was carried out using UV-accelerated light-aging equipment with a UV lamp (1000 W, λmax = 365 nm), and the corresponding FT-IR spectra were also recorded to evaluate the light-aging process every 10 min.3. Results and discussion
3.1. Structural analysis of Zn2Al-LDH nanofillers
Fig. 1 shows the XRD patterns of the five Zn2Al-LDH samples, and Table S1 (see ESI†) lists the associated crystallographic data and chemical compositions. In each case, the characteristic Bragg reflection peaks of Zn2Al-LDH exhibit a series of basal reflections (00l) at low angles and the (110) reflection at a higher angle, indicating that a layered structure and an intra-layer structural ordering are formed.33 The HALS-LDH and DBHP-LDH exhibit diffraction reflections (003) at 7.5° and 3.3°/2θ, corresponding to the d-spacing distances of 1.17 and 2.63 nm, respectively. The basal spacing of co-intercalated HxDy-LDH is almost the same as that of DBHP-LDH because the DBHP anion is of larger size than the HALS anion. Furthermore, compared to those of DBHP-LDH and HxDy-LDH, the corresponding Bragg peaks of HALS-LDH are significantly broader in terms of (00l); this is correlated to a diffraction intensity much lower, thus resulting, in this case, in a strong structural disorder in the LDH platelet stacking. The XRD patterns of the five Zn2Al-LDH samples show the absence of the carbonate phase (Zn2Al-CO3-LDH presents a basal spacing value of 7.6 Å),34 indicating that the amount of Zn2Al-CO3-LDH impurity is lower than the detection limit of XRD equipment. That is to say, the co-precipitation method is available to prepare the intercalated LDH.The co-existence of HALS and DBHP anions in the vicinity of LDH platelets is further confirmed by the FT-IR spectra, as shown in Fig. 2, knowing that IR spectroscopy cannot address adsorption versus intercalation. All Zn2Al-LDH samples show characteristic IR absorption bands of Zn2Al-LDH. Lattice vibration (M–O and O–M–O) appears at 427 cm−1, indicating the formation of the Zn2Al-LDH platelet structure. The broadband around 3420 cm−1 is attributed to the hydroxyl group present in the brucite-like layer and the O–H stretching vibration of water molecules. Simultaneously, the carbonyl stretching vibrations coming from HALS and DBHP species are also observed. The position of vibration bands of COO− is shifted from 1644 and 1391 cm−1 to 1618 and 1415 cm−1 for HALSs, 1707 and 1432 cm−1 to 1532 and 1408 cm−1 for DBHPs. The splitting between an asymmetrical and symmetrical mode Δν(COO−) = νas − νs is changed between the guest molecule and its intercalated LDHs, which means the coordination mode between the functional group of the carboxylate ion and the metal laminate is modified.35 The coordination mode changed from the monodentate character to the bridging character (from Δν = 253 cm−1 of the HALS to Δν = 203 cm−1 of HALS-LDH) and from the monodentate character to a more chelating character (from Δν = 275 cm−1 of DBHPs to Δν = 124 cm−1 of DBHP-LDH). Also, similar shifts in the coordination model are also found in co-intercalated HxDy-LDH. That is to say that upon intercalation, there is a change in the symmetry of the carboxylation functional group because the HALS and DBHP interact electrostatically with the inner OH-bearing LDH layer surface. In addition, no absorption bands of carbonate or nitrate anions are observed, neither –CO3 (1357 cm−1) nor –NO3 (1383 cm−1), indicating the absence of these two contaminations.
