Developments in conducting polymer fibres: from established spinning methods toward advanced applications†
Azadeh Mirabedini
,
Javad Foroughi
* and
Gordon G. Wallace
 *
*
ARC Centre of Excellence for Electromaterials Science, Intelligent Polymer Research Institute, AIIM Facility, University of Wollongong, Fairy Meadow, NSW 2519, Australia. E-mail: foroughi@uow.edu.au; gwallace@uow.edu.au
First published on 20th April 2016
Abstract
Conducting polymers have received increasing attention in both fundamental research and various fields of application in recent decades, ranging in use from biomaterials to renewable energy storage devices. Processing of conducting polymers into fibrillar structures through spinning has provided some unique capabilities to their final applications. Compared with non fibrillar forms, conducting polymer fibres are expected to display improved properties arising mainly from their low dimensions, well-oriented polymer chains, light weight and large surface area to volume ratio. Spinning methods have been employed effectively to produce technological conducting fibres from nanoscale to hundreds of micrometre sizes with controlled properties. This review considers the history, categories, the latest research and development, pristine and composite conducting polymer fibres and current/future applications of them while focus on spinning methods related to conducting polymer fibres.
1. Introduction
1.1. Historical background
Although polymers have traditionally been considered to be electrical insulators, conducting polymers (CPs) were shown to exhibit semiconducting behaviour not long ago.1,2 The fundamental feature of all conducting polymers is their extended conjugated π-system along the polymer backbone, which leads to metal-like electronic, magnetic and optical properties, while properties commonly associated with conventional polymers, such as flexibility, are maintained.3,4 Undoped forms of CPs represent semiconducting characteristics before they undergo a subsequent process so-called “doping” which involves oxidizing or reducing the material. Doping greatly increases the number of charge carriers within their internal structures for the purpose of modulating its electrical properties. ICPs have been studied extensively due to their intriguing electronic and redox properties, good environmental stability and numerous potential applications in many fields since their discovery in 1970s.5–7 The field has evolved from the early discovery of metallic conductivity in polyacetylene to a focus on soluble and processable polymers and copolymers.8Knowledge surrounding the early developments in textiles is meagre due to insufficient records. Before the 18th century, all textile fabrics were made of natural fibres such as wool, silk, cotton and linen. Mass production of fibres and their fabrication into textiles grew out of the early stages of the industrial revolution as the demand for cloth increased.9 It was found that many physical and chemical properties of polymers are improved mostly due to the alignment of polymer chains along the fibre axis compared to the non fibrillar structures. To achieve that, a specialised form of extrusion using spinneret, known as spinning, was utilised extensively to form multiple continuous filaments. The subsequent merging of fibre spinning and conducting polymer technologies introduced a new era of so-called “electronic textiles”.10 Polyaniline was the first among the conducting polymers to formed into a fibre.11 Thus far, CPFs have been produced and utilised for a wide range of applications such as energy storage (batteries, capacitors),12–16 energy conversion (photovoltaic, thermal energy harvesting),17,18 biology from tissue engineering19,20 to biomedical monitoring4,21–23 and also diagnosis and treatment (including controlled drug delivery).24–28
1.2. Conducting polymers
ICPs were discovered in 1977 with the 109 times increase in electrical conductivity (σ) of polyacetylene (PAc) through halogen doping to as high as 105 S cm−1.7 To date, a tremendous amount of research has been carried out in the field of conducting polymers, while the broader significance of the field was recognised in the year 2000 with the awarding of the Nobel Prize for Chemistry to the three discoverers of ICPs, Shirakawa, MacDiarmid and Heeger.3 Since the discovery of conducting PAc, a number of additional ICPs have been developed, including polypyrrole (PPy),30–34 polyaniline (PAni),35–37 polythiophene (PTh),38,39 poly(p-phenylenevinylene) (PPV),40,41 poly(3,4-ethylene dioxythiophene) (PEDOT),3,42–44 and polyfuran (PF).45 The structures of selected conducting polymers are illustrated in Fig. 1. The most significant conducting polymers with regard to technological fibres are PAni, PPy, PTh and PEDOT.1.3. Current achievements in the fabrication of ICPs
Conducting polymers must undergo processing steps in order to attain the desired form. The precise nature of such processing steps is guided by the intended use.10 Printing and fibre spinning technologies are two of the most prominent methods which are being investigated for the development of devices based on ICPs.Printing is a fast, old and inexpensive method that is used for mass fabrication of advanced conducting components.10 In recent years, increasing efforts have been focused on the printing of conducting polymer-based devices.46 Printing is a reproduction process in which ink is applied to a substrate in order to transmit information such as images, graphics and text. Printed materials must form a solid, continuous conducting film following solvent removal. The solvent plays significant roles such as compatibility with the conducting polymer, stability in solution and appropriate rheological and surface energy characteristics. Printing technologies that require a printing plate are known as conventional methods and include lithography (offset), gravure, letterpress and screen-printing. Non-impact printing (NIP), such as inkjet printing or electrophotography, uses laser technology and does not require a printing plate.47 Printing provides a convenient route to the deposition of conducting polymers with spatial resolution in the x, y plane in the order of tens of microns and makes layer thicknesses in the order of 100 nm feasible. The birth of 3D-printing goes back to 1984 when as Charles Hull invented stereolithography which enabled a tangible 3D object to be created from a 3D model.48 Varieties of conducting polymers have been processed earlier to become printable including PAni,49,50 PPy,51,52 and PTh.53
Spinning of polymer fibres has witnessed great progress over the past few decades as an interdisciplinary field that applies the principles of engineering and material science toward the development of textile substitutes.54 It is a specialised form of extrusion that uses a spinneret to form multiple continuous filaments or mono filaments. All fibre forming processes – regardless of the materials involved – are irreversible processes involving the rapid and continuous solidification of a liquid with a very restricted size in two directions. The solidification is brought about by the removal of heat and/or solvent by contacting the liquid with a suitable moving fluid, which can be a gas or a liquid. Considering fibres as continuous threadlike filaments with large L/Ds (typically L/D > 5), several other polymerisation methods reported for the production of short fibres are not considered in this review. The first step to produce fibres is to convert the polymer into a processable and spinnable state. Thermoplastic polymers can be converted into the melt-state and melt-spun. Other polymers may be dissolved in a solvent or chemically treated to form soluble or thermoplastic derivatives and subsequently spun via wet spinning, dry spinning or electrospinning.
Extensive advances have been made during the last three decades in the fundamental understanding of fibre spinning using conducting polymers. The very first attempts to achieve optimal conditions for the spinning of fibres from PAni were begun in the late 1980s.11,55,56 A few years later Mattes et al. pioneered the processing of PAni into fibre form through a dry–wet spinning process.57 Yet to date, CPFs lack an inclusive published report which wraps the origins of their emergence, the fabrication methods and their developments from the beginning to recent time despite printed conducting polymers.46 Hence, this paper attempted to provide an overview and perspective on the field of conducting polymer fibres with a particular emphasis on major spinning methods as key techniques to produce them.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS63,64 were wet-spun. A schematic of wet spinning is shown in Fig. 2.
PSS63,64 were wet-spun. A schematic of wet spinning is shown in Fig. 2.
2. Spinnable conducting polymers
Many researchers have investigated improved processing techniques for the preparation of conducting polymer fibrillar structures. Two main categories may be defined, the first being fibres spun purely from conducting polymers, termed “pristine conducting polymer fibres”. The second category refers to composite fibres that are comprised of conducting polymer(s) and one or more other constituents. These may be fabricated either by blending of the components, or by coating, electrospraying or polymerising dissimilar materials onto the outer surface of a fibre. This category is referred to as “conducting composite fibres”. The two main categories of conducting polymer fibrillar structures are described in detail below.2.1. Pristine conducting polymer fibres
PAni may be the considered to be the first conducting polymer spun into a fibrillar form.57 The spinning of PAc, PAni, PPy, PTh and PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres is described in detail in the following sections.
PSS fibres is described in detail in the following sections.
Due to the aforementioned processability issues surrounding PAc, few studies have reported on the successful preparation of PAc fibres. Sliva et al. first described a method for making continuous PAc fibres using a thin film evaporator to volatilise the reaction mixture of oxidatively coupled diethynyl organo compounds.81 The resulting concentrate could then be spun to produce PAc fibres that were easily converted into high strength carbon fibres. Akagi et al. reported the synthesis of hierarchical helical PAc fibres79 under an asymmetric reaction field consisting of chiral nematic liquid crystal. The prepared PAc helical fibrillar structure may be considered as the only such structure to be reported so far. The relatively high electrical conductivities of ∼1500–1800 S cm−1 obtained following iodine doping suggest that these fibres may find electromagnetic and optical applications.79 Kim et al. attempted to prepare a PAc fibre network from a low density foam-like PAc later on.82
PAni may exist in one of three well-defined oxidation states: leucoemeraldine, emeraldine and pernigraniline (Fig. 5). Leucoemeraldine and pernigraniline are the fully reduced (all nitrogen atoms in amine form) and the fully oxidised (all nitrogen atoms in imine form) forms of PAni, respectively. Green, protonated emeraldine is the only conducting form of PAni, and contains reduced amine and oxidised imine nitrogens in equal amounts i.e. –NH–/–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ratio ∼0.5.87 The blue, insulating emeraldine form can be transformed into the conducting form by lowering the pH of the medium and vice versa.83 Another interesting feature is that using an organic counterion (X−) as the dopant (e.g. camphor sulfonic acid), PAni may be retained in solution in the doped conducting form, further enhancing its versatility.88,89 PAni fibres may be spun from emeraldine base27,57,60,90 and leucoemeraldine base91–93 solutions and converted to the conducting form using aqueous protonating acids following processing.
