A low-cost, highly-conductive polyvinyl alcohol flexible film with Ag-microsheets and AgNWs as fillers†
Yongyun Mao*ab,
Junmei Guob,
Changyi Hub,
Hongwei Yangb,
Yuwen Yangb and
Song Chenb
aInstitute of Applied Physics and Materials Engineering, Faculty of Science and Technology, University of Macau, Avenida da Universidade, Taipa, Macau, China. E-mail: maoyongyun123@126.com
bState Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals, Kunming Institute of Precious Metals, Kunming, 650106, China
First published on 22nd August 2016
Abstract
To fabricate the conductive composites with the low resistivity required for conductive films, a multi-layer network of AgNWs must be produced which may not be cost effective. Recently, we have counteracted the problem by using AgNWs and Ag-microsheets to form novel overlapping-junctions-nanostructures, which can significantly improve the electrical properties of conductive films. Subsequently, low-cost, highly-conductivity flexible conductive films were fabricated using Ag-microsheets, Ag-nanowires (AgNWs) and polyvinyl alcohol (PVA) as conducting agents. Interestingly, the interconnected AgNWs and Ag-microsheets within the reciprocal architecture could effectively accommodate strain and show good conductivity under stretching (stretchability of 35%) before they ruptured into patches; thus having major commercial implications.
Recent advances in unconventional foldable and stretchable electronics have attracted increasing attention, including stretchable organic light-emitting diodes,1,2 electronic circuits,3 stretchable solar cells,4 flexible transparent electrodes5 and stretchable strain sensors.6,7 Consequently, highly stretchable materials with good conductivity and low-cost are highly desirable. Randomly distributed metallic nanowires in flexible polymers matrices, which allows high mechanical flexibility and good conductivity have thus become an attractive alternative for flexibility electronic devices and conductive films. The prominent conductivity property of AgNWs has made them promising materials for applications as conductive nano-fillers for flexible and stretchable electronic and devices.5,8,9 However, with the development of portable intelligent electronic devices, the research studies of AgNWs-based flexibility electronic devices no longer just focus on the using of nanowires, but also typically introduce some special morphologies conductive fillers to extend their electrical properties.10,11
Traditionally, the most common way to increase the electrical conductivity of conductive film is to introduce highly conductive fillers into the polymer matrix. But up to today, high loading of the fillers is required to achieve high electrical conductivity which sacrifices the lightweight, excellent processability and low cost of polymeric materials. Therefore, it is critical that forming the novel overlapping-junctions-nanostructures within the polymer matrix and decrease the loading of fillers, which could help the formation of electron conductive pathways. And more recently, a novel architecture that consist of one-dimensional (1D) AgNWs and two-dimensional (2D) microsheets have attracted increasing concerns since 2D microsheets play the role of framework to enhance the electrical conductivity and mechanical flexibility of the hybrid films.9,12–15 For instance, Shi et al. demonstrated a method of molecular level controlled fabrication of hybrid TCFs composed of 1D AgNWs and 2D reduced grapheme oxide sheets.16 Jurewicz et al. fabricated a conductive film with low sheet resistance by modifying the electrical properties of an ultra-low density nanowire network through local deposition of conducting grapheme platelets. Using the approach the amount of nanowires needed to produce viable transparent electrodes could be more than fifty times less than the equivalent pristine high density nanowires networks.17 Additionally, to achieve a low sheet resistance for conductive films, much effort has been expended to further reduce the contact