 | ||
Fig. 2 FT-IR spectra of (a) HALS, DBHP, Zn2Al-HALS-LDH and Zn2Al-DBHP-LDH and (b) Zn2Al-HxDy-LDH (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 1 y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
Solid-state 13C NMR CPMAS spectra of the HALS and DBHP before and after their incorporation between LDH layers are also compared (Fig. 3). Also, the peak assignment of the HALS and DBHP anion carbon nuclei is shown in the figure. After the intercalation of HALS and DBHP molecules into LDHs, some resonance peaks are shifted. Between the HALS and HALS-LDH, large shifts in the resonance lines of HALS-LDH are observed for most of the carbon nuclei close to the carboxylate group, C8. Indeed, its value is deshielded when the HALS molecule is interleaved. This is usually explained by the electrostatic interaction between the carboxyl function and the LDH inner-surface, and this effect propagates along the alkyl chain since C6 and C7 are both high-field shifted, as well as C5 to less extent because C5 is bearing a carbonyl function. The carbon nuclei from the piperidine cycle are found unmodified, and having in mind the fact that the CP sequence is not quantitative, the change in intensity is not discussed. A similar observation may be carried out between the spectrum of the DBHP molecule and when it is interleaved, the C9 carboxylate is also down-field shifted while the C7 and C8 carbon nuclei next to C9 are shifted to a higher field. For the other carbon nuclei, their chemical shift is found unchanged, this arising from the butylphenol cycle averaging the interaction and by the fact that the tert-butyl groups are too far from the tethering carboxylate group. Altogether, one notes that the down-field shift on the carboxylate is larger for the HALS than for the DBHP, and so the high-field shifts of both carbon nuclei of the alkyl chain next to carboxylate. This may be interpreted by an electrostatic interaction more efficiently for the HALS than for the DBHP, and this is tentatively explained here by the more cumbersome DBHP molecule (a larger basal spacing) which is also strongly hydrophobic in the presence of tert-butyl compared with tetra-methyl for the HALS. The net difference in the electrostatic interaction between the organic anions and the host Zn2Al LDH sheets was also confirmed by FT-IR spectra, as well as between both interleaved families with Δν(COO−) = 203 and 124 cm−1 for HALS- and DBHP-LDH, respectively (vide supra). Similar changes are also depicted in the series of co-intercalated HxDy-LDH samples.
 | ||
Fig. 3
13C NMR CPMAS spectra of (a) HALS, Zn2Al-HALS-LDH and DBHP, Zn2Al-DBHP-LDH and (b) Zn2Al-HxDy-LDH (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 1 y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
Besides, the thermal behavior has been assessed by thermogravimetry. Fig. 4 depicts the thermogravimetric curves of Zn2Al-LDHs, and Table S2 (see the ESI†) lists the corresponding data. The TG curves of the HALS and DBHP show a single mass loss step (200–350 °C), corresponding to the DTA exothermic peak at 300 and 306 °C, due to the decomposition of HALS and DBHP molecules.28,29 The thermal decomposition of LDHs occurs in different steps: (1) the removal of absorbed and interlayer water molecules (below 220 °C); (2) the loss of the metal hydroxide layer (220–360 °C); and (3) the decomposition of the interlayer organic anion (330–500 °C). A partial overlap between the second and third mass loss is commonly observed. From the total weight loss, one can estimate the organic content that results in the chemical formulae of the Zn2Al-LDHs, as reported in Table S1 (see the ESI†). The residual mass at 700 °C is considered to be “Zn2AlO7/2”, which is related to the molecular weight at ambient temperature by the loss percentage.36 According to the feed conditions, all guest molecules appear to be present in a ratio of 1/1 relative to Al3+. The thermal stability is also expressed by DTA and the temperatures at the mass loss of a determined amount (e.g., 50%, at T50). LDHs have high T50, within the range from 371 °C (for DBHP-LDH) up to 411 °C (for H1D1-LDH), and the DTA exothermic peak of H1D1-LDH at 344 and 435 °C. After intercalating into the LDH layers, the decomposition temperature of HALS and DBHP molecules is increased, and the series of co-intercalated HxDy-LDH exhibit higher thermal stability compared to that of HALS(DBHP)-LDH, especially H1D1-LDH. All these results suggest that the intercalated LDH structure significantly improves the thermal stability of the guest species due to the interaction between the guest species and the LDH host sheet. Certainly, the interaction favorably inhibits the migration of the guest species from the intercalated LDH.