ratio ∼0.5.87 The blue, insulating emeraldine form can be transformed into the conducting form by lowering the pH of the medium and vice versa.83 Another interesting feature is that using an organic counterion (X−) as the dopant (e.g. camphor sulfonic acid), PAni may be retained in solution in the doped conducting form, further enhancing its versatility.88,89 PAni fibres may be spun from emeraldine base27,57,60,90 and leucoemeraldine base91–93 solutions and converted to the conducting form using aqueous protonating acids following processing.
 | ||
| Fig. 5 (a) Emeraldine (y = 0.5), (b) leucoemeraldine and (c) pernigraniline oxidation states of polyaniline. | ||
Researchers have investigated various features of PAni, from stability in solution and different spinning methods through to electrochemical properties, actuating characteristics, and biomedical applications.57,60,90,91,94–100 Wet spinning has probably been the most important spinning method used to produce PAni fibres.57,60,92,95,96,100 However, several processing problems were found, such as poor solubility in organic polymers and rapid polymer gelation at low solids content.11,56,101 Andreatta et al. reported the complete solubility of PAni (emeraldine salt or base) in concentrated sulfuric acid and demonstrated the feasibility of solution processing of crystalline, electrically conducting PAni fibres and films.100 Hsu et al. were probably first to successfully spin the basic undoped form of PAni into fibre form,101 reporting electrical conductivity values of 320.5 S cm−1 and 157.8 S cm−1 of stretched fibres that had been doped with aqueous H2SO4 and HCl, respectively. To overcome the fast gelation of PAni, researchers found that selected Lewis-base organic solvents have a better solvency compared to N-methyl-2-pyrrolidinone (NMP).102,103 Years later, the preparation of stable spinning solutions for low molecular weight emeraldine base was reported using N,N′-dimethyl propylene urea (DMPU) instead of NMP,56 while Mattes et al. developed an approach to circumvent processing problems by addition of secondary amines to act as gel inhibitors in high molecular weight PAni solutions with concentrations of >20% (w/w) (Fig. 6).57,104
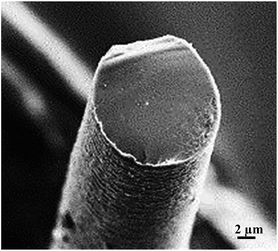 | ||
| Fig. 6 Scanning electron micrographs of cross-section of wet-spun polyaniline fibre. Reproduced with permission from ref. 14 Copyright© 2006, Elsevier. | ||
Up to the time of the work of Mattes et al.,57 the standard method for making conducting PAni fibres from the emeraldine base form was to convert to the conducting salt form using an aqueous protonic acid. This method had several difficulties, including inhomogeneous protonation, relative ease of de-doping, and adverse effects on material properties.101 In 1998, a new acid processing route to PAni was reported by Adams et al., using 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPSA) as both protonating acid and solvating group, and dichloroacetic acid (DCA) as solvent.105 One year later, in what may be considered as a first, PAni fibres were produced using a one-step wet spinning method,95 which eliminated the need for further protonation. Subsequently, various coagulation solvents (e.g. acetone, butyl acetate, 4-methyl-2-pentanone) were trialled in order to achieve a range of mechanical properties and electrical conductivities for different applications.60
Many research groups have also attempted to fabricate nano-sized PAni fibres.90,91,106–109 Cárdenas et al. were pioneers in the successful use of electrospinning to produce PAni nanofibres.27 This method produced fibres with diameters ranging from hundreds of nanometres to a few micrometres. This was a significant advance at the time, not only because pure PAni fibres were obtained, but also because the fibre was collected in an innovative manner involving the placement of an acetone bath on the electrode. In addition, the further treatment of fibres with radiation or gas without concern for side reactions with doping agents is an advantage. PAni fibres have found broad application, particularly sensors and biosensors,107,110–112 actuators15,93,99 and electrochemical mechanism investigations.92 A summary of PAni fibre production using different methods is presented in Table 1.
| No. | Dopants used | Focus of the research | Spinning method | Reported electrical conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| a YM-Young's Modulus, UTS-Ultimate Tensile Stress, US-Ultimate Strain. | ||||||
| 1 | H2SO4 | Preparation of conducting fibres of PAni from solutions in concentrated sulfuric acid | Wet spinning | 20–60 S cm−1 | 100 | |
| 2 | NMP | Preparation of concentrated (10–25%) spinnable solutions of PAni in the emeraldine base form for fibre spinning | Dry-jet wet spinning | H2SO4 and HCl are 320.5 and 157.8 S cm−1 | Average tenacity of 3.9 GPa (∼400 MPa) | 101 |
| 3 | NMP and DMPU | Improvement of solution stability and spinnability of concentrated PAni solutions | Wet spinning (DMPU, NMP, NMP/LICl) | — | Approaching that of nylon-6 | 56 |
| 4 | BPA | Investigation of the effect of dopant on preserving the mechanical properties and improvement of conductivity values | Dry–wet spinning (water) | 10−2 S cm−1-stretched acetic acid doped 10−6 S cm−1-unstretched acetic acid doped 1 S cm−1-HCl and BPA doped | YMa 0.55–13.1 GPa, USa ∼ 2–9% | 57 |
| 5 | AMPSA | Measuring electrical and mechanical properties of wet-spun PAni fibres in various coagulation solvents | Wet spinning (DCA) | 70–150 S cm−1 | YM 40–60 MPa, UTS 20–60 MPa | 60 |
| 6 | HClO4 | The development of a linear actuator prototype based on PAni fibres | Wet spinning (water) | 1–3 S cm−1 | YM ∼ 2–3 MPa | 86 |
| 7 | AMPSA | Study the processing routes for preparation of conducting PAni fibres | Wet spinning (acetone) | 900–1500 S cm−1 | YM 0.2 GPa, UTSa 97 MPa, US ∼ 550% | 95 |
| 8 | AMPSA | Study the temperature dependence of conductivity behaviour of the fibres | Wet spinning (acetone) | 760–1060 S cm−1 at 363 K | — | 54 |
| 9 | AMPSA | Introducing a new one-step acid-solution processing route for production of PAni fibres | Wet spinning (dichloroacetic acid) | 70–150 S cm−1 | YM 40–60 MPa, UTS 20–60 MPa, US ∼ 500% | 29 |
| 10 | NMP containing heptamethyleneimine as a gel inhibitor | Controlling macrovoid formation in wet-spun PAni fibres | Wet spinning (water) | — | YM 550–900 MPa, UTS 5.9–12.8 MPa, US ∼ 1.1–1.45% | 113 |
| 11 | AMPSA | Electrochemically characterisation of PAni fibre microelectrodes for the first time | Wet spinning (dichloroacetic acid) | +0.20 to +0.60 V versus SCE | — | 30 |
| 12 | CSA | Study the influence of dopants and cis-1,2-ethenesulfonate family of twin-tailed surfactants on properties of PAni nanofibre | Interfacial polymerisation | ∼1–5 S cm−1 | — | 91 |
| 13 | — | Using the electrospinning technique to produce sub-micron fibres of pure PAni | Electrospinning (acetone bath placed on the electrode) | 10−3 to 102 S cm−1 | — | 27 |
| 14 | Sulfonated β-cyclodextrin and sulfonated α-cyclodextrin | Preparation and characterisation of PAni nanofibres containing sulfonated cyclodextrin group | Interfacial polymerisation | 3.2 × 10−2 to 8.5 × 10−3 S cm−1 | — | 4 |
| 15 | HCSA | Electrospinning and evaluating PAni fibres doped with different levels of HCSA evaluated as chemiresistive gas sensors | Coaxial electrospinning | Up to 50 ± 30 S cm−1 | — | 112 |
| 16 | APS | Controlling the morphological structure of the synthesized fibres by tuning the reaction time | Self-assembly process | Electroactive −0.6 V to +0.3 V | — | 106 |
Although not considered in this review, it is worth noting that several other polymerisation methods have been reported for the production of discontinuous PAni nanofibres (diameter < 100 nm), such as photolithographic synthesis (ultraviolet irradiation of aqueous aniline solutions),114,115 chemical polymerisation (with prevention of secondary polymer growth),116–119 nanofibre seeding through interfacial polymerisation,120–122 and chemical oxidation polymerisation of doped aniline.83
PPy demonstrates high electrical conductivity, good electrochemical properties, strong adhesion to substrates and thermal stability.89,126 The heteroatomic and extended π-conjugated backbone structure of PPy provides it with chemical stability and electrical conductivity, respectively.4,125 PPy exhibits a wide range of surface electrical conductivities (10−3 S cm−1 < σ < 100 S cm−1) depending on the functionality and substitution pattern of the monomer and the nature of the counterion or dopant.127 Not surprisingly therefore PPy has already been applied in a wide variety of areas such as rechargeable lithium batteries,16,128 low temperature fuel cell technology,129 medical applications,130–132 and volatile organic compound detection.133,134 It has also been investigated as a material for “artificial muscles” that would offer numerous advantages over traditional motor actuation.135,136
PPy may be switched between its oxidised and reduced states, thereby allowing dynamic control of electrical, chemical and mechanical properties. Reduced, non-conducting PPy has a resonance structure that resembles the aromatic or quinoid forms, and may be converted to the conducting form upon oxidation. The charge associated with the oxidised state is typically delocalised over several monomer units and can form a radical cation (polaron) or a dication (bipolaron), as depicted in Fig. 7. In general, small anionic species are incorporated into the PPy chains upon oxidation and are expelled upon reduction in order to maintain charge neutrality.125
 | ||
| Fig. 7 Chemical structures of polypyrrole in neutral (a) aromatic and (b) quinoid forms, and in oxidised (c) polaron and (d) bipolaron forms. | ||
PPy usually takes the form of an intractable powder following chemical polymerisation and an insoluble film following electropolymerisation.137 PPy prepared by conventional methods is insoluble in most organic solvents.61,138 These characteristics may be largely attributed to the presence of strong interchain interactions and a rigid structure. Difficulties associated with poor processability have motivated researchers to identify methods to render PPy processable. These methods include direct polymerisation onto polymers sheets, glass, polymer and inorganic particles, clays, zeolites, porous membranes, fibres and textiles, and soluble matrices.137 Furthermore attempts to improve polymer solubility have been made involving alkyl group substitution at the 3- and 4-positions or at the nitrogen atom of the pyrrole ring.137 Another technique that has proven successful has been the use of long chain surfactant dopants such as sodium dodecyl benzene sulfonate (DDS),139,140 di(2-ethylhexyl)sulfosuccinate sodium salt (DEHS),141 and polystyrene sulfonate.142 PPy doped with such surfactants were soluble in a number of solvents including m-cresol, NMP, dimethyl sulfoxide (DMSO), dimethyl formamide (DMF) and tetrahydrofuran (THF).137
The fabrication of continuous electrically conducting PPy fibres was first achieved using electrospinning,76 in contrast to PAni. The electrospun fibres were in a web form, with average diameter of 3 μm and conductivity of ca. 0.5 S cm−1. Chronakis et al. used a different dopant and oxidant to similarly electrospun PPy nanofibres with diameters ranging between 70 and 300 nm.143 Recently solid-phase extraction was described based on electrospun conducting PPy hollow fibres for the extraction of different classes of compounds, where the application was attractive due to its low consumption of organic solvents, simplicity, high recovery and ease of automation and operation.144,145
Few reports exist that consider the wet spinning of soluble PPy into continuous fibres, despite initial attempts.146 This question was essentially abandoned for a number of years until Foroughi et al. published the first report on the production of continuous conducting PPy fibres (Fig. 8) through wet spinning,61 which showed electrical conductivity of ∼3 S cm−1 and elastic modulus of ∼1.5 GPa. Later on the mechanical and electrical properties of these fibres were also studied.147 Although a number of researchers continue to seek new methods to produce wet-spun PPy fibres, no additional reports have been published. Previous studies into the preparation of PPy fibres are summarised in Table 2.