resistance at overlapping junctions.18–21 However, the inherent junction resistance associated with electron transport between wires is one of the major limiting factors on the electrical conductivity of percolating assemblies of AgNWs. Although this may be overcome by welding at overlapping junctions, such method represents an expensive post-fabrication process.22–24 Fortunately, another possibility is to combine AgNWs with other nanostructured materials such as nanotube or microsheets.25,26 However, as a conductor, graphene sheet or nanotube has a poor compatibility with AgNWs and unevenly disperses in the matrix thus affecting the mechanical and electrical properties of the conductive films.14
As a conductive fillers, plate-like silver nanoparticles contact with each other at the boundary, which is superior to spherical particles.27 To reduce the percolation threshold for conductivity and enhance the electrical properties, Ag-microsheets with a high aspect ratio are preferred. Therefore, Ag-microsheets can be widely applied in various fields, such as pigments, optical materials, conductive-adhesives and so on.28–32 As we all know, both AgNWs and Ag-microsheets are the same metal materials, are processed very easily and are perfectly combined to each other. Therefore, Ag-microsheets have many advantages over the graphene sheet. However, few researches on the using of Ag-microsheets and AgNWs to form the novel overlapping-junctions-nanostructures, which can significant improve the electrical properties of conductive films thus far. Herein, we report a facile, scalable and relatively inexpensive method to obtain the highly conducting Ag-microsheets/AgNWs/PVA conductive films employing low-cost solution-processed Ag-microsheets and AgNWs. The results reveal that the synergic effect of AgNWs and Ag-microsheets via overlapping junctions for enhancing conductivity can be observed when they were introduced into the flexible conductive film. More interestingly, the overlapping-junctions-nanostructures reinforce the conducting network and exhibit no obvious change in electrical conductivity in the stretching or folding with elongation strains up to 35%.
AgNWs of large quantity were prepared according to our previous work.33 The large scale of Ag-microsheets were prepared in our laboratory and the detailed synthetic procedures and characterization methods are listed in the ESI.† Fig. 1 shows the typical scanning electron microscopy (SEM) images for the as-prepared Ag-microsheets and AgNWs. Fig. 1A and B demonstrate that the Ag-microsheets are uniform and flower-like shape, with a particle size about 2 μm. The thickness of the as-prepared Ag-microsheets is about 150–200 nm, as shown in Fig. 1B. It is clearly observed in Fig. 1A and B that the as-prepared relative to the previously reported micrometer-sized Ag sheets,12,34 the as-prepared Ag-microsheets are more uniform and better dispersity. Fig. 1C and C-1 present AgNWs with high aspect ratio and the diameter of ca. 50 nm and the length of ca. 50 μm can be synthesized, thus forming efficient percolating network for electrons transferring. Additionally, X-ray diffraction (XRD) was conducted to investigate the microstructure of the products. Fig. 1D depicts the XRD pattern of the uniform AgNWs and Ag-microsheets. All the diffraction peaks can be indexed to the (111), (200), (220), (311) and (222) planes of pure face-centered-cubic silver crystals, which is consistent with the standard value accrording to JCPDS card no. 04-0783.35 No impurities are detected, indicating the formation of highly pure AgNWs and Ag-microsheets.
 | ||
| Fig. 1 Low and high magnification SEM images of Ag-microsheets (A and B) and AgNWs (C and C-1); (D) XRD of fresh prepared AgNWs and Ag-microsheets. | ||
