A mobile laboratory for rapid on-site analysis of catechols from water samples with real-time results production
Yun Wanga,
Yuanyuan Lia,
Yan Liu*a,
Juan Hanb,
Jinchen Xiaa,
Xu Baoc,
Liang Nia and
Xu Tanga
aSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, PR China. E-mail: lyan@ujs.edu.cn; Fax: +86-0511-88791800; Tel: +86-137-7553-9306
bSchool of Food and Biological Engineering, Jiangsu University, Zhenjiang 212013, PR China
cSchool of Computer and Communications Engineering, Jiangsu University, Zhenjiang 212013, PR China
First published on 9th August 2016
Abstract
The search for a practical method to analyze cis-diol-containing compounds outside laboratory settings remains a substantially scientific challenge. Herein, we used a “mobile laboratory”, wherein a filter paper-based colorimetric sensor array, a smartphone, and a remote server were combined together, for rapid on-site analysis of catechols from water samples with real-time results production. A smallest-scale filter paper-based 2 × 2 colorimetric sensor array composed of pH indicators and phenylboronic acid was configured. The array was able to distinguish 7 water-soluble catechols at 7 serial concentrations, through simultaneous treatment via principal component analysis, hierarchical cluster analysis, and linear discriminant analysis. After both the discriminatory power of the array and the prediction ability of the partial least squares quantitative models were proven to be predominant, the smartphone was coupled to the remote server. All the ΔRGB data were uploaded to the remote server wherein linear discriminant analysis and partial least squares processing modules were established to provide qualitative discrimination and quantitative calculation, respectively, of the analytes in real time. The applicability of this novel method to a real-life scenario was confirmed by the on-site analysis of various catechols from a water sample of the Yangtze River; the feedback result in the smartphone showed that the method was able to identify catechols with 100% accuracy and predict the concentrations to within 0.484–4.08 standard deviation.
Introduction
Catechols are widely produced and employed in industries worldwide wherein they are important for pharmaceuticals and fine chemical intermediates, which also include 1,2,4-benzenetriol and 4-methylcatechol. While the tremendous value of catechols is embodied, the environmental pollution caused by the massive illegal discharge and accidental leakage of catechols cannot be ignored. This is because most catechols have good water solubility and maximum concentration of water-soluble catechols in the water is always over 300 mM. Once leaked into a natural water source in high concentrations, these catechols cause great harm to human health through the food chain.1 Catechol, being a significant environmental pollutant with high toxicity, can irritate human eyes and skin through contact. Even at low concentrations in foods, catechol can give an undesirable taste and it is readily adsorbed from the gastrointestinal tract, adversely resulting in liver function decrease and renal tube degeneration.2 Dopamine hydrochloride, on addition in large amounts to the feed or the drinking water, can lower the fat content in pigs. Meat from these pigs can cause adverse health effects if consumed by humans over long periods, which may include headache, nausea, weakness, and convulsions of the limbs.3In recent years, drinking water safety has become a great concern since growing evidences indicate that long-term exposure to contaminated drinking water can induce adverse effects on both humans and wildlife.4,5 The Yangtze River is the longest river in China, providing the source of drinking water for most cities along the river. However, contamination incidents, which are caused by the accidental leaks occurring during ship transportation of various chemicals, such as organic compounds6 and heavy metals,7 are emerging in this river. In 2012, a serious contamination of the drinking water supply occurred in the city of Zhenjiang, China. It took a long time to discover that the pollution was caused by phenol leaking into the Yangtze River in the Zhenjiang section. Therefore, rapid methods to detect water contaminants such as catechols are highly desirable in terms of accomplishing a timely treatment of pollutants at high concentrations.