 | ||
| Fig. 8 Scanning electron micrographs of (a) wet-spun polypyrrole fibre cross-section and (b) polypyrrole hollow fibres polymerised under ultrasonication. Reproduced with permission from ref. 61 and 144 Copyright© 2008 and 2012; Elsevier and The Royal Society of Chemistry Publishing, respectively. | ||
| No. | Dopants used | Focus of the research | Spinning method | Reported electrical conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| 1 | Sodium dodecyl sulfate (SDS) | Growth of dendrite-like fibres at PPy/Pt electrode interface | Electropolymerisation (galvanostatically polymerisation) | 20 S cm−1 | — | 148 |
| 2 | DBSA as dopant, APS as the oxidant | Fabrication of electrically conducting PPy nonwoven web | Electrospinning | 0.5 S cm−1 | — | 76 |
| 3 | DEHS | Fabrication of electrically conducting PPy | Electrospinning | 2.7 × 10−2 S cm−1 | — | 143 |
| 4 | DEHS | Fabrication of continuous PPy fibre | Wet spinning (dichloroacetic acid (DCAA)) | ∼3 S cm−1 | YM 1.5 GPa, UTS 25 MPa, US 2% | 61 |
| 5 | DEHS | Effect of synthesis conditions on the properties of wet-spun PPy fibre | Wet spinning | — | YM 4.2 GPa, UTS 136 MPa, US 40% at 100 °C | 62 |
| 6 | DEHS | Investigation of mechanical and the electrical properties of PPy fibres | Wet spinning | 60 < T < 200, 566.8 S cm−1 T < 60, 419.9 S cm−1 | YM ∼ 4.2 GPa, UTS 136 MPa, US 5% | 147 |
PThs have been prepared since the 1980s by means of two main routes, namely chemical, and cathodic or anodic electrochemical synthesis.149 The first chemical synthesis using metal-catalysed polymerisation of thermostable 2,5-dibromothiophene was reported by two research groups independently.150,151 Yamamoto et al. also reported on the polycondensation of 2,5-dibromothiophene catalysed by Ni(bipy)Cl2.150 Lin and Dudek have attempted several catalytic systems such as Ni, Pd, Co, and Fe salts.151 Among the electrochemical synthesis methods, anodic electropolymerisation in particular presents several distinct advantages such as absence of catalyst, direct grafting of the doped conducting polymer onto the electrode surface, easy control of film thickness by controlling the deposition charge, and the possibility to perform in situ characterisation of the polymerisation process by electrochemical and/or spectroscopic techniques. The electropolymerisation of bithiophene was initially addressed in 1980.149
Amongst the wide variety of conducting polymers, those derived from thiophene and its derivatives show good stability toward oxygen and moisture in both doped and neutral states.152 This combined with favourable electrical and optical properties has led to the application of PThs in electrochromic displays, protection of semiconductors against photocorrosion, and energy storage systems.153 Similarly to PPy, PTh is utilised in solid phase extraction applications. To the best of our knowledge, the only published work on the preparation of PTh fibres is by Zhang et al., who described the preparation of PTh nanofibres via seeding as a general synthetic approach for bulk nanofibre production.154
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS is now commercially available and applied in antistatic coatings,157 electrode materials,158 organic electronics,159 transparent electrodes, capacitors,160 touchscreens, organic light-emitting diodes, microelectrodes and sensors.155,161
PSS is now commercially available and applied in antistatic coatings,157 electrode materials,158 organic electronics,159 transparent electrodes, capacitors,160 touchscreens, organic light-emitting diodes, microelectrodes and sensors.155,161Initial attempts to prepare fibrillar structures from PEDOT started in 1994, when Sailor et al. reported electrosynthesis techniques for the fabrication of complex PEDOT interconnects on Pt arrays.162 Okuzaki and Ishihara later reported the first preparation of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS microfibres via wet spinning with acetone as the coagulant (Fig. 10(a)),163 where the effects of spinning conditions on fibre diameter (which ranged between 180 and 410 μm), electrical conductivity, microstructure and mechanical properties were investigated. The fabrication of nanotubes from electrochemically synthesised PEDOT using alumina as the template was subsequently addressed by Zhang et al. as another novel approach.161 PEDOT nanofibres with diameters ranging between 100 and 180 nm were later produced using vanadium pentoxide nanofibres by a one-step nanofibre seeding method.155 In this procedure EDOT is dissolved in aqueous camphorsulfonic acid (HCSA) together with a vanadium pentoxide nanofibre sol–gel, before radical cationic polymerisation was initiated by addition of ammonium persulfate (APS). Of special note in the preparation of PEDOT fibres is the work of Baik and co-workers, who developed a method to synthesise PEDOT nanofibres by simple chemical polymerisation without employing a template.164 Shortly thereafter, Okuzaki et al. fabricated highly conducting PEDOT
PSS microfibres via wet spinning with acetone as the coagulant (Fig. 10(a)),163 where the effects of spinning conditions on fibre diameter (which ranged between 180 and 410 μm), electrical conductivity, microstructure and mechanical properties were investigated. The fabrication of nanotubes from electrochemically synthesised PEDOT using alumina as the template was subsequently addressed by Zhang et al. as another novel approach.161 PEDOT nanofibres with diameters ranging between 100 and 180 nm were later produced using vanadium pentoxide nanofibres by a one-step nanofibre seeding method.155 In this procedure EDOT is dissolved in aqueous camphorsulfonic acid (HCSA) together with a vanadium pentoxide nanofibre sol–gel, before radical cationic polymerisation was initiated by addition of ammonium persulfate (APS). Of special note in the preparation of PEDOT fibres is the work of Baik and co-workers, who developed a method to synthesise PEDOT nanofibres by simple chemical polymerisation without employing a template.164 Shortly thereafter, Okuzaki et al. fabricated highly conducting PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS microfibres with 5 μm diameter and up to 467 S cm−1 electrical conductivity by wet spinning followed by ethylene glycol post-treatment.64 Dipping in ethylene glycol (two-step wet spinning process) resulted in a 2–6 fold increase in electrical conductivity from 195 S cm−1 to 467 S cm−1 and a 25% increase in tensile strength after drying from 94 MPa to 130 MPa. Characterisation with X-ray photoelectron spectroscopy, X-ray diffractometry and atomic force microscopy led to the conclusion that the removal of insulating PSS from PEDOT
PSS microfibres with 5 μm diameter and up to 467 S cm−1 electrical conductivity by wet spinning followed by ethylene glycol post-treatment.64 Dipping in ethylene glycol (two-step wet spinning process) resulted in a 2–6 fold increase in electrical conductivity from 195 S cm−1 to 467 S cm−1 and a 25% increase in tensile strength after drying from 94 MPa to 130 MPa. Characterisation with X-ray photoelectron spectroscopy, X-ray diffractometry and atomic force microscopy led to the conclusion that the removal of insulating PSS from PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS grain surfaces and crystallization were responsible for the enhanced electrical and mechanical properties of the microfibres. This work opened a new way for scientists to prepare relatively long PEDOT
PSS grain surfaces and crystallization were responsible for the enhanced electrical and mechanical properties of the microfibres. This work opened a new way for scientists to prepare relatively long PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres using a straightforward method. Jalili et al. simplified the method to a one-step process to prepare microfibres (Fig. 10) by employing a wet spinning formulation consisting of an aqueous blend of PEDOT
PSS fibres using a straightforward method. Jalili et al. simplified the method to a one-step process to prepare microfibres (Fig. 10) by employing a wet spinning formulation consisting of an aqueous blend of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS and poly(ethylene glycol), where the need for post-spinning treatment with ethylene glycol was eliminated and fairly high electrical conductivities of up to 264 S cm−1 were achieved.63 Table 3 summarises efforts made in the preparation of PEDOT
PSS and poly(ethylene glycol), where the need for post-spinning treatment with ethylene glycol was eliminated and fairly high electrical conductivities of up to 264 S cm−1 were achieved.63 Table 3 summarises efforts made in the preparation of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres.