 | ||
Fig. 4 (a) TG and (b) DTA curves of Zn2Al-HALS-LDH, Zn2Al-DBHP-LDH and Zn2Al-HxDy-LDH (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 1 y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2 1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 2). | ||
3.2. Structure analysis of LDH/PP composites
Fig. 5a and b demonstrate the XRD patterns of the LDH/PP composite films by the solvent mixing method following the hot-pressed process. PP free of LDHs is XRD silent in the low 2θ angular domain (2–10°). When the hybrid LDH materials are dispersed into PP, the corresponding XRD patterns for DBHP-LDH/PP and HxDy-LDH/PP show some characteristic 00l reflections. Compared to those of LDH pristine samples, their positions are slightly shifted to lower 2θ angles corresponding to a change in the basal distance from 2.69 nm (Fig. 1) to 2.95 nm (Fig. 5a), thus indicating that some polymer chains are possibly diffusing within the LDH gap.37 However, for the HALS-LDH/PP and (H-LDH)x(D-LDH)y/PP composites, one notes the quasi-absence of (00l) peaks of LDHs, and this indicates that the layers of LDHs initially stacked are extensively dispersed and even exfoliated into the PP matrix. At a larger angular domain, X-ray patterns exhibit the characteristic Bragg reflections assigned to the (110), (040), (130), (111) and (131)/(041) planes of the α-form of the PP crystal. The diffraction peak positions are modified by neither their position nor their FWHM intensity, thus indicating that the incorporation of LDHs does not affect the crystallized domains of PP. Fig. 5c shows the IR spectra of LDH/PP composites. All the samples demonstrate the methyl and methylene characteristic bands of PP, e.g. 2950, 2915, 2868, 2837, 1454, and 1375 cm−1. Similar situations have also been found in PP composites prepared by extrusion mixing, see ESI Fig. S1.† As a result, the addition of LDHs is found to lead the well-dispersed platelets into PP, and their effect on the molecular vibrational motions and the overall structure of PP was negligible. | ||
| Fig. 5 XRD patterns of LDH/PP composites in (a) 2–10°, (b) 11–25°/2θ and (c) FT-IR spectra of LDH/PP composites prepared by the solvent mixing method. | ||
Besides, Fig. 6 shows the TEM images of H1D1-LDH/PP and (H-LDH)1(D-LDH)1/PP composites prepared by the solvent mixing method. At low magnification images (a, b), some speckles are randomly distributed in PP, suggesting that the LDH fillers are evenly dispersed in PP. At high magnification images (a′, b′), the edges of LDH fillers appear rather blurry, indicating a thorough mixing between both the components, most probably arising from the chemical modification by an organic solvent that improves the compatibility of LDHs in PP. It is worth noting that the distinct layer structure of LDHs can be observed in H1D1-LDH/PP, with a basal distance of ∼3 nm. H1D1-LDH shows a certain degree of incomplete exfoliation in PP. However, this situation is not observed in (H-LDH)1(D-LDH)1/PP, where only small aggregates are depicted. In both cases, it is consistent with the XRD analysis results at low 2θ values.
 | ||
| Fig. 6 TEM images of H1D1-LDH/PP (a and a′) and (H-LDH)1(D-LDH)1/PP (b and b′) prepared by the solvent mixing method. Insets present the enlarged region for clear observation. | ||
To further unravel the structural arrangement of the fillers into PP, SAXS is conducted and the corresponding results are described in Fig. 7. For the series using the solvent mixing, most of the samples present a hump located at a diffusion value q of 0.033 Å−1, corresponding to a distance of 3.0 nm. For HALS-LDH and the physical mixture, such a hump is absent, underling a degree of exfoliation, as already observed in the wide-angle XRD analysis. When decreasing q, the slope becomes constant showing that there is no other particle size in the scanned domain (up to 100 nm). At q < 0.07 Å−1, the good linear fit