Traditional methods of detecting catechols, such as HPLC and spectrophotometric method, are inconvenient due to high costs, cumbersome operation of the analytical instruments, and slow analysis speed, which hinders their use as rapid detection methods. In recent years, many colorimetric sensor arrays have been developed as powerful tools to rapidly detect cis-diol-containing compounds. Lim and co-workers reported a low-cost, simple colorimetric sensor array capable of identifying 15 different monosaccharides, disaccharides, and artificial sweeteners.8 Zhang et al. employed a colorimetric sensor array to successfully discriminate 8 cis-diol-containing flavonoids.9 This sensor array methodology is famous for its low cost, simple processing steps, and rapid detection. When utilized to analyze real-life samples, however, one drawback of this sensor array method is that the collection of data involves a time-consuming process of image collection with a camera or a scanner, and later processing of those images with a computer. In addition, these sensor arrays can only be used for the identification of various analytes coupled with principal component analysis (PCA),10 hierarchical cluster analysis (HCA),11 or linear discriminant analysis (LDA),12 which imposes some restrictions on their application. So the development of a “mobile laboratory” where cis-diol-containing compounds can be qualitatively and quantitatively analyzed and experimental results can be produced in real time would be a great improvement for the use of colorimetric sensor arrays outside laboratory settings.
In this work, a “mobile laboratory”, where a filter paper-based colorimetric sensor array, a smartphone, and a remote server were combined together, was created for rapid on-site analysis of catechols from water samples with real-time results production. The smallest-scale 2 × 2 colorimetric sensor array composed of pH indicators and phenylboronic acid was configured and the smartphone was functionalized as a reader for the colorimetric sensor array. The smallest-scale 2 × 2 colorimetric sensor array was used to qualitatively identify 7 water-soluble catechols at 7 serial concentrations coupled with PCA, HCA, and LDA. Concentrations of these analytes were then quantitatively determined coupling with partial least squares (PLS).13 After the simultaneous treatment via PCA, HCA, LDA, and PLS, the ΔRGB data of these 7 different catechols at 7 serial concentrations were uploaded to a remote server using the smartphone to establish the LDA and PLS processing modules, which allowed the rapid and convenient on-site analysis of catechols from water samples in the Yangtze River. Finally, the analysis results, including the classification and concentration information, are fed back to the smartphone by the remote server in real time. To the best of our knowledge, this is the first paper dealing with the on-site detection of analytes using a smartphone-based colorimetric reader coupled to a remote server.
Materials and methods
Chemicals and instruments
Phenylboronic acid was purchased from Sigma (U.S.). Methyl orange, congo red, xylenol orange, and rhodizonic acid were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Catechol, 4-methylcatechol, 1,2,4-benzenetriol, 3,4-dihydroxybenzaldehyde, dopamine hydrochloride, 3-fluorocatechol, and 3,4-dihydroxybenzoic acid were purchased from Shanghai Haorui Chemical Co., Ltd. (Shanghai, China). Phosphate buffer (50 mM, pH 9.0) was prepared from phosphoric acid by titration with NaOH solution. Without further pH adjustment, this buffer was used to prepare the phenylboronic acid (50 mM) solution for the configuration of the colorimetric sensor array. Solutions of the catechols (10, 20, 50, 100, 150, 200, 250 mM) were prepared in de-ionized water. pH indicator solutions (0.1%, w/v) were prepared in de-ionized water and alcohol–water solutions.Qualitative filter paper 102 (Φ = 7 cm) was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). A commercially available STAEDTLER Lumocolor permanent universal pen and a white light-emitting diode (LED) light were used. The smartphone (Samsung Galaxy A8) with a 1.5 GHz Qualcomm Snapdragon 615 CPU and a 16 MP camera was employed in this study. Its high-performance CPU was capable of image recognition and calculation in real-time, and the high-resolution camera produced finely digitized images. An app on the smartphone was coded using Java and Android Studio 1.3.2 in order to achieve image rendering, automatic color recognition, and calibration.