PSS fibres.
 | ||
Fig. 10 Scanning electron micrographs of (a) fibre surface of first wet-spun PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS microfibre, (b) cross-section of PEDOT PSS microfibre, (b) cross-section of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibre spun into acetone. Reproduced with permission from ref. 64 and 63 Copyright© 2008 and 2011, Science Direct and 2011; WILEY-VCH Verlag GmbH & Co., respectively. PSS fibre spun into acetone. Reproduced with permission from ref. 64 and 63 Copyright© 2008 and 2011, Science Direct and 2011; WILEY-VCH Verlag GmbH & Co., respectively. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres
PSS fibres
| No. | Dopants used | Focus of the research | Spinning method | Reported electrical conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| 1 | Poly(4-styrenesulfonate) | Fabrication and characterisation of conducting PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS microfibres PSS microfibres |
Wet spinning (acetone) | ∼10−1 S cm−1 | YM 1.1 ± 0.3 GPa, UTS 17.2 ± 5.1 MPa, US 4.3 ± 2.3% | 163 |
| 2 | Poly(4-styrenesulfonate) | Fabrication microfibres of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS PSS |
Wet spinning (acetone) | 0.4–1 S cm−1 | 165 | |
| 3 | Poly(4-styrenesulfonate) | Fabrication of highly conducting PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS microfibres and study the effect of dip-treatment in ethylene glycol on its properties PSS microfibres and study the effect of dip-treatment in ethylene glycol on its properties |
Wet spinning (acetone) | 74–467 S cm−1 (after post-treatment) | YM 4.0 GPa, UTS 130 MPa | 64 |
| 4 | Boron trifluoride | Synthesis of highly conducting poly(3,4-ethylenedioxythiophene) fibre by simple chemical polymerisation | In situ polymerisation | In the range of 150–250 S cm−1 | — | 164 |
| 5 | — | Production of continuous PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS in both acetone/IPA coagulation bathes PSS in both acetone/IPA coagulation bathes |
Wet spinning (acetone and isopropanol (IPA)) | <264 S cm−1 | IPA: YM 3.3 ± 0.3 GPa, UTS 125 ± 7 MPa, US 15.8 ± 1.2%, acetone: YM 2.5 GPa, UTS 98 MPa, US 12.5% | 63 |
2.2. Composite conducting fibres
Following the discovery of conducting polymers in 1977,1,166 their processability has been one of the major barriers to their widespread use. Consequently, the combination of conducting polymers with other, more processable materials in composite structures has become one of the most effective ways to produce conducting fibres. By employing this approach, not only difficulties related to processability could be surmounted, but the complementary characteristics of other individual component(s) improved the final properties. Not surprisingly, a large proportion of reported high-quality conducting fibres have been composite fibres.Techniques for preparing composite conducting fibres are diverse, from dispersing conducting fillers in a thermoplastic polymer via mechanical mixing and blending, to coating a layer of conducting polymer on a fibrillar substrate via chemical or electrochemical polymerisation of a suitable precursor. Based on a survey of the available literature, composite conducting polymers may be categorised into two main sub-groups. The first category may be referred to as “composite conducting polyblend fibres”, that is fibres consisting of conducting polymers blended with natural/synthetic polymers. The second group encompasses composite fibres made by combining conducting polymers and carbon-based materials (carbon nanotubes (CNTs) in particular), which may be referred to as “conducting polymer–carbon nanotube fibres”. Historical backgrounds and the most recent advances in the field are discussed in detail below.
2.2.1.1. Composite polyaniline fibres. Composite PAni fibres contain a natural or synthetic polymer in addition to PAni, and thus represent a large category of composite fibres. Composite PAni fibres were first reported in the late 1980s.167 Segonds and Epstein prepared composite fibres from poly(para-phenylenediamine) (PPD)–terephthalic acid (T) (Kevlar® aramid) and the emeraldine salt of PAni by mixing emeraldine base PAni in PPD–T/H2SO4 solution and extruding this solution via wet spinning.167 Although the fibres showed insulating properties because of the low loading levels of emeraldine salt, the authors demonstrated the feasibility of making multicomponent systems. Years later, Zhang et al. prepared composite PAni fibres by wet spinning based on a blend of PAni and poly-ω-aminoundecanoyle in concentrated H2S04. These fibres showed high strength and relatively high electrical conductivity (∼10−7 S cm−1) compared to related fibres reported up to that time. Subsequently, various composite PAni fibres have been prepared using a range of polymers via wet spinning.90,168,169 The highest electrical conductivity was stated from a wet-spun composite PAni fibre (∼1000 S cm−1) belonged to 200% stretched PAni/Au bilayers which was doped with AMPSA.93
Most reports concerned with the electrospinning of conducting polymers focus on PAni and blends thereof.170 This technique was especially used in recent years to produce nanofibrillar films for bio-related applications. The first report of composite PAni fibres prepared by electrospinning was that of HCSA-doped polyaniline (PAni·HCSA)/polyethylene oxide (PEO) blend nanofibres.171 As-spun fibres demonstrated electrical conductivities in the order of ∼10−2 S cm−1, which was quite an achievement at the time.
Several applications of electrospun composite PAni fibres have been reported. PAni was blended with insulating polymer fibres (PEO, polyvinylpyrrolidone (PVP), and polystyrene (PS)) to prepare sensors with a range of response.111,172 Nanofibrillar blends of a copolymer of PAni and benzoic acid and poly(lactic acid) (PLA) were also used as tissue engineering scaffolds.173 Uniform electrospun fibres of PAni/poly[(L-lactide)-co-(ε-caprolactone)] were developed for electrically conducting, engineered nerve grafts.174 Electrospinning was also used to prepare photocatalytically active TiO2/PAni composite fibres (Fig. 11(a)).175
 | ||
| Fig. 11 Scanning electron micrographs of (a) TiO2/polyaniline composite fibres and (b) polyester fibres coated with polyaniline. Reproduced with permission from ref. 175 and 176 Copyright© 2011 and 2005; Elsevier and University of Bielsko-Biala, respectively. | ||
The approach of coating insulating fibres with conducting PAni as a route to conducting fibres was first considered in a report where PET fibres were coated with a layer of PAni/dodecylbenzene sulfonic acid (DBSA) (Fig. 11(b)),176 although no conductivity values were reported. In subsequent efforts, ultra-high molecular weight polyethylene, stainless steel, polycaprolactam and polyester were used as substrates on which a layer of PAni was coated.177–180 In situ oxidative polymerisation was used to prepare PAni-coated short nylon/natural rubber fibres,181 while researchers have also employed this method to prepare conducting fibres based on polyurethane, kenaf, polyacrylamide, cellulose, coconut, poly(methyl methacrylate) and detonation nanodiamond fibre substrates.182–188
Recently, PAni nanofibre/silver nanoparticle composite networks were prepared via well-known seeding polymerisation method with reported electrical conductivities in the range of 2 × 10−3 to 0.196 S cm−1 applied as an antibacterial agent.189
Melt spinning is another method that has been used for making composite PAni fibres. However, the use of this method has been restricted because of several issues mentioned earlier, such as relatively low decomposition temperatures, poor control over the exact temperature of the polymer melt during spinning, thermo-mechanical history of the melt and final fibre structure. Kim et al. were the first to consider the spinning of a melted blend containing PAni, followed by a coating process.70 Table 4 summarises research concerning composite PAni fibres.