of HALS-LDH/PP and (H-LDH)x(D-LDH)y/PP confirms full exfoliation of LDH clay platelets. The slope of −2 for (H-LDH)1(D-LDH)1/PP and (H-LDH)1(D-LDH)2/PP and −2.2 for HALS-LDH/PP and (H-LDH)2(D-LDH)1/PP are consistent with scattering from isolated two-dimensional platelets.38 For the series of extrusion-made samples, the characteristic hump at around q = 0.03 Å−1 is visible with pronounced shifts in some cases. Only HALS-LDH does not present such a hump. Additional changes of the slope are indicative of correlative distances. For instance, it is observed at q = 0.0065 Å−1 for H2D1-LDH, that corresponds to a distance of 15.4 nm. Below this value (in q), the slope is more horizontal, indicative that there is no more aggregation/correlation at a larger distance scale.39
 | ||
| Fig. 7 SAXS data for PP composites prepared by (a) the solvent mixing method and (b) the extrusion mixing method. | ||
To further investigate the microstructural changes of PP composites, rheology analysis in the PP molten state was scrutinized. The rheological properties of composites are affected by many factors, such as dispersion state, nano-structure and the interaction at the interface here between filler platelets and polymer chains. The rheological data in the Cole–Cole representation, η′′ − η′ (ω), are chosen to express viscoelastic behavior, because any change in the molecular weight (Mw) and molecular weight distribution (MWD) is directly evidenced on the viscoelastic behavior η′′ − η′ (ω).40 From the Cole–Cole plots of LDH/PP composites (Fig. 8a and b), a depressed semi-circle associated with a Newtonian behavior is observed. The convex downward semi-circular profile at the intercept η′′ tending to 0 corresponds to the Newtonian zero-shear viscosity η′0 when ω tends to 0, and related to the apparent Mw value using the empirical relationship η′0 = K·Mw3.4, where K = 4.7 × 10−9. When LDH nanofillers are incorporated into a PP matrix, the Newtonian zero-shear viscosity of LDH/PP composites is shifted to a higher value. This can be interpreted as a chain extender effect of the LDH/PP composites and related to an efficient interfacial interaction between LDH and PP chains. LDH fillers play a reinforcing role toward PP and appear to be tunable as the function of the nature of the organic molecule tethered to the inorganic platelets.
 | ||
| Fig. 8 Cole–Cole plots of PP composites prepared by (a) the solvent mixing method and (b) the extrusion mixing method. (c) Correlation between both dispersion processes. | ||
LDH/PP composites are prepared by the solvent mixing method, and the values of viscosity range in the order: PP < HALS-LDH/PP < HALS/PP < DBHP/PP < (H-LDH)1(D-LDH)2/PP < (H-LDH)2(D-LDH)1/PP < (H-LDH)1(D-LDH)1/PP < DBHP-LDH/PP < H1D2-LDH/PP < H2D1-LDH/PP < H1D1-LDH/PP. LDH/PP composites prepared by extrusion mixing present a much lower viscosity compared with solvent mixing composites, with the viscosities ranging in the order: PP < HALS/PP < HALS-LDH/PP < (H-LDH)2(D-LDH)1/PP < DBHP-LDH/PP < (H-LDH)1(D-LDH)1/PP < DBHP/PP < H2D1-LDH/PP < H1D1-LDH/PP < (H-LDH)1(D-LDH)2/PP < H1D2-LDH/PP. The auto-correlation curve of η′0 between both mixing methods is shown in Fig. 8c. Indeed comparing both methods of dispersion, η′0 values of the solvent mixing composites are found to be much larger than those of the extrusion mixing composites, indicating that there is no linear relationship between the two mixing methods. Therefore, co-intercalated HxDy-LDH fillers induce a strong chain extension effect on PP, even more pronounced for H1D1 co-added guest molecules. At the same 4 wt% loading, the solvent mixing LDH/PP composites seem to perform better than extrusion mixing LDH/PP composites. These results suggest an efficient dispersion of LDHs in PP with an interfacial interaction being largely developed through solvent mixing and an improvement in mechanical properties for the polymer materials.