Configuration of filter paper-based 2 × 2 colorimetric sensor array
In this report, a common filter paper was utilized as the substrate material for the preparation of the filter paper-based colorimetric sensor array. 4 circles with a 6 mm inner diameter were drawn onto the filter paper surface by the STAEDTLER Lumocolor permanent universal pen to build a 2 × 2 matrix (Fig. 1a). It is well-known that a filter paper can easily wick and absorb fluids.14 Since the ink of the pen is hydrophobic, the 4 circles on the filter paper could create hydrophobic barriers that would block the diffusion of the fluids inside the circles. Phenylboronic acid and one pH indicator (methyl orange, congo red, xylenol orange, rhodizonic acid) served as an ensemble probe. 4 pH indicator solutions (0.15 mL each) were respectively added to 4 glass tubes containing the phenylboronic acid phosphate buffer solution (50 mM, 0.1 mL), forming 4 ensemble probes. Next, 7 different catechol solutions with specific concentrations (10, 20, 50, 100, 150, 200, 250 mM, 0.25 mL each) including water (blank control), were each added to the 4 glass tubes. In this way, a 10 mM phosphate buffer with 10 mM phenylboronic acid in the absence of catechols (water control) at an initial pH of 7.5 was finalized as the condition, and the final concentrations of catechols that were detected at this condition were 5, 10, 25, 50, 75, 100, 125 mM. After 5 min of mixing for full color development, 2 μL of the mixtures in the 4 glass tubes were taken out and dropped in the 4 circles on the filter paper, thus constituting a filter paper-based 2 × 2 colorimetric sensor array. | ||
| Fig. 1 Schematic of (a) filter paper-based 2 × 2 colorimetric sensor array and, (b) smartphone-based colorimetric reader. | ||
Processing of data
A schematic data acquisition and processing by the smartphone/colorimetric sensor array ensemble, designed in-house by our research group, is shown in Fig. 1b. The filter paper was transferred to a light-tight box containing a white LED light at the bottom and a smartphone fixed at the top. With this equipment, ambient lighting conditions and the imaging distance/angle were kept constant when capturing the images of the sensor array. The “before” image consisted of a shot taken with the 16 megapixel (MP) smartphone camera capturing the color of each ensemble probe and water; the “after” image consisted of a camera shot directed towards the 2 × 2 array of ensemble probe/analyte mixtures. Using our laboratory designed app on the smartphone, a color difference map was obtained by subtracting the “before” image from the “after” image (red minus red, green minus green, blue minus blue). To prevent subtraction artifacts caused by acquisitions near the spot edge, only the spot center was included in the calculation. All experiments were run in quintuplicate for each of the 7 analytes and the blank control. Next, the ΔRGB data was processed by PCA, HCA, LDA, and PLS analysis using a computer before being uploaded to a remote server.Creation of “mobile laboratory” for on-site analysis of catechols
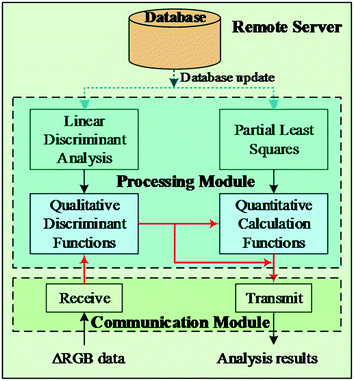
The smartphone was connected to a remote server via a wireless-network using a user datagram protocol (UDP). As shown in Fig. 2, the remote server consisted of three parts: the database, processing modules, and communication modules. The database in the remote server plays a role in storing the array data (ΔRGB). It must be noted that the ΔRGB data from the array has been processed by PCA, HCA, LDA, and PLS analysis before connecting the smartphone to the remote server. The processing module is programmed and linked into the Java package using the Matlab 2013a software. The Eclipse Integrated Development Environment (IDE) for Java Developers was used to create the communication module in order to process the analyte data by linking with the Java created processing module. The communication module functions by receiving the ΔRGB data of one unknown analyte and transmitting the analysis results to the smartphone.To qualitatively and quantitatively analyze unknown samples, all the ΔRGB data previously subjected to PCA, HCA, LDA, and PLS analysis are uploaded to the database of the remote server, which can be used to form the analysis polynomials for LDA and PLS. Once the database is updated after the investigation of more of the 7 catechol serial concentrations, the analysis polynomials are re-derived correspondingly. When new analyte data arrives at the receive module of the remote server, it is first sent to the LDA processing module, where the data undergoes classification discriminant analysis. Next, the analyte data and the classification result are forwarded to the PLS processing module for detailed calculation of the concentration. Finally, the analysis results, including the classification and concentration information, are fed back to the smartphone. So in this “mobile laboratory” where a filter paper-based colorimetric sensor array, a smartphone, and a remote server are combined together, rapid on-site analysis of catechols from water samples with real-time results production can be carried out anytime and anywhere.