SEM micrographs were also taken to examine the morphologies of PVA composites films. The fracture surface morphologies of Ag-microsheets/PVA, AgNWs/PVA and Ag-microsheets/AgNWs/PVA nanocomposites were studied to compare with pure PVA. PVA conductive film materials were fabricated by a facile one strategy method of the aqueous dispersions of AgNWs and Ag-microsheets. The density of Ag-microsheets and AgNWs loading in PVA matrix can easily be controlled by varying the concentration of materials. Details of the preparation method for each solution are provided in the experimental section (ESI†). Once uniformly mixed, these solutions were poured into the Teflon plate. The Teflon plate was subsequently placed in the oven cured for about 1 h at 100 °C to give the conductive film. The neat PVA fracture surface exhibits a relatively smooth surface, as shown in Fig. 2A. The freeze-fractured Ag-microsheets/PVA composite film prepared with 20 wt% Ag-microsheets showed few agglomerations of Ag-microsheets, indicating the uneven dispersion in PVA matrix, as shown in Fig. 2B. Fig. 2C and C-1 show the SEM images of the as prepared AgNWs/PVA composite (10 wt% AgNWs) and the cross-section of AgNWs/PVA. It should be noted that the AgNWs of the resultant films were fully-embeded in the PVA polymer, and therefore distinct from previous reports in which AgNWs were buried in the polymer surface (as shown in Fig. 2C-1).5 Additionally, the connections between the crossed AgNWs were mainly due to van der Waals interactions between the AgNWs. The AgNWs were stacked closely, with no spaces between them, and thus form the conducting network and exhibit better electrical conductivity. Simultaneously, due to the addition of AgNWs, the Ag-microsheets can be dispersed in PVA matrix more uniformly combined with AgNWs (Fig. 2D) than the using of Ag-microsheets alone (Fig. 2B). As a consequence we believe that the enhanced electrical conductivity of the Ag-microsheets/AgNWs/PVA (10 wt% AgNWs and 5 wt% Ag-microsheets) composite film could be explained by the lack of agglomerations and more homogenous dispersion of the Ag-microsheets and AgNWs in the matrix compared to the using of Ag-microsheets alone. In other words, a high number of overlapping junctions between AgNWs and Ag-microsheets exist in the interconnected reciprocal architecture. Therefore we propose that the unusual conductivity performance of the Ag-microsheets/AgNWs/PVA conductive film is due to the synergic effect of AgNWs and Ag-microsheets.
Fig. 3 displays a typical high magnification SEM image of Ag-microsheets/AgNWs/PVA conductive film and schematic illustration of the overlapping-junctions-nanostructures. It clearly illustrates that the heavily intertwined AgNWs and Ag-microsheets may result in increased wire-sheet-wire contact, as shown in Fig. 3A. Simultaneously, many parts of AgNW are “adsorbed” on the Ag-microsheets interface it has been shown in red rectangle in Fig. 3A and the novel overlapping-junctions-nanostructures were formed. Such novel overlapping-junctions-nanostructures can lead to an enhancement in conduction via “percolation doping” within the hybrid structure.36 Moreover, as marked by the red rectangle in Fig. 3A, the Ag-microsheets efficiently bridging the very small amount of crossed AgNWs can provide enough conductive pathways. Therefore, the improvement of conductivity can be attributed to the bridging of AgNWs by Ag-microsheets, providing additional conduction channels through the overlapping-junctions-nanostructures. From the analysis above, it seems that the Ag-microsheet-bridging phenomenon existed in the overlapping-junctions-nanostructures and the Ag-microsheets look like conductive-islands, which serve as an electrical connector between so many AgNWs, as shown in Fig. 3B. Therefore, the conductivity of as-prepared Ag-microsheets/AgNWs/PVA (silver content of 15 wt%) conductive film is better than previously reported conductive films (silver content of 80 wt%), which usually show resistivity in the range of about 10−3 to 10−4 Ω cm.37–39 Obviously, the morphology of the conductive-islands has a strong influence on electrical conductance. The above results clearly demonstrate the potential role of the novel overlapping-junctions-nanostructures in conductive films.