| No. | Spinning solution blend | Focus of the research | Synthesis method | Reported conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| a YM-Young's Modulus, UTS-Ultimate Tensile Stress, US-Ultimate Strain, UEB-Ultimate Elongation at Break | ||||||
| 1 | PAni/PPD–T/H2S04 spin dope | Preparation of composite fibres of poly(para-phenylenediamine)–terephthalic acid and emeraldine salt | Wet spinning (water) | — | YMa 62 GPa, UTSa 28 GPa | 167 |
| 2 | Lyotropic PAni/poly(p-phenylene terephthalamide), PPD–T, sulfuric acid solutions | Preparation of PAni composite fibres are of lyotropic PAni/poly(p-phenylene terephthalamide), PPD–T, sulfuric acid solutions | Air-gap spinning (at ∼80 °C) | ∼0.1 S cm−1 (30% wt PAn) | YM 270 GPa | 190 |
| 3 | PAni/poly-ω-aminoundecanoyle | Preparation of conducting PAni/poly-ω-aminoundecanoyle fibre | Wet spinning (concentrated H2S04) | ∼10−7 S cm−1 (5% wt PAn) | — | 191 |
| 4 | PAni/HCSA/polyethylene oxide blends | Electrostatic fabrication of ultrafine conducting fibres: PAni/polyethylene oxide blends | Electrospinning | ∼10−4 to 10−1 S cm−1 (∼20–100% wt PAn·HCSA) | — | 171 |
| 5 | PAni/polystyrene–polybutadiene–polystyrene (SBS) | Production of electrically conducting fibres in PAn–SBS blends | Capillary extruded | ∼10−4 S cm−1 (∼20% wt PAn) | — | 169 |
| 6 | HCSA/PAni/polyethylene oxide (PEO) | Experimental observation of FET behavior in doped PAni/polyethylene oxide PAni/PEO nanofibre | Electrospinning | ∼10−3 S cm−1 | — | 108 |
| 7 | PAni doped with DBSA, polypyrrole (PPy) and graphite | Electrical and morphological properties of PP and PET conducting polymer fibre | Melt spinning and coating process | 2.88 × 10−7 S cm−1 (40% wt PPy) | — | 70 |
| 8 | PAni doped with DBSA | Early attempts to obtain polyester (PET) fibres with antielectrostatic properties | Coating (fibres were padded with PAn) | — | — | 176 |
| 9 | EB powder was dissolved in N-methyl-2-pyrrolidinone (NMP) to form a 5 wt% solution | Actuation of PAni (AMPS) fibres | Wet spinning (2-butanone) | ∼1000 S cm−1 (200% stretched fibres) | Poor! | 93 |
| 10 | PAni/nylon 6 in both concentrated sulphuric formic acids | Investigation on the coagulation rate of PAni/nylon 6 fibre in different acids and the influence of Li2SO4 on mechanical properties of the fibres | Wet spinning (Li2SO4) | Electrical resistance 0.665–0.015 Ω cm−1 (∼5–25% wt PAn) | Improved using Li2SO4 in the bath | 168 |
| 11 | PAni-ES and DBSA mixtures dissolved in xylene and coated with the solution of UHMWPE | Preparation and characterisation of PAni-coated ultra-high-molecular-weight polyethylene (UHMWPE) yarns | Coating | ∼10−1 | — | 177 |
| 12 | Chitosan/acetic acid was wet-spun and aniline was polymerised on resulting fibres in the presence of HCl and APS | Electrochemical actuation in chitosan/PAni microfibres for artificial muscles | Wet spinning (NaOH) followed by in situ oxidative polymerisation | 2.856 × 10−2 S cm−1 | USa 0.39% | 192 |
| 13 | Two phase blend of PAni-complex and fibre grade polypropylene in melt-state | Investigation of different processing conditions such as mixing parameters (time and temperature) and draw ratio on polypropylene/PAni fibres | Melt spinning | — | — | 193 |
| 14 | PAni film was deposited on the surface of stainless steel from H2SO4/aniline solution | Extraction of the target analytes from aqueous benzene derivatives systems in water samples using coated stainless steel with PAn | Coating | — | — | 178 |
| 15 | PAni emeraldine base (PAni-EB)/HCSA blended with (PEO, PVP and PS) | PAni fibres blended with different polymers were prepared to mimic structures like olfactory cilia possessing high surface to volume ratio. They were and tested for gas sensing | Electrospinning | — | — | 111 |
| 16 | Poly(L-lactide-co-3-caprolactone) (PLCL)/PAni | Composite fibres of poly(L-lactide-co-3-caprolactone) (PLCL)/PAni for the culture of PC12 cells | Electrospinning | ∼0.1–0.15 S cm−1 | UEBa 160 ± 14.4%, UTSa 15 ± 3 MPa | −174 |
| 17 | Poly(aniline-co-3-aminobenzoic acid)/HCl/poly(aniline-co-3-aminobenzoic acid)/pure poly(lactic acid) (PLA) | Fabrication of nanofibres of HCl-doped poly(aniline-co-3-aminobenzoic acid) (3ABAPANI) and poly(lactic acid) (PLA) blends and their potential applications in tissue engineering | Electrospinning | In the range of 0.9–8.1 mS cm−1 | — | 173 |
| 18 | PAni-EB in formic acid/PAni-coated polycaprolactam (PA6) fibres doped by HCl, H2SO4, HCOOH and TSA | The effect of solvent concentration and roller speed on the mechanical and electrical properties of PAni-coated PA6 fibre were discussed | Coating | PAni-coated PA6 fibre has good permanent conductivity volume resistivity (Ω cm = 101 to 102) | — | 179 |
| 19 | Aniline in HCl/piece of TiO2 multi-pore fibre film/APS in HCl was added to the aniline mixture | Preparation of TiO2/PAni composite fibre | Electrospinning in situ polymerisation | — | — | 175 |
| 20 | Aniline/ammonium peroxydisulfate in the presence of short nylon-6 fibre | Preparation and characterisation of natural rubber–PAni coated short nylon-6 fibre (PAni–N6) composites | In situ oxidative polymerisation | ∼1.99 × 10−6 S cm−1 | Depend on PAn concentration | 181 |
| 21 | Aniline/potassium peroxydisulfate solution coated on polyester fabrics washed with an aqueous acid solution (H2SO4 or HCl) | Chemical and electrochemical characterisation of PAni coated conducting fabrics | Coating | −1 to +2 V | — | 180 |
| 22 | HCPSA/PAni/PLCL dissolved in hexafluoropropanol (HFP) | Electroactive electrospun PAni/poly[(L-lactide)-co-(ε-caprolactone)] fibres for control of neural cell function | Electrospinning | ∼0.00641 S cm−1 | — | 174 |
| 23 | A blend of PP/PA6/PAni-complex (polypropylene/polyam-ide-6/PAni-complex) | Preparation of conducting polyblend filaments by melt spinning of PP/PA6 blends/modified with poly-aniline-complex and characterised | Melt spinning | ∼10−3 S cm−1 (draw ratio of 5) | Tenacity < 28 cN per tex | 194 |
| 24 | PU fibres immersed in aniline/HCl solution | Preparation of PAni/PU fibre by in situ chemical oxidative polymerisation of PAni on PU fibre and investigation of their piezoresistive properties | In situ oxidative polymerisation | 10−2 S cm−1 | US 400% | 182 |
| 25 | Kenaf fibres (UKF) in NaOH solution/aniline monomer in HCl/APS | Modification of natural kenaf fibre by PAni | In situ oxidative polymerisation | ∼10−4 to 10−3 S cm−1 | UTS 100 ± 50 N mm−2 | 183 |
| 26 | Cellulose fibre in HCl/aniline and APS | Preparation of PAni/cellulose composite fibre for the treatment of Cr(VI)-contaminated water, and its effect | In situ oxidative polymerisation | — | — | 184 |
| 27 | Cellulose acetate (CA)–PAni | Preparation of dual-layer hollow fibre of PAni–cellulose acetate | Wet spinning (HCl and APS) | −2 to +2 V | — | 185 |
| 28 | A blend of aniline, acrylamide and M acids/N,N0-methylenebisacrylamide (NMBA) and potassium peroxydisulfate (KPS) added | Synthesising hexagonal PAni fibres with polyacrylamide pendants in PAAm oligomer (oligo-PAAm) colloid | In situ oxidative polymerisation | 2.2 S cm−1 | — | 186 |
| 29 | Aniline in presence of detonation nanodiamond (DND)/SDS/APS | Characterisations of morphological, electrical and mechanical properties of PAni/DND nanofibres | Precipitation polymerisation technique | 10–100 S cm−1 | — | 187 |
| 30 | Aniline in presence of CF using FeCl3·6H2O or APS as oxidant | Investigation PAni-coated coconut fibres properties as additives in matrix of polyurethane | In situ oxidative polymerisation | 1.5 × 10−1 S cm−1 (FeCl3·6H2O) 1.9 × 10−2 (APS) | — | 188 |
| 31 | Silver nanoparticles embedded PAni (AgNO3 aq. solutions + V2O5 nanofibrous seeding agent) | Synthesis and characterisation of PAni nanofibre/silver composite networks as antibacterial agents | Seeding polymerisation reaction | 2 × 10−3 to 0.196 S cm−1 | — | 189 |
| 32 | PAni/HCSA | Electrospun PAni fibres as highly sensitive chemiresistive sensors for NH3 and NO2 gases | Electrospinning | 2 × 10−6 to 50 S cm−1 | — | 112 |
| 33 | PAni/HCSA blended with PMMA or PEO | Introduce a novel method to calculate fibre conductivity using IDE applied on electrospun conductive nanofibres | Coaxial electrospinning | Up to 50 S cm−1 | — | 172 |
| 34 | Spherical-shaped Ag nanoparticles decorated PAni nanofibre | Preparation of Ag-decorated BDP fibres as a sensitive material for the detection of 4-mercaptobenzoic acid and rhodamine 6G | Solution dipping method | — | — | 195 |
| 35 | Acidic solutions of aniline in HCl polymerised on nonwoven electrospun PS mats | Preparation of hierarchical PAni–polystyrene composite for water remediation | In situ polymerisation | — | — | 196 |