Results and discussion
Filter paper-based 2 × 2 colorimetric sensor array responses
The selective association of boronic acids with diols has been extensively studied.15–17 As shown in Fig. 3, boronic acids can form reversible cyclic ester complexes with cis-diols with concomitant hydronium ion generation, thereby lowering the pH of the solution. Different catechols (Fig. 4) generate very different changes in the pH when reacting with boronic acid because of the different binding constants; therefore, boronic acid and pH indicators can serve as ensemble probes to facilitate discrimination of different catechols.Herein, 4 pH indicators and phenylboronic acid (pKa 8.8)18 were used as ensemble probes for full discrimination of 7 catechols. It is well known that the pKa values of phenylboronic acid vary upon complexation to different cis-diols. This decrease in pKa translates into an increased acidity of the solution; the presence of pH indicators in the ensemble probes therefore reflects this change through the variation in RGB values obtained from the colorimetric sensor array responses.
The color difference maps for the 7 different catechols at 7 serial concentrations, which prove to be unique for each analyte, are shown in the inset maps of Fig. 6a–g. Although the concentration of catechols reaches as low as 5 mM, the smallest-scale 2 × 2 colorimetric sensor array displays a high overall response, which indicates that phenylboronic acid has a great affinity for catechols in this work. In previous studies, probe sets composed of various pH indicators and one or more boronic acids were required to produce desirable discrimination of the cis-diol-containing analytes.8,16,19 In this work, an attempt was made to differentiate the greatest number of water-soluble analytes with the fewest number of ensemble probes. Only 4 ensemble probes were used to configure the smallest-scale 2 × 2 colorimetric sensor array to differentiate as many as 7 catechols with 7 serial concentrations, which made great progress in the number of the probes. Moreover, the substrate material for the preparation of the 2 × 2 colorimetric sensor array was a common filter paper, which was portable and low-cost. Therefore, the filter paper-based 2 × 2 colorimetric sensor array in this paper is associated with some prime advantages, including simplifying the operation steps and reducing the costs when applied to detect practical samples.
PCA analysis of filter paper-based 2 × 2 colorimetric sensor array
The filter paper-based 2 × 2 colorimetric sensor array was used for the discrimination of 7 catechols at 7 serial concentrations (5, 10, 25, 50, 75, 100, 125 mM). Each analyte in the array is represented as a 12-dimensional vector since the color difference map is a 3N-dimensional vector, where N is the number of pH indicators in the array, i.e. (4 red, blue and green difference values in a 2 × 2 colorimetric sensor array). Then, PCA was used to compress and visualize the high-dimensional discriminant information using the Matlab software.The color difference maps shown in the inset maps of Fig. 6a–g were easily distinguished by the naked eye even without statistical analysis. At a first glance, the changes in pH values in the acidic or alkaline solution environment accounted for the colorimetric changes of the array. When we classified the analytes by PCA, however, a single dimension (i.e. pH values) and even two dimensions failed to achieve a satisfactory discrimination of the 7 catechols with 7 serial concentrations (5, 10, 25, 50, 75, 100, 125 mM). Therefore, colorimetric array data with more independent dimensions were required for the optimal identification of closely related catechols. According to the PCA analysis, 4–6 principal components were required to contain over 99% of all data variance of the 7 serial concentrations. This high dimensionality indicates that the colorimetric array senses more than the changes in pH, which is consistent with conclusions of previous studies.8,16,20 In addition to the selective association between phenylboronic acid and catechols, there are other non-pH-related analyte–dye interactions that assist in the discrimination process. These include Lewis acid–base interactions, hydrogen-bonds, π–π bonds, dipolar bonds, etc.
Many sensor arrays based on specific or selective interactions between receptors and analytes have been developed. Although PCA showed that for these sensor arrays, over 90% of all discriminatory information was contained within the two principal components and the ability to differentiate similar analytes was limited.21–24 Other sensor arrays that are able to identify the analytes unambiguously, but require the use of multiple sets of probes have been configured. In other words, an optimized colorimetric sensor array for the detection of a wide range of analytes is unavailable.8,20,25–28 In contrast, only 4 ensemble probes were used in this paper, making up a smallest-scale 2 × 2 array. PCA analysis shows that the first three-dimensional principal components account for 98.55%, 98.67%, 98.38%, 95.99%, 95.7%, 93.42%, 94.06% of the total discrimination information, respectively, of the 7 serial concentrations. These high percentages demonstrate an extraordinary chemical discrimination ability for the smallest-scale 2 × 2 colorimetric sensor array. As shown in Fig. 5a–g, 7 catechols at 7 serial concentrations and 1 control are visually clustered together and separated from each other in the three-dimensional plot, which indicates the strong reproducibility and discriminatory power of the colorimetric sensor array. Moreover, the concentration of catechols that can be visually distinguished in the PCA space could be as low as 5 mM, which indicates that the filter paper-based 2 × 2 array can be applied for microanalysis.