3.3. Thermal properties of LDH/PP composites
In the following, the series of composites prepared only by the solvent mixing method will be considered. Fig. 9a reports the thermogravimetric analysis (TGA) of the LDH/PP composites, and Table S3 (see the ESI†) lists the data concerning the decomposition temperatures. The main mass loss process for all the samples takes place in the range of 250–400 °C. The degradation process at Tonset for LDH/PP located at higher temperatures than that of PP, which indicates that the incorporation of LDHs improves the thermal stability of the composites. The temperature at which the composites lose a determined amount of weight (50%, T50) are considered here for comparison, T50 ranges as HxDy-LDH/PP > (H-LDH)x(D-LDH)y/PP > HALS(DBHP)-LDH/PP > HALS(DBHP)/PP > PP. In all the cases, T50 gradually increases from 331 (for PP) up to 399 °C (for H1D1-LDH). For the latter H1D1-LDH filler, Tonset is of 376 °C, also higher than the other fillers. Compared with the single molecule (H or D) intercalated LDH filler or physically mixed LDH filler, the series of co-intercalated HxDy-LDH fillers endow PP with remarkable improvement in the thermal stability, most probably arising from strong interfacial interaction as already underlined by rheology. | ||
| Fig. 9 (a) TGA curves of LDH/PP composites. (b) FT-IR spectra of pure PP during thermal aging. The carbonyl peak area of LDH/PP composites during (c) thermal aging and (d) light aging. | ||
Another evaluation of thermal stability is to perform accelerated thermal aging. LDH/PP films were employed at 150 °C and tested with FT-IR every 80 min. For example, Fig. 9b shows the FT-IR spectra of PP film (without a LDH filler) at different aging times. The PP film gradually discolored, hardened and finally completely cracked within 240 minutes, and this is associated with the carbonyl absorption band (1810–1660 cm−1) becoming larger and larger. Therefore, the area of the carbonyl absorption band can be used to evaluate the thermal aging degradation behavior of PP. Fig. 9c further depicts the integrated area of some LDH/PP composites after different aging times. There are three time scales observed during the thermal-oxidative degradation: (1) a very short period during which PP film is totally broken after a thermal aging time of 240 min, that shows that PP has no ability against thermal aging; (2) a medium period as HALS-LDH/PP presents certain ability (about 720 min) but not enough to be stabilized on a longer time, and (3) a much longer period for other LDH fillers that show efficient effect on the long-term stability of LDH/PP composites and ranging as H1D1-LDH/PP > DBHP-LDH/PP > H1D2-LDH/PP > (H-LDH)1(D-LDH)2/PP > (H-LDH)1(D-LDH)1/PP. Thus, the HxDy-LDH series present much higher promoter action against the thermal aging of PP compared to that of HALS/DBHP-LDH and of the simple physical mixture (H-LDH)x(D-LDH)y. It underlines that the beneficial effect of the ratio between HALS and DBHP in the interlayer and their co-intercalated structure both retard the thermal-aging degradation for PP, most presumably in a synergistic way.
3.4. Light stability of LDH/PP composites
Furthermore, Fig. 9d shows the light-aging degradation of LDH/PP. In time, all the LDH/PP composites present a gradually increasing light oxidation following the aging time until 100 min, and accordingly the carbonyl peak region is increased. The aging process is differentiated in two ways: (1) the integrated area of PP is slightly smaller than that of LDH/PP before 40 min, highlighting that LDHs do not obviously succeed in inhibiting the light-oxidation degradation for PP at early exposure time. However, the difference of all samples is very small. (2) With the extension of the aging time, PP exhibits faster growth and larger intensity after 40 min. It is indicated that LDH fillers have a positive effect on the long-term light-aging degradation of PP under the investigated conditions.4. Conclusions
The preparation of HALS and DBHP co-intercalated into LDHs to produce the inorganic–organic hybrid co-intercalated Zn2Al-HxDy-LDH material by adjusting the guest anion ratio (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y = 1
y = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was reported. LDH/PP nanocomposites based on HALS, DBHP and PP have been prepared by dispersing different LDH fillers in PP through two mixing methods: solvent mixing and extrusion mixing. The fillers are found to be well dispersed into polymers without influencing their crystallization state but providing a strong chain extension effect on PP, and this is even more pronounced for the series of co-intercalated HxDy-LDH. Among the samples, the thermal stability of HxDy-LDH composites is improved. Through the accelerated aging tests (thermal and UV exposure), the co-intercalated hybrid materials are found to decelerate the oxidative degradation for PP. The study highlights the beneficial and synergistic effect of using a co-intercalated HxDy-LDH hybrid structure as a new kind of toolbox, extending even further the possibility of this host structure, with, here, a tunable ratio for the co-added guest molecule for a better performance of PP in the selected applications.