2.2.1.2. Composite polypyrrole fibres. The low water solubility and poor processability of PPy mean that there are few reports of pristine PPy fibres.197 It follows that PPy may be considered as the most utilised conducting polymer in making composite fibres. Over the past two decades, a variety of materials have been demonstrated as appealing substrates for PPy. Due to the good adhesion force between PPy and various substrates,18 conducting composites may be prepared that retain the inherent properties of both PPy and the substrate.198 These substrates include carbon, graphite,199 glass,200 and polymeric fibres.201,202 In general, the conductivity of PPy/fibre composites is directly related to PPy loading, ratio of oxidant to dopant, and fibre structure.203
In 1989 Kuhn et al. were the first to perform a remarkably effective, in situ, solution-based, and commercially feasible process for coating each individual fibre in woven, knitted or nonwoven textiles with a thin layer of PPy, with the results published some years later in 1993.204 This method was subsequently applied to a variety of textiles. Forder et al. applied the technique on polyester, nylon and cotton, leading to electrical conductivities ranging between 35 and 160 S cm−1.205 Others have endeavoured to coat graphite fibres with PPy through this method.199 One of the earliest reports of the deposition of PPy onto fibres involved a two-step process whereby the substrate was soaked in a ferric chloride solution before immersion in a pyrrole solution.206 Silver nanowires,207 ultra-high molecular weight polyethylene fibre (Fig. 12(a)),208 silica short fibres,28 cotton fibres209 and yarns,210 short nylon fibre/natural rubber,211 cellulose203 and banana fibres212 have been used as alternative substrates for the oxidative polymerisation process to create a PPy layer on them. Using reactive wet spinning, Foroughi et al. reported the fabrication of electrically conducting fibres comprised of an alginate biopolymer and PPy (Fig. 13(b)).213 Recently Wang et al. described the preparation of novel actuators based on graphene fibres coated with electropolymerised PPy.214 Furthermore, a wet spinning approach was lately employed to produce conductive composite fibres from reduced graphene oxide and polypyrrole nanoparticles resulted in conductivities of ∼20 S cm−1.215
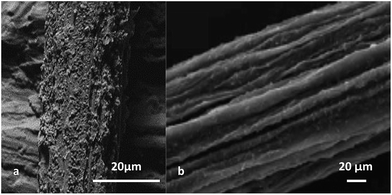 | ||
| Fig. 12 Scanning electron micrographs of (a) polypyrrole-coated ultra-high molecular weight polyethylene fibre, (b) polypyrrole–(APS/DEHS)–alginate fibre. Reproduced with permission from ref. 208 and 213 Copyrights© 2011; Elsevier and RSC Publishing, respectively. | ||
 | ||
Fig. 13 Scanning electron micrographs of (a) composite PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS/polyacrylonitrile conducting fibres with 0.38 wt% PEDOT PSS/polyacrylonitrile conducting fibres with 0.38 wt% PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS content and (b) PEDOT PSS content and (b) PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS–chitosan fibre. Reproduced with permission from ref. 239 and 242 Copyrights© 2013 and, Elsevier and WILEY-VCH Verlag GmbH & Co., respectively. PSS–chitosan fibre. Reproduced with permission from ref. 239 and 242 Copyrights© 2013 and, Elsevier and WILEY-VCH Verlag GmbH & Co., respectively. | ||
Chemical vapour deposition (CVD) (also known as vapour phase polymerisation) is another straightforward and rapid method to deposit PPy onto various substrates, and has been used widely to produce composite PPy fibres.18,202,216–220 Although this method has the advantage of simplicity, the highest reported electrical conductivity of fibres prepared this way was only 0.68 S cm−1,216 likely due to the formation of only a thin layer of conducting PPy. Nair and co-workers were the first to merge electrospinning with CVD for the synthesis of electrically conducting composite PPy nanofibres.202 This approach provided the advantages of electrospinning while at the same time circumventing the intractability of PPy. A year later, Chronakis et al. reported for the first time a method to prepare nanofibres using a mixture of PPy and PEO.143 In 2007, a microfluidic approach was described by others for fabricating hollow and core/sheath PPy nanofibres by electrospinning.221 The benefits of using microfluidic devices for nanofibre synthesis include rapid prototyping, ease of fabrication, and the ability to spin multiple fibres in parallel through arrays of individual microchannels. PPy composite core–shell nanostructures were also successfully prepared using PAn, PS and polyamide 6 (PA6) solutions.222 It is worth noting that a large number of prepared PPy composite fibres have been employed for sensor applications.18,134,223 An overview of the studies performed on composite PPy fibres is given in Table 5.
| No. | Sample name | Focus of the research | Synthesis method | Reported electrical conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| 1 | PPy–polyester and PPy–quartz composite fibre | Utilization of time-of-flight secondary ion mass spectrometry to examine the nature of the aromatic sulphonate dopant anion(s) in PPy overlayers deposited onto both polyester fibres and quartz fibres | — | — | — | 224 |
| 2 | Aqueous colloidal magnetite particles onto (polyester, nylon, cotton etc.) fibres from utilising a simple dip-coat procedure and then treated them with a PPy coating | Preparation of superparamagnetic-conducting textile composites by a facile, two-step solution deposition process | Two-step solution deposition process | 35–160 S cm−1 | — | 205 |
| 3 | Graphite fibre PPy coatings | Fabrication of graphite fibre PPy coatings by aqueous electrochemical polymerisation | Aqueous electrochemical polymerisation | — | — | 199 |
| 4 | PPy/poly(p-phenylene terephthalamide) | Continuous fast vapor phase polymerisation method to prepare electrically conducting PPy/PPTA | Vapor phase polymerisation | 0.68 S cm−1 | UTS 2.644 kPa, US 4.4% | 216 |
| 5 | Aramid fibres were immersed in FeCl3·6H2O solutions and were then exposed to pyrrole/H2O vapour at 20 °C | Preparation of conducting aramid/PPy composite fibres by vapour-phase polymerisation | Vapour phase polymerisation | ∼1.3 × 10−3 S cm−1 | — | 217 |
| 6 | Insulating natural cotton, silk, and wool fibres were electrochemically coated with pyrrole in acetonitrile (containing p-toluenesulfonic acid) | Fabrication of conducting fibres from natural polymers | Electrochemical polymerisation | — | — | 225 |
| 7 | PPy coated fabrics (83% Tactel blended with 17% (40 denier) Lycra in) | Preparation of a flexible strain sensor from PPy-coated fabrics | Chemical vapour deposition (CVD) | Change with several factors such as dopant, temperature, etc. | — | 18 |
| 8 | PPy–poly(ethyl-ene oxide) (PPy–PEO) composite nanofibre | Fabrication of electrically conducting PPy–poly(ethylene oxide) composite nanofibre | Electrospinning and vapour phase polymerisation | ∼10−3 S cm−1 | — | 202 |
| 9 | Aqueous titanium tetraisopropoxide + ethanol contained polyvinylpyrrolidone (PVP), then exposed to the saturated pyrrole vapor | Fabrication and characterisation of coaxial nanocables of PPy (PPy)/TiO2 and their surface properties | Electrospinning followed by vapor-phase polymerisation | — | — | 226 |
| 10 | Organic solvent soluble PPy, [(PPy3)+ (DEHS)−]x + NaDEHS | Preparation of PPy nanofibres with the practical application for construction of nanoelectronic devices | Electrospinning | Pure [(PPy3)+ (DEHS)−]x 2.7 × 10−2 S cm−1 for the composite fibre 3.5 × 10−4 S cm−1 | — | 143 |
| 11 | A stock solution of PVP, pyrrole and FeCl3 in ethanol + DMF as the core channel layer device and PVP in ethanol + DMF as the sheath | Fabrication of core/sheath nanofibres of (PPy)/PVP | Electrospinning with multi-spinneret microfluidic devices | — | — | 221 |
| 12 | Pyrrole monomer + PAn polymers in DMF, H2O2 was slowly added depending on pyrrole amount | Fabrication of polyacrylonitrile/PPy (PAn/PPy) composite nanofibres | Electrospinning | — | — | 24 |
| 13 | Fibres were dipped in solution of FeCl3 anhydrous in dried acetonitrile and then exposed to pyrrole vapour | Preparation of continuous vapour deposited PPy–cotton and PPy–silk yarns | Vapour phase polymerisation | ∼10−4 S cm−1 | — | 218 |
| 14 | Steel fibre coated with PPy (PPy) doped with polyphosphate | Development of polyphosphate-doped PPy coated on steel fibre for the GC determination of a group of organochlorine pesticides (OCPs) in water | Solid-phase microextraction (electrochemical coating) | — | — | 227 |
| 15 | Pyrrole, 3,4-diethyl pyrrole, PVA as the steric stabiliser polystyrene/FeCl3 fibres coated with PPy polystyrene/PPy suspension fibre in DMF | Production of conducting nanofibres by electrospinning based on PPy | Electrospinning | — | — | 222 |
| 16 | Polyamide 6/PPy nanofibres | Fabrication of composite polyamide 6/PPy conducting nanofibres | Electrospinning | — | — | 201 |
| 17 | Pyrrole, N-methylpyrrole, 3-methylthiophene, poly(3,4-ethylene dioxythiophene), deposited on substrates (Pt, stainless steel) | Preparation of poly-N-methylpyrrole microfibre | Electrodeposition | — | — | 228 |
| 18 | Silk fabrics were coated with electrically conducting doped PPy | Preparation of bio-based conducting composites ((PPy)-coated silk) fabrics | In situ oxidative polymerisation | — | — | 20 |