HCA analysis of filter paper-based 2 × 2 colorimetric sensor array
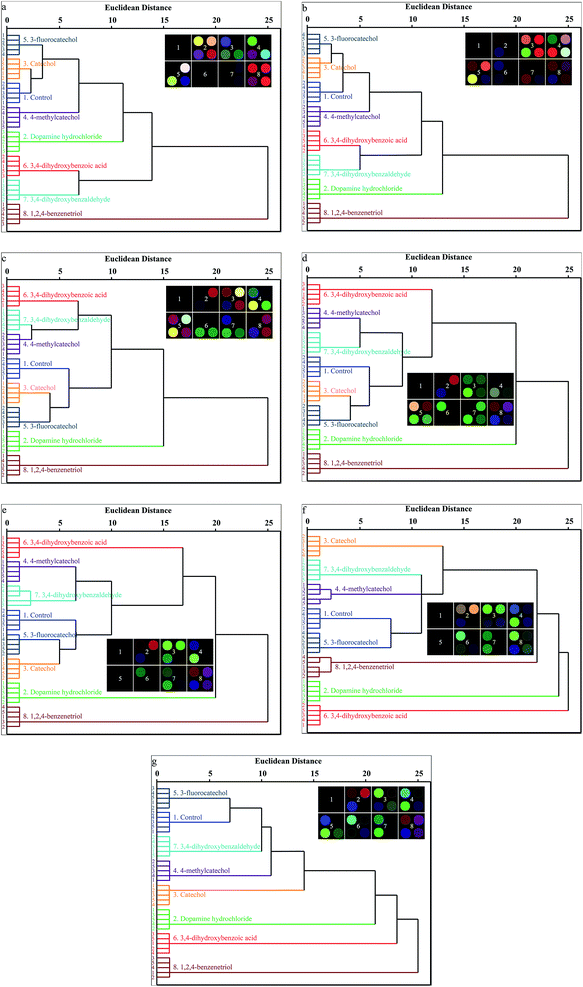
The high dispersion of the colorimetric sensor array data requires a classification algorithm that takes advantages of the full dimensionality of the data. The HCA is a simple and model-free approach, which forms dendrograms based on clustering of the array response data in the 12-dimensional ΔRGB color space in this work. HCA analysis was performed using the SPSS Statistics 20 software to group the 7 catechols with 7 serial concentrations (5, 10, 25, 50, 75, 100, 125 mM) based on the similarity in the response pattern of the 2 × 2 array. Clustering was defined according to the Euclidean distance. Quintuplicate trials were run for each analyte. All the 7 catechols at 7 serial concentrations plus 1 control were accurately identified against one another (Fig. 6a–g). Remarkably, for the 40 total cases (7 catechols and 1 control × 5 replicates) for each concentration, there were no errors and zero misclassifications. Both the properties of the substituents (electron-donating and electron-withdrawing groups) and the locations of the substituents on the phenyl rings of the catechols can affect the binding affinities of the catechols to phenylboronic acid. This binding affinity and other non-pH related analyte–dye interactions (Lewis acid–base, dipolar, hydrogen-bond, etc.) assist in the appropriate clustering and discrimination of the 7 analytes in the HCA dendrogram.LDA analysis of filter paper-based 2 × 2 colorimetric sensor array
LDA is commonly used for data classification and dimensionality reduction. Discriminant functions are established as a linear combination of descriptors that maximize the ratio of inter-class variance and minimize the ratio of intra-class variance.28 The data from the sensor array were processed using the SPSS Statistics 20 software, and LDA scatter plots were obtained using the discriminant functions. Fig. 7a–g show the distributions of the 7 catechols at 7 serial concentrations plus 1 control in a 2D space defined by the first two calculated discriminant functions (Function 1 and Function 2). Quintuplicate trials were run for each analyte. In the scatter plot, the overlapping of the points demonstrates the reproducibility of the analysis and the divided groups suggest that the filter paper-based 2 × 2 colorimetric sensor array displays high discrimination among the 7 catechols at 7 serial concentrations.LDA serves as a useful method for testing the accuracy of the colorimetric sensor array since it can be utilized to not only visualize the discriminatory ability of the colorimetric sensor array but also identify the unknown samples. Leave-one-out cross-validation was used to evaluate the predictive power of LDA. According to the results from the classification matrix of the LDA model, there were no incorrect classifications among the 40 cases (7 catechols and 1 control × 5 replicates) for each concentration, affording a prediction accuracy of 100%.