 | ||
| Fig. 3 SEM image of Ag-microsheets/AgNWs/PVA (A) and schematic illustration of the overlapping-junctions-nanostructures (B). | ||
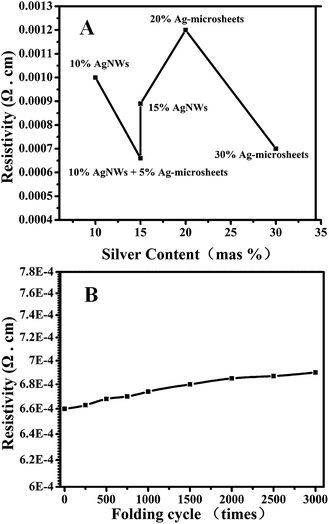
Electrical measurements of hybrid conductive films were conducted using the four-probe technique. The resistivity of Ag-microsheets/PVA (20 wt% and 30 wt% Ag-microsheets), AgNWs/PVA (10 wt% and 15 wt% AgNWs) and Ag-microsheets/AgNWs/PVA (10 wt% AgNWs and 5 wt% Ag-microsheets) conductive films as a function of the filler content are plotted in Fig. 4A. For AgNWs/PVA conductive films, the resistivity decreases slowly from 1.0 × 10−3 Ω cm to 0.89 × 10−3 Ω cm as AgNWs content increases from 10 wt% to 15 wt%. More interesting, to investigate the possible changes induced by the loading of Ag-microsheets conductive filler, the resistivity of the Ag-microsheets/AgNWs/PVA sample was compared. This found that the loading of Ag-microsheets conductive filler markedly reduced the resistivity from 0.79 × 10−3 Ω cm (AgNWs/PVA 15 wt% AgNWs) to 0.66 × 10−3 Ω cm (Ag-microsheets/AgNWs/PVA, 10 wt% AgNWs and 5 wt% Ag-microsheets) when they loading the same weight ratio of silver (Fig. 4A). The lower resistivity of the Ag-microsheets/AgNWs/PVA conductive film could be explained by the lack of agglomerations and more homogenous dispersion of the Ag-microsheets and AgNWs in the matrix compared to the using of Ag-microsheets alone. In the meantime, the using of AgNWs and Ag-microsheets can form the novel overlapping-junctions-nanostructures, which can significantly improve the electrical properties of conductive film compared to the AgNWs/PVA conductive film (15 wt% AgNWs). Additionally, the resistivity of Ag-microsheets/PVA films with different Ag-microsheets concentrations was also characterized and the resistivity decreases slowly from 1.2 × 10−3 Ω cm to 0.7 × 10−3 Ω cm as Ag-microsheets content increases from 20 wt% to 30 wt%. After comparison, at the very low Ag-microsheets contents, the resistivity of the Ag-microsheets/AgNWs/PVA film decreased evidently with the using of Ag-microsheets and AgNWs. The quality of the electrically conductive properties is determined by the synergic effect of AgNWs and Ag-microsheets and the forming of the novel overlapping-junctions-nanostructures. That is, the electrically conductive networks are formed through the contact points and contact areas between/among AgNWs and Ag-microsheets.
We also studied the resistivity vary according to the cycles of the composite bend until the sample was folded. As shown in Fig. 4B, the resistivity remained stable at ca. 0.66–0.69 × 10−3 Ω cm until the folding cycles reached 3000 times. The result demonstrate that the resistivity at each folding times don not show obvious change, indicating that the Ag-microsheets/AgNWs/PVA conductive film can be used as ideal interconnects in flexible electronic applications. Based upon the results in this work, it is believed that the Ag-microsheets/AgNWs/PVA conductive film should be a promising conductive material in electronics industry, which requires high electrical conductivity and foldability.
The mechanical properties of neat PVA and conductive nanocomposites films were systematically investigated at room temperature. Fig. 5 shows the stress–strain curves of different conductive nanocomposites films and pure PVA film. After the loading of silver conductive fillers, the tensile stress of all the conductive films decreased compared to the pure PVA polymer. In addition, it was important to mention that the PVA composites which loading AgNWs exhibited the largest elongation-at-break because of the good movement of the PVA chains. However due to the agglomerations and uneven dispersion of Ag-microsheets in PVA matrix (as shown in Fig. 2B), the tensile stress and elongation-at-break declined sharply. The possible cause of this problem is that the agglomerations and uneven dispersion of Ag-microsheets restricts the movement of the PVA chains.14 Additionally, the overlapping-junctions-nanostructures facilitated high stretchability of 25% without obvious degrading the conductivity of Ag-microsheets/AgNWs/PVA conductive film.