2) was reported. LDH/PP nanocomposites based on HALS, DBHP and PP have been prepared by dispersing different LDH fillers in PP through two mixing methods: solvent mixing and extrusion mixing. The fillers are found to be well dispersed into polymers without influencing their crystallization state but providing a strong chain extension effect on PP, and this is even more pronounced for the series of co-intercalated HxDy-LDH. Among the samples, the thermal stability of HxDy-LDH composites is improved. Through the accelerated aging tests (thermal and UV exposure), the co-intercalated hybrid materials are found to decelerate the oxidative degradation for PP. The study highlights the beneficial and synergistic effect of using a co-intercalated HxDy-LDH hybrid structure as a new kind of toolbox, extending even further the possibility of this host structure, with, here, a tunable ratio for the co-added guest molecule for a better performance of PP in the selected applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (21571015, 21627813), the Fundamental Research Funds for the Central Universities (JD1716, 12060093063), and the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1205). The authors would like to thank CPER (challenge MMaSyF 2016) through the project METAPROFILE for the SAXS equipment.Notes and references
- S. D. Anuar Sharuddin, F. Abnisa, W. M. A. Wan Daud and M. K. Aroua, Energy Convers. Manage., 2016, 115, 308–326 CrossRef CAS
.
- T. V. Duncan, ACS Appl. Mater. Interfaces, 2015, 7, 20–39 CrossRef CAS PubMed
.
- Y. Peng, R. Liu and J. Cao, Appl. Surf. Sci., 2015, 332, 253–259 CrossRef CAS
.
- G. Zhang, C. Nam, T. C. M. Chung, L. Petersson and H. Hillborg, Macromolecules, 2017, 50, 7041–7051 CrossRef CAS
.
- M. Norouzi, Y. Zare and P. Kiany, Polym. Rev., 2015, 55, 531–560 CrossRef CAS
.
- M. Baron, J. C. Morris, S. Telitel, J. L. Clement, J. Lalevee, F. Morlet-Savary, A. Spangenberg, J. P. Malval, O. Soppera, D. Gigmes and Y. Guillaneuf, J. Am. Chem. Soc., 2018, 140, 3339–3344 CrossRef CAS PubMed
.
- E. A. Haidasz, D. Meng, R. Amorati, A. Baschieri, K. U. Ingold, L. Valgimigli and D. A. Pratt, J. Am. Chem. Soc., 2016, 138, 5290–5298 CrossRef CAS PubMed
.
- W. A. Yehye, N. A. Rahman, A. Ariffin, S. B. Abd Hamid, A. A. Alhadi, F. A. Kadir and M. Yaeghoobi, Eur. J. Med. Chem., 2015, 101, 295–312 CrossRef CAS PubMed
.
- E. A. Haidasz, R. Shah and D. A. Pratt, J. Am. Chem. Soc., 2014, 136, 16643–16650 CrossRef CAS PubMed
.
- J. Yang, Y. Huang, Y. Lv, S. Li, Q. Yang and G. Li, Carbon, 2015, 89, 340–349 CrossRef CAS
.
- R. Li, K. Shi, L. Ye and G. Li, Composites, Part B, 2019, 162, 11–20 CrossRef CAS
.
- Y. Yang, C. Hu, H. Zhong, X. Chen, R. Chen and K. L. Yam, J. Agric. Food Chem., 2016, 64, 7866–7873 CrossRef CAS PubMed
.
- K. A. Iyer, J. Lechanski and J. M. Torkelson, Composites, Part A, 2016, 83, 47–55 CrossRef CAS
.
- J. Liu, H. Pu, S. Liu, J. Kan and C. Jin, Carbohydr. Polym., 2017, 174, 999–1017 CrossRef CAS PubMed
.
- G. Zhang, C. Nam, L. Petersson, J. Jämbeck, H. Hillborg and T. C. M. Chung, Macromolecules, 2018, 51, 1927–1936 CrossRef CAS
.
- N. T. Dintcheva, R. Arrigo, E. Morici, C. Gambarotti, S. Carroccio, F. Cicogna and G. Filippone, Composites, Part B, 2015, 82, 196–204 CrossRef CAS
.
- J. Bu, X. Huang, S. Li and P. Jiang, Carbon, 2016, 106, 218–227 CrossRef CAS
.
- M. Kotal and A. K. Bhowmick, Prog. Polym. Sci., 2015, 51, 127–187 CrossRef CAS
.