Creation of PLS quantitative models
PLS is a multivariate calibration method that searches for factors which explain as much covariance as possible between a set of predictor variables, X, and response variables, Y.13 In this section, PLS quantitative models were established based on the ΔRGB data of the 7 catechols with 8 serial concentrations (0, 5, 10, 25, 50, 75, 100, 125 mM). The principal components have an important influence on the predictive ability of the PLS quantitative models. To obtain the optimal models for accurately predicting the concentrations of the 7 catechols, a cross-validation method was used to select an appropriate number of principal components. The effect of the principal components on the root mean squared error (RMSE) value of each PLS quantitative model of the 7 catechols was studied. The smaller the RMSE value is, the more precise the PLS quantitative model created, according to the number of the principal components. According to the RMSE value, the number of the principal components (5–7) was eventually selected to create the 7 PLS quantitative models.The models were assessed with further samples in order to evaluate the accuracy and precision of the created prediction models. Fig. 8a–g display the actual analyte concentrations (mM) against the predicted concentrations (mM) using the PLS algorithm. The usefulness of the PLS algorithm was clearly demonstrated by subjecting the experimental data to linear regression analysis to obtain a line with the best fit. As shown in Fig. 8a–g, both the values of the slopes of the fitting lines (1.0763, 0.9733, 1.0545, 0.9547, 0.9997, 1.0184, 0.9733) and the values of the regression coefficients (0.9988, 0.9996, 0.9957, 0.9896, 0.9995, 0.9979, 0.9976) are very close to 1, which demonstrate the accuracy of the 7 PLS quantitative models. The values of RMSEs (1.029, 2.3277, 2.1221, 4.9542, 0.4369, 1.1076, 0.7281) of the 7 PLS quantitative models are low, which proves their high precision. In conclusion, the PLS quantitative models in this paper are a powerful tool for predicting the concentrations of catechols.
On-site analysis of practical samples
In an attempt to evaluate the feasibility of this novel method to real-life examples, 7 catechols were all analyzed on site from a sample of water from the Yangtze River. Each analyte was added to a separate solution of water from the Yangtze River at two concentrations (50, 125 mM). The Yangtze River water solution was regarded as a blank control, which contained no catechols (0 mM). According to the steps described in the Section “Configuration of filter paper-based 2 × 2 colorimetric sensor array” and “Processing of data”, the filter paper-based 2 × 2 colorimetric sensor arrays were configured and the ΔRGB data of unknown samples were subsequently obtained using the smartphone. The new ΔRGB data were uploaded to the remote server and the feedback information was displayed on the smartphone in real time. Analysis was performed three times per analyte concentration.We were gratified to learn that all analytes at all concentrations were identified without even one instance of misclassification, as shown in Table 1. Although there were some unavoidable deviations from the predicted and actual concentrations, particularly for low concentrations, the results of quantitative analysis gave a very good estimate of the degree of water pollution. As mentioned above, most catechols have good water solubility and the maximum concentration of water-soluble catechols in water is always over 300 mM. To detect catechols in natural water, the water sample containing an unknown analyte with an unknown concentration will be firstly diluted to prepare several dilute solutions. Then, these dilute solutions will be analyzed by our device. The smartphone will show the detection results as long as the concentration of one of these dilute solutions is in the appropriate range (5–125 mM). The use of this method to accurately analyze catechols and other cis-diol-containing compounds at trace levels is currently under study.