Fig. 6A shows the deformation of Ag-microsheets/AgNWs/PVA nanocomposite conductive film after the stretching of 33%. At ε (stretchability) = 33%, a number of micro-cracks (red rectangle in Fig. 6A) and newly created micro-cracks by the applied strain were opened with width from several micrometers to a few tens of micrometers in a perpendicular orientation to the direction of elongation. In order to confirm whether the interconnected AgNWs and Ag-microsheets within the reciprocal architecture could effectively accommodate strain and show good conductivity under stretching. The change tendency of resistivity of the conductive film under various stretching conditions has been preliminary studied. Fig. 6B shows the resistivity remained stable at ca. 0.66–2 × 10−3 Ω cm until the ε (stretchability) = 25%, and then the resistivity sharply increased to 0.019 Ω cm when the ε reached to 33%. That is because the electrical current path thus becomes narrower and longer due to the hindrance by the increased micro-cracks rather than fracture of current path. Additionally, it can be seen that the surface of the nanocomposite was flat without any noticeable ruptures when the nanocomposite stretched within the scope of ε = 30% (Fig. 6A). The result demonstrate that the resistivity at each stretching condition don not show obvious change and the Ag-microsheets/AgNWs/PVA conductive film should be a promising conductive material in electronics industry, which requires high electrical conductivity and flexibility.
 | ||
| Fig. 6 The deformation SEM image of Ag-microsheets/AgNWs/PVA conductive film after the stretching of 30% (A), changes of the resistivity with the tensile strain (B). | ||
To summarize, we have fabricated a novel overlapping-junctions-nanostructures through a facile, scalable and relatively inexpensive method. The interconnected AgNWs and Ag-microsheets within the reciprocal architecture could effectively accommodate strain and show good conductivity under stretching (stretchability of 35%) before they ruptured into patches. By comparison, it seems that the perfect two-dimensional shape of the Ag-microsheets filler favors the formation of novel overlapping-junctions-nanostructures and benefits the formation of the electron conductive pathways, thus helps to improve the electrical conductivity of the composites. This result open the way to new highly conducting polymer-based conductive film efficiently based on the overlapping-junctions-nanostructures and decrease the loading of conductive fillers.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 61540074), the Basic Applied Research Foundation of Yunnan Province, China (Grant No. 2016FD126) and the fund of the State Key Laboratory of Advanced Technologies for Comprehensive Utilization of Platinum Metals (No. SKL-SPM-201521).Notes and references
- M. S. White, M. Kaltenbrunner and E. D. Glowacki, et al., Nat. Photonics, 2013, 7, 811 CrossRef CAS.
- H.-G. Cheong, R. E. Triambulo and G.-H. Lee, et al., ACS Appl. Mater. Interfaces, 2014, 6, 7846 CAS.
- Y. Mao, C. Wang and H. Yang, Mater. Lett., 2015, 150, 101 CrossRef CAS.
- D. J. Lipomi, B. C. K. Tee and M. Vosgueritchian, et al., Adv. Mater., 2011, 23, 1771 CrossRef CAS PubMed.
- S. M. Jeong, J.-H. Kim and S. Song, et al., RSC Adv., 2015, 5, 51086 RSC.
- H. Lee, B. Seong and H. Moon, et al., RSC Adv., 2015, 5, 28379 RSC.
- M. Amjadi, A. Pichitpajongkit and S. Lee, et al., ACS Nano, 2014, 8, 5154 CrossRef CAS PubMed.
- H.-W. Cui, J.-T. Jiu and K. Suganuma, et al., RSC Adv., 2015, 5, 7200 RSC.
- Z. Luo, Z. Cai and Y. Wang, et al., RSC Adv., 2016, 6, 37124 RSC.
- L. Shen, L. Du and S. Tan, et al., Chem. Commun., 2016, 52, 6296 RSC.
- N. Li, G.-W. Huang and H.-M. Xiao, et al., Composites, Part A, 2015, 77, 87 CrossRef CAS.