- D. Basu, A. Das, K. W. Stöckelhuber, U. Wagenknecht and G. Heinrich, Prog. Polym. Sci., 2014, 39, 594–626 CrossRef CAS
.
- Q. Wang and D. O'Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed
.
- C. Li, M. Wei, D. G. Evans and X. Duan, Small, 2014, 10, 4469–4486 CrossRef CAS PubMed
.
- H. Zhong, C. Campos-Roldán, Y. Zhao, S. Zhang, Y. Feng and N. Alonso-Vante, Catalysts, 2018, 8, 559 CrossRef
.
- X. Mei, W. Wang, L. Yan, T. Hu, R. Liang, D. Yan, M. Wei, D. G. Evans and X. Duan, Biomaterials, 2018, 165, 14–24 CrossRef CAS PubMed
.
- E. N. Kalali, X. Wang and D. Wang, J. Mater. Chem. A, 2016, 4, 2147–2157 RSC
.
- D. Li, L. Qian, Y. Feng, P. Tang and L. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 20603–20611 CrossRef CAS PubMed
.
- Z. Gu, J. J. Atherton and Z. P. Xu, Chem. Commun., 2015, 51, 3024–3036 RSC
.
- C. Taviot-Guého, V. Prévot, C. Forano, G. Renaudin, C. Mousty and F. Leroux, Adv. Funct. Mater., 2018, 28, 1703868 CrossRef
.
- Q. Zhang, F. Leroux, P. Tang, D. Li and Y. Feng, Polym. Degrad. Stab., 2018, 154, 55–61 CrossRef CAS
.
- Q. Zhang, Q. Jiao, F. Leroux, P. Tang, D. Li and Y. Feng, New J. Chem., 2017, 41, 2364–2371 RSC
.
- Y. Gao, J. Wu, Z. Zhang, R. Jin, X. Zhang, X. Yan, A. Umar, Z. Guo and Q. Wang, J. Mater. Chem. A, 2013, 1, 9928–9934 RSC
.
- F. Leroux, A. Dalod, M. Hennous, L. Sisti, G. Totaro, A. Celli, C. Coehlo and V. Verney, Appl. Clay Sci., 2014, 100, 102–111 CrossRef CAS
.
- Y. Feng, Y. Jiang, Q. Huang, S. Chen, F. Zhang, P. Tang and D. Li, Ind. Eng. Chem. Res., 2014, 53, 2287–2292 CrossRef CAS
.
- Y. Zhao, G. Chen, T. Bian, C. Zhou, G. I. N. Waterhouse, L. Wu, C. Tung, L. J. Smith, D. O'Hare and T. Zhang, Adv. Mater., 2015, 27, 7824–7831 CrossRef CAS PubMed
.
- R. M. M. Santos, J. Tronto, V. Briois and C. V. Santilli, J. Mater. Chem. A, 2017, 5, 9998–10009 RSC
.
- Q. Zhou, V. Verney, S. Commereuc, I. J. Chin and F. Leroux, J. Colloid Interface Sci., 2010, 349, 127–133 CrossRef CAS PubMed
.
- L. Liu, M. Cheng and Z. Yang, Electrochim. Acta, 2018, 277, 67–76 CrossRef CAS
.
- J. H. Yang, W. Zhang, H. Ryu, J. H. Lee, D. H. Park, J. Y. Choi, A. Vinu, A. A. Elzatahry and J. H. Choy, J. Mater. Chem. A, 2015, 3, 22730–22738 RSC
.
- D. W. Schaefer and R. S. Justice, Macromolecules, 2007, 40, 8501–8517 CrossRef CAS
.
- P. J. Purohit, J. E. Huacuja-Sánchez, D. Y. Wang, F. Emmerling, A. Thünemann, G. Heinrich and A. Schönhals, Macromolecules, 2011, 44, 4342–4354 CrossRef CAS
.
- G. Totaro, L. Sisti, A. Celli, I. Aloisio, D. Di Gioia, A. A. Marek, V. Verney and F. Leroux, Dalton Trans., 2018, 47, 3155–3165 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qi00601j |
| This journal is © the Partner Organisations 2019 |