| 19 | Ag nanowires (NWs) dispersed in solution of (Cu(Ac)2), then oxidise pyrrole monomers to make uniform PPy sheath | Preparation of Ag/PPy coaxial nanocables | Ion adsorption/oxidative polymerisation | — | — | 207 |
| 20 | PPy and PTh coating on stainless steels as working electrodes | Application of PPy and PTh as an adsorbent in solid-phase microextraction sampling of five adrenolytic drugs | Solid-phase microextraction electropolymerisation (SPME) coating | — | — | 229 |
| 21 | FeCl3-doped PPy into a collagen-based polyelectrolyte complexation (PEC) fibre | Fabrication of PPy-incorporated collagen-based fibres to make 3D electroactive fibrous scaffolds | Interfacial polyelectrolyte complexation (IPC) | — | UTS 304 ± 61.0 MPa, YM 10.4 ± 4.3 GPa | 230 |
| 22 | PPy/hexagonally ordered silica (PPy/SBA15) coated on stainless steel wire | Fabrication of PPy/hexagonally ordered silica nanocomposite for solid-phase microextraction | Vapour phase polymerisation | — | — | 219 |
| 23 | Zn(S2CNEt2)2/pyrrole monomer | Spinning PPy/ZnS core/shell coaxial nanowires | Two-step process by anodic aluminum oxide (AAO) templates | — | — | 231 |
| 24 | FeCl3-doped UHMWPE fibres soaked in an aqueous solution of hexahydrate (catalyst) treated by pyrrole monomer | Production of PPy-coated UHMWPE fibre to improve adhesion and structure properties of UHMWPE fibre | Oxidative polymerisation coating | — | — | 208 |
| 25 | Silica fibres treated with silane agent and then coated with PPy | Production of PPy-coated amorphous silica short fibres (PPy–ASF) | In situ oxidative polymerisation coating | 0.32 S cm−1 | — | 28 |
| 26 | Admixing silver nitrate and pyrrole in presence of cotton fabric at different mole ratios | Synthesis of PPy silver nanocomposite on cotton fabrics | In situ oxidative polymerisation | ∼10−3 S cm−1 | — | 209 |
| 27 | MWCNT fillers + polystyrene matrix PPy was coated on fibres | Fabrication of conducting electrospun polymer fibres with nano features | Electrospinning/in situ polymerisation | 3.7 × 10−4 S cm−1 | — | 232 |
| 28 | PPy coated layer on Ag–TiO2 nanofibre | Protecting Ag nanoparticles from being oxidised by a PPy layer | Electrospinning | — | — | 233 |
| 29 | PPy coated on E-glass fibre, FeCl3 (oxidant)/TsO− (dopant) | Fabrication of coaxial fibres to shield the house hold appliances | Vapor deposition | 99.23% of the EM incident radiations can be blocked | — | 234 |
| 30 | Pyrrole (Py) polymerised on graphene fibres | Fabricating of novel electrochemical flexible and wearable graphene/PPy fibres as an actuator | In situ oxidative polymerisation | Highly active within the ±0.8 V range | — | 214 |
| 31 | Py polymerised on the BF surface using (FeCl3·6H2O) as oxidant | Preparation and characterisation of PPy-coated banana fibres | In situ oxidative polymerisation | 1.8 × 105 S cm−1 (electrical resistivity) | — | 212 |
| 32 | Py in presence of FeCl3 (oxidant)/p-toluene sulphonic acid (dopant) | Fabricating and characterising natural rubber/PPy and natural rubber/PPy/PPy-coated short nylon fibre | In situ oxidative polymerisation | 8.3 × 10−4 S cm−1 NR/PPy 6.25 × 10−2 S cm−1 (for NR/PPy/F-PPy) | — | 211 |
| 33 | FeCl3 and anthraquinone-2-sulfonic acid sodium salt as oxidant/dopant | Preparation of PPy/cellulose fibre composites | In situ oxidative polymerisation | 203 | ||
| 34 | PCL + dichloromethane and N,N-dimethylformamide (DMF), PPy was coated on fibres/FeCl3 as an oxidant | Fabrication of PPy hollow fibres to extract two important neuroendocrine markers from plasma samples | Electrospinning and in situ polymerisation | — | — | 144 |
| 35 | Cotton, wool, polyester exposed to pyrrole using FeCl3 (oxidant) | Investigation of the response of conductive woven, knitted, and nonwoven composite fabrics of pH, humidity, and mechanical strain | In situ polymerisation | — | — | 223 |
| 36 | Aq. Py with FeCl3 (oxidant)/pTSA (dopant) | Fabrication of electroconductive cotton yarn | In situ polymerisation | Resistivity 182.63 kΩ m−1 | — | 210 |
| 37 | Aq. Py with FeCl3 (oxidant)/pTSA (dopant) | Preparation and characterisation of electrically conductive textiles for heat generation | In situ polymerisation | Resistivity 1013.08 Ω m−1 | — | 235 |
| 38 | Aq. Py with FeCl3 (oxidant)/pTSA (dopant) | Investigation of chitosan/polypyrrole composite fibre as biocompatible artificial muscles | In situ polymerisation | — | — | 236 |
| 39 | PPy coated with ferric tosylate (oxidant)/n-butanol/EDOT deposited | Soft linear electroactive polymer actuators based on polypyrrole | Two-step chemical–electrochemical synthesis | — | — | 220 |
| 40 | Ppy nanoparticles dispersed in aq. GO solution | Preparation of conductive composite fibres from reduced graphene oxide and polypyrrole | Wet spinning | ∼20 S cm−1 | YM 8 ± 4 GPa, UTS 175 ± 8 MPa | 215 |
2.2.1.3. Composite poly(3,4-ethylene dioxythiophene)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) poly(styrenesulfonic acid) fibres. PEDOT is a well-studied semiconducting polymer that is rendered solution-processable when doped with acidic PSS.237 The processability of PEDOT
poly(styrenesulfonic acid) fibres. PEDOT is a well-studied semiconducting polymer that is rendered solution-processable when doped with acidic PSS.237 The processability of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS has naturally meant that relatively few studies have considered PEDOT
PSS has naturally meant that relatively few studies have considered PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS within composite fibres. Nevertheless, composite PEDOT
PSS within composite fibres. Nevertheless, composite PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres are at the centre of attention due to their high conductivity and multiple applications.
PSS fibres are at the centre of attention due to their high conductivity and multiple applications.Dip-coating has been the main method used for preparing hybrid PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres for the past few years. This method was first employed by Irwin et al. to deposit PEDOT
PSS fibres for the past few years. This method was first employed by Irwin et al. to deposit PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS onto silk fibres from an ethylene glycol solution,17 yielding a composite fibre exhibiting 8.5 S cm−1 electrical conductivity, which was considerably higher compared to previous literature values for ICP-coated fibres. Zampetti et al. coated an electrospun titania membrane mesh with PEDOT
PSS onto silk fibres from an ethylene glycol solution,17 yielding a composite fibre exhibiting 8.5 S cm−1 electrical conductivity, which was considerably higher compared to previous literature values for ICP-coated fibres. Zampetti et al. coated an electrospun titania membrane mesh with PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS using dip-coating,238 which was then used as a nitric oxide sensor for asthma monitoring. Recently, PEDOT
PSS using dip-coating,238 which was then used as a nitric oxide sensor for asthma monitoring. Recently, PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS-coated chitosan hybrid fibres was developed,239 which showed relatively high conductivity values of ca. 60 S cm−1 (Fig. 13(a)). A few researchers have just addressed preparation of electrically conductive textiles based on poly(ethylene terephthalate) (PET), polyurethane and polyacrylate fabrics coated with PEDOT
PSS-coated chitosan hybrid fibres was developed,239 which showed relatively high conductivity values of ca. 60 S cm−1 (Fig. 13(a)). A few researchers have just addressed preparation of electrically conductive textiles based on poly(ethylene terephthalate) (PET), polyurethane and polyacrylate fabrics coated with PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS.157,240,241
PSS.157,240,241
In recent time, limited cases described preparation of composite PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres through wet spinning method. Liu and colleagues recently described a novel approach to prepare conducting fibres of PEDOT
PSS fibres through wet spinning method. Liu and colleagues recently described a novel approach to prepare conducting fibres of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS blended with PAn via wet spinning (Fig. 13(b)).242 Not long ago, polyurethane/PEDOT
PSS blended with PAn via wet spinning (Fig. 13(b)).242 Not long ago, polyurethane/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS elastomeric fibres with high electrical conductivities in the range of ca. 2–25 S cm−1 were reported.243 Seyedin et al. claimed as-prepared fibres as potential strain-responsive sensors. A summary of composite PEDOT
PSS elastomeric fibres with high electrical conductivities in the range of ca. 2–25 S cm−1 were reported.243 Seyedin et al. claimed as-prepared fibres as potential strain-responsive sensors. A summary of composite PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibre investigations is presented in Table 6.