| Actual sample | Actual concentration (mM) | Predicted result | |
|---|---|---|---|
| Compound | Concentrationa (mM) | ||
| a Means ± SD. | |||
| Dopamine hydrochloride | 125 | Dopamine hydrochloride | 131.03 ± 1.825 |
| 50 | Dopamine hydrochloride | 51.06 ± 2.934 | |
| Catechol | 125 | Catechol | 123.95 ± 0.484 |
| 50 | Catechol | 50.23 ± 1.031 | |
| 4-Methylcatechol | 125 | 4-Methylcatechol | 128.82 ± 1.874 |
| 50 | 4-Methylcatechol | 48.202 ± 0.930 | |
| 3-Fluorocatechol | 125 | 3-Fluorocatechol | 115.39 ± 1.684 |
| 50 | 3-Fluorocatechol | 51.35 ± 3.926 | |
| 3,4-Dihydroxybenzoic acid | 125 | 3,4-Dihydroxybenzoic acid | 124.34 ± 0.708 |
| 50 | 3,4-Dihydroxybenzoic acid | 51.45 ± 0.780 | |
| 3,4-Dihydroxybenzaldehyde | 125 | 3,4-Dihydroxybenzaldehyde | 126.56 ± 4.08 |
| 50 | 3,4-Dihydroxybenzaldehyde | 48.40 ± 3.30 | |
| 1,2,4-Benzenetriol | 125 | 1,2,4-Benzenetriol | 121.12 ± 1.16 |
| 50 | 1,2,4-Benzenetriol | 47.14 ± 2.17 | |
| Control | 0 | Control | 2.46 ± 0.876 |
In recent years, colorimetric sensor arrays8,9 have been developed into powerful tools for the analysis of compounds bearing cis-diols, present in compounds such as catechols and carbohydrates. Although these sensor arrays are vastly superior to traditional methods including HPLC and spectrophotometric methods because of their low cost and simple processing steps, they can only be used to do qualitative identification of compounds bearing cis-diols, which limits their practical application. Additionally, probe sets composed of various pH indicators and one or more boronic acids were required to produce desirable discrimination of the cis-diol-containing analytes in previous studies. In contrast, only 4 ensemble probes were used to configure the smallest-scale 2 × 2 colorimetric sensor array to differentiate as many as 7 catechols with 7 serial concentrations in this work, which simplified the operation steps and reduced the costs. More importantly, a “mobile laboratory”, where a filter paper-based colorimetric sensor array, a smartphone, and a remote server were combined together, was created for rapid, on-site analysis of catechols from water samples with real-time results production. This mobile laboratory enables the colorimetric sensor array to do both qualitative and quantitative analysis in the field, expanding its application fields. For future suspected cases of water catechols contamination, the simple, convenient, and rapid method described in this paper will allow individuals to conveniently analyze water samples in a qualitative and quantitative manner on-site.
Conclusions
In this study, a “mobile laboratory”, wherein a low-cost, simple to arrange filter paper-based colorimetric sensor array/smartphone ensemble was coupled to a remote server, was created for rapid on-site analysis of catechols from water samples with real-time results. The smallest-scale filter paper-based 2 × 2 colorimetric sensor array was able to distinguish all 7 water-soluble catechols at 7 serial concentrations, through simultaneous treatment via PCA, HCA, and LDA. The results from the multivariate data analysis demonstrated the ability of the proposed array for reliable identification and classification of catechols. A particularly noteworthy aspect of our work is the novel coupling of a smartphone to a remote server that allows for the on-site classification, discriminant analysis, and quantitative measurement of concentrations of unknown samples to be undertaken. This is the first time such a system has been established. The smartphone is a uniquely practical portable device that is able to read the array, upload the data, and receive the results in real time. This simple, convenient approach shown here is a real life example suitable for the simple, rapid, qualitative and quantitative analysis of catechols on-site with real-time results production.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos 31470434, 21406090, and 21576124).References
- M. Russell and S. G. Burton, Anal. Chim. Acta, 1999, 389, 161–170 CrossRef.
- M. Aziz, T. Selvaraju and H. Yang, Electroanalysis, 2007, 19, 1543–1546 CrossRef.
- F. Ramos, A. Cristino, P. Carrola, T. Eloy, J. M. Silva, M. da Conceição Castilho and M. I. N. da Silveira, Anal. Chim. Acta, 2003, 483, 207–213 CrossRef CAS.
- J. Yin, F. Zhao, X.-X. Zhang, Y. Chen, W. Li, B. Wu and H. Ren, Int. J. Environ. Sci. Technol., 2015, 12, 847–856 CrossRef CAS.