- H.-M. Ren, Y. Guo and S.-Y. Huang, et al., ACS Appl. Mater. Interfaces, 2015, 7, 13685 CAS.
- R.-Z. Li, A. Hu and D. Bridges, et al., Nanoscale, 2015, 7, 7368 RSC.
- S. Yao, Y. Li and Z. Zhou, et al., RSC Adv., 2015, 5, 91878 RSC.
- X. Tian, G. Li and Z. Tu, et al., RSC Adv., 2016, 6, 597 RSC.
- L. Shi, J. Yang and T. Yang, et al., RSC Adv., 2014, 4, 43270 RSC.
- I. Jurewicz, A. Fahimi and P. E. Lyons, et al., Adv. Funct. Mater., 2014, 24, 7580 CrossRef CAS.
- M. Mol Menamparambath, C. Muhammed Ajmal and K. Hee Kim, et al., Sci. Rep., 2015, 5, 16371 CrossRef PubMed.
- W. Leclerc, P. Karamian and A. Vivet, et al., Technische Mechanik, 2012, 32, 358 Search PubMed.
- W. Xu, W. Chen and H. Chen, et al., Phys. A, 2014, 399, 126 CrossRef.
- W. Leclerc, P. Karamian-Surville and A. Vivet, Comput. Mater. Sci., 2013, 69, 481 CrossRef.
- E. C. Garnett, W. Cai and J. J. Cha, et al., Nat. Mater., 2012, 11, 241 CrossRef CAS PubMed.
- S. Zhu, Y. Gao and B. Hu, et al., Nanotechnology, 2013, 24, 335202 CrossRef PubMed.
- J. Lee, I. Lee and T. S. Kim, et al., Small, 2013, 9, 2887 CrossRef CAS PubMed.
- T. Tokuno, M. Nogi and J. Jiu, et al., Nanoscale Res. Lett., 2012, 7, 1 CrossRef PubMed.
- R. Chen, S. R. Das and C. Jeong, et al., Adv. Funct. Mater., 2013, 23, 5150 CrossRef CAS.
- Y. Liguo and Z. Yanhua, Rare Met. Mater. Eng., 2010, 39, 401 CrossRef.
- B. Sadeghi, M. Sadjadi and R. Vahdati, Superlattices Microstruct., 2009, 46, 858 CrossRef CAS.
- Z. Cao, H. Fu and L. Kang, et al., J. Mater. Chem., 2008, 18, 2673 RSC.
- Y.-K. Kim and D.-H. Min, RSC Adv., 2014, 4, 6950 RSC.
- J. Sun, X. Wang and J. Liu, et al., RSC Adv., 2014, 4, 35263 RSC.
- X.-Y. Zhang, A. Hu and T. Zhang, et al., ACS Nano, 2011, 5, 9082 CrossRef CAS PubMed.
- Y. Mao, C. Wang and H. Yang, Mater. Lett., 2015, 142, 102 CrossRef CAS.
- M. H. Kim, D. K. Yoon and S. H. Im, RSC Adv., 2015, 5, 14266 RSC.
- K. L. Kelly, E. Coronado and L. L. Zhao, et al., J. Phys. Chem. B, 2002, 107, 668 CrossRef.
- C. Jeong, P. Nair and M. Khan, et al., Nano Lett., 2011, 11, 5020 CrossRef CAS PubMed.
- C. Yang, W. Lin and Z. Li, et al., Adv. Funct. Mater., 2011, 21, 4582 CrossRef CAS.
- Y.-H. Ji, Y. Liu and G.-W. Huang, et al., ACS Appl. Mater. Interfaces, 2015, 7, 8041 CAS.
- A. Mahajan, W. J. Hyun and S. B. Walker, et al., ACS Appl. Mater. Interfaces, 2015, 7, 1841 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c6ra17851k |
| This journal is © The Royal Society of Chemistry 2016 |