PSS fibre investigations is presented in Table 6.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibre investigations
PSS fibre investigations
| No. | Spinning or coating solution | Focus of the research | Synthesis method | Reported electrical conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ionomer mixture poly(3,4-ethylenedioxythiophene)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(styrenesulfonate) (PEDOT poly(styrenesulfonate) (PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS; 1 PSS; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5, w 2.5, w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) w) |
Preparation and characterisation of silk casted PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres from a ethylene glycol solution PSS fibres from a ethylene glycol solution |
Dip-coating | 8.5 S cm−1 | UTS 1000 cN | 17 |
| 2 | PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS solution, mixed with 0.1% EDOT, which was infiltrated into silk thread PSS solution, mixed with 0.1% EDOT, which was infiltrated into silk thread |
Preparation of composite fibres of PEDOT–PSS/silk for signal recording | Electrochemically deposition | 0.102 S cm−1 | UTS 1239 cN | 244 |
| 3 | Aqueous PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS dispersion injected into chitosan coagulation bath and then treated with ethylene glycol (EG) PSS dispersion injected into chitosan coagulation bath and then treated with ethylene glycol (EG) |
Producing coaxial conducting polymer fibres loaded with an antibiotic drug by electropolymerisation for smart release applications | Dip-coating | 56 ± 7 S cm−1 | YM 2 ± 0.3 GPa, UTS 99 ± 7 MPa, US 20.6 ± 1.2% | 239 |
| 4 | PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS blended with polyacrylonitrile (PAn) PSS blended with polyacrylonitrile (PAn) |
Characterisation of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS–PAn composite fibre in terms of electrical conductivity, thermal stability and mechanical properties PSS–PAn composite fibre in terms of electrical conductivity, thermal stability and mechanical properties |
Wet spinning | 5.0 S cm−1 | YM 3.32 cN per dtex, UTS 0.36 cN per dtex, US 36.73% | 242 |
| 5 | Electrospun nanofibres of titania onto an interdigitated electrode (IDE) coated with an ultra-thin film of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS PSS |
Preparation of a chemosensor as a potential sensing material for NO (asthma monitoring) | Electrospinning followed by dip-coating | — | — | 238 |
| 6 | Polyethylene terephthalate (PET) textiles coat with aq. PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS solutions PSS solutions |
Fabrication of e-textiles based on polyethylene terephthalate (PET) textiles with different formulations of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS PSS |
Knife-coating (direct), pad-coating (impregnation) and screen printing | Resistivity 10–20 Ω m−2 | — | 157 |
| 7 | Different compositions of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS (2.9–25%) and PU PSS (2.9–25%) and PU |
Preparation of highly conductive polyurethane/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS elastomeric fibres for sensor applications PSS elastomeric fibres for sensor applications |
Wet spinning | 2–25 S cm−1 | YM ∼ 7.2–247 MPa, UTS ∼ 0.5–9.1 MPa, US up to ∼400% | 243 |
| 8 | Polyvinyl alcohol (PVA) and PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS aq. solution PSS aq. solution |
Investigation of tensile strength and conductivity of PVA/PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres PSS fibres |
Wet spinning | 10–79 S cm−1 | UTS ∼ 39–84 MPa | 245 |
 | ||
| Fig. 14 Scanning electron micrograph of an APS/DEHS-doped polypyrrole–alginate–carbon nanotube fibre. Reproduced with permission from ref. 213 Copyright© 2011; The Royal Society of Chemistry. | ||
Electrospinning was employed for the first time by Kim et al. for producing one-dimensional multi-walled carbon nanotube (MWNT)-filled PAni/PEO nanocomposite fibres with improved electrical properties.252 Improvements in electrical and mechanical properties of electrospun PAni/PEO/MWCNT composite fibres were later described by Lin and Wu.253 Zhang et al. have recently reported on preparation of nanocomposite PAni/polyacrylonitrile/multiwalled carbon nanotubes fibres with conductivities up to 7.97 S m−1 via electrospinning.254
In situ polymerisation of conducting polymers on CNTs is as another method used extensively to fabricate composite fibres. Fan et al. synthesised PPy on CNTs using (NH4)2S2O8 as the oxidant and reported modification of the electrical, magnetic and thermal properties of the CNTs by PPy.255,256 Ju et al. described a two-step method for producing aligned nano-sized PPy/activated carbon composite fibres for supercapacitor applications using electrospinning followed by an in situ chemical polymerisation method.257 Foroughi and co-workers prepared PPy–MWNT yarns by chemical and electrochemical polymerisation of pyrrole on the surface and within the porous interior of twisted MWNT yarns. The composite yarn produced may be used for applications where electrical conductivity and good mechanical properties are of primary importance.258 Wet spinning of composite formulations based on functionalised PEG–SWNT and PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS was investigated recently, yielding composite fibres exhibiting 22.8 GPa modulus and 254 MPa ultimate stress (Fig. 15(b)).259 Dorraji et al. described PAni/MWCNT/chitosan nanofibres manufactured via polymerisation of PAni/MWCNT on wet-spun chitosan fibres.260 These fibres yielded conductivities of ca. 5.3 × 10−2 S cm−1. Table 7 summarises investigations into composite conducting polymer–carbon nanotube fibres.
PSS was investigated recently, yielding composite fibres exhibiting 22.8 GPa modulus and 254 MPa ultimate stress (Fig. 15(b)).259 Dorraji et al. described PAni/MWCNT/chitosan nanofibres manufactured via polymerisation of PAni/MWCNT on wet-spun chitosan fibres.260 These fibres yielded conductivities of ca. 5.3 × 10−2 S cm−1. Table 7 summarises investigations into composite conducting polymer–carbon nanotube fibres.
 | ||
| Fig. 15 Scanning electron micrograph of chemically prepared PPy–CNT yarn showing the surface morphology, scale bar shows 1 μm; reproduced with permission from ref. 258 Copyright© 2012; The Royal Society of Chemistry. | ||
| No. | Spinning or coating solution | Focus of the research | Spinning method | Reported electrical conductivity/conduction potential window | Mechanical properties | Ref. |
|---|---|---|---|---|---|---|
| 1 | PAni–CNT–DMPU dispersion | Producing PAni–CNT composite fibres for potential use in e-textiles | Wet spinning | (2 wt% CNT) 32 ± 3 S cm−1 | (2 wt% CNT) after doping UTS 229 ± 22 MPa, YM 5.2 ± 0.2 GPa, US 8 ± 3% | 248 |
| 2 | AMPSA | Producing PAni–SWCNT composite fibres for potential use in e-textiles | Wet spinning | 750 S cm−1 | UTS 250–300 MPa, YM 7–8 GPa | 249 |
| 3 | PAni–CNT–DMPU dispersion | Improvement of electromechanical actuation of fibres when used as artificial muscles | Wet spinning | 128.0 ± 25 S cm−1 | UTS 260 MPa, YM 17 GPa | 247 |
| 4 | PAni (AMPSA)–CNT–DMPU dispersion | Producing high-strength conducting PAni–CNT composite fibres | Wet spinning | 716 ± 36 S cm−1 | UTS 255 ± 32 MPa, US 4 ± 0.6%, YM 7.3 ± 0.4 GPa | 250 |
| 5 | Chitosan/PAni/SWNT (DCA–AMPSA) | Producing a fibre which shows both pH and electrochemical actuation | Wet spinning | 1.8 S cm−1 | UTS 95 MPa, US 60% | 251 |
| 6 | Alg–PPy–CNT APS/DEHS (dopant) FeCl3/DEHS (dopant) APS/pTS (dopant) FeCl3/pTS (dopant) | Producing electrically conducting, robust fibres of PPy–Alg–CNT | Reactive wet spinning | 3.0 ± 0.5 S cm−1 | UTS 250 ± 5 MPa, US 10 ± 1.3%, YM 10 ± 0.5 GPa | 213 |
| 7 | 2.0 ± 0.4 S cm−1 | UTS 95 ± 4 MPa, US 10 ± 1.3%, YM 10 ± 0.5 GPa | ||||
| 8 | 4.0 ± 0.8 S cm−1 | UTS 250 ± 5 MPa, US 5 ± 1%, YM 11.8 ± 2 GPa | ||||
| 9 | 10.0 ± 1.5 S cm−1 | UTS 65 ± 8 MPa, US 4 ± 0.7%, YM 3.7 ± 0.8 GPa | ||||
| 10 | PAni–polyethylene oxide (PEO)–MWNT | Producing hybrid PAni/PEO/MWNT fibres with improved electrical properties using electrospinning | Electrospinning | 8.89 μS cm−1 (with 0.5 wt% MWNTs) | — | 252 |
| 11 | PAni–PEO–MWNT | To increase the electrical conductivity of PAni/PEO nanofibre | Electrospinning | Up to 2.77 μS cm−1 | — | 261 |
| 12 | PAni–PEO–MWNT | Improving the electrical and mechanical properties of PAni/PEO/MWNT composite fibre | Electrospinning | 9 × 10−6 S cm−1 | YM 23.6 MPa | 253 |
| 13 | CNT–PPy (NH4)2S2O8 (oxidant) | Producing CNT–PPy composite fibre | In situ polymerisation | 16 S cm−1 | — | 255 |
| 14 | CNT–PPy APS (oxidant) | Improvement in electrical, magnetic and thermal properties of CNTs by coating them with PPy | In situ polymerisation | 16 S cm−1 | — | 256 |
| 15 | PPy-activated carbon nanofiber (ACNF)–CNT | Producing nano composite electrodes for supercapacitors | Electrospinning followed by in situ chemical polymerisation | PPy/ACNF: 0.79 S cm−1, PPy/ACNF/CNT: 1.33 S cm−1 | — | 257 |
| 16 | PAni–PAn–MWCNTs solution DBSA (dopant) | Electrospinning and microwave absorption of polyaniline/polyacrylonitrile/multiwalled carbon nanotubes nanocomposite | Electrospinning | Up to ∼8 S m−1 | — | 254 |
| 17 | PAni m-cresol solutions for coating carbon fabrics | Preparation of PAni/CF and characterization of them for removal of Cr(VI) from wastewater | Dip-coating | — | — | 262 |
| 18 | MWCNTs–aniline in HCl chemically deposited on chitosan fibres | Chitosan/polyaniline/MWCNT nanofibre as electrodes for electrical double layer capacitors | Chemical oxidative polymerization | 5.3 × 10−2 S cm−1 | — | 260 |
| 19 | PPy coating on CF-filled polytetrafluoroethylene composites | Polypyrrole coating effect on mechanical properties of carbon fibre-filled polytetrafluoroethylene | Dip-coating | — | Very poor | 263 |
| 20 | PPy–MWNT | Producing hybrid PPy–MWNT yarns with good conductivity and mechanical properties | Chemical and electrochemical polymerisation | Electrochemically prepared PPy–CNT yarn | Single PPy–CNT yarn (chemically prepared PPy–CNT) | 258 |
| 220 ± 9 S cm−1 | UTS 510 ± 7 MPa, US 2.5 ± 0.4%, YM 35 ± 3 GPa | |||||
| Chemically prepared PPy–CNT yarn | Two-ply PPy–CNT yarn chemically prepared PPy–CNT | |||||
| 235 ± 15 S cm−1 | UTS 740 ± 18 MPa, US 1.5 ± 0.6%, YM 57 ± 6 GPa | |||||
| Electrochemically prepared PPy–CNT | ||||||
| UTS 273 ± 9 MPa, US 4.5 ± 1%, YM 7.6 ± 0.4 GPa |
3. Current and future applications of CPFs
Thus far, conjugated conducting polymer fibres have found many applications due to combination of properties similar to those of metals along with their great formability via the