- B. Wu, X. Zhang, X. Zhang, A. Yasun, Y. Zhang, D. Zhao, T. Ford and S. Cheng, Ecotoxicology, 2009, 18, 707–714 CrossRef CAS PubMed.
- M. Liu, P. Baugh, S. Hutchinson, L. Yu and S. Xu, Environ. Pollut., 2000, 110, 357–365 CrossRef CAS PubMed.
- B. Müller, M. Berg, Z. P. Yao, X. F. Zhang, D. Wang and A. Pfluger, Sci. Total Environ., 2008, 402, 232–247 CrossRef PubMed.
- S. H. Lim, C. J. Musto, E. Park, W. Zhong and K. S. Suslick, Org. Lett., 2008, 10, 4405–4408 CrossRef CAS PubMed.
- X. Zhang, E. V. Anslyn and X. Qian, Supramol. Chem., 2012, 24, 520–525 CrossRef CAS.
- J. R. Carey, K. S. Suslick, K. I. Hulkower, J. A. Imlay, K. R. Imlay, C. K. Ingison, J. B. Ponder, A. Sen and A. E. Wittrig, J. Am. Chem. Soc., 2011, 133, 7571–7576 CrossRef CAS PubMed.
- M. A. Palacios, R. Nishiyabu, M. Marquez and P. Anzenbacher, J. Am. Chem. Soc., 2007, 129, 7538–7544 CrossRef CAS PubMed.
- G. L. Feudo, B. Macchione, A. Naccarato, G. Sindona and A. Tagarelli, Food Res. Int., 2011, 44, 781–788 CrossRef.
- H. Xiao-wei, L. Zhi-hua, Z. Xiao-bo, S. Ji-yong, M. Han-ping, Z. Jie-wen, H. Li-min and M. Holmes, Food Chem., 2016, 197, 930–936 CrossRef PubMed.
- A. W. Martinez, S. T. Phillips, G. M. Whitesides and E. Carrilho, Anal. Chem., 2009, 82, 3–10 CrossRef PubMed.
- T. D. James, K. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed., 1996, 35, 1910–1922 CrossRef.
- J. W. Lee, J. S. Lee and Y. T. Chang, Angew. Chem., Int. Ed., 2006, 45, 6485–6487 CrossRef CAS PubMed.
- M. Dowlut and D. G. Hall, J. Am. Chem. Soc., 2006, 128, 4226–4227 CrossRef CAS PubMed.
- L. Bosch, T. Fyles and T. D. James, Tetrahedron, 2004, 60, 11175–11190 CrossRef CAS.
- K. KantaáGhosh, Chem. Commun., 2011, 47, 4001–4003 RSC.
- C. J. Musto, S. H. Lim and K. S. Suslick, Anal. Chem., 2009, 81, 6526–6533 CrossRef CAS PubMed.
- A. Ziyatdinov, S. Marco, A. Chaudry, K. Persaud, P. Caminal and A. Perera, Sens. Actuators, B, 2010, 146, 460–465 CrossRef CAS.
- I. Sasaki, H. Tsuchiya, M. Nishioka, M. Sadakata and T. Okubo, Sens. Actuators, B, 2002, 86, 26–33 CrossRef CAS.
- J. Cui, W. Zhu, N. Gao, J. Li, H. Yang, Y. Jiang, P. Seidel, B. J. Ravoo and G. Li, Angew. Chem., Int. Ed., 2014, 53, 3844–3848 CrossRef CAS PubMed.
- R. B. Roy, B. Tudu, L. Shaw, A. Jana, N. Bhattacharyya and R. Bandyopadhyay, J. Food Eng., 2012, 110, 356–363 CrossRef.
- H. Zhou, L. Baldini, J. Hong, A. J. Wilson and A. D. Hamilton, J. Am. Chem. Soc., 2006, 128, 2421–2425 CrossRef CAS PubMed.
- L. Feng, H. Li, X. Li, L. Chen, Z. Shen and Y. Guan, Anal. Chim. Acta, 2012, 743, 1–8 CrossRef CAS PubMed.
- Z. Li, W. P. Bassett, J. R. Askim and K. S. Suslick, Chem. Commun., 2015, 51, 15312–15315 RSC.
- J. R. Askim and K. S. Suslick, Anal. Chem., 2015, 87, 7810–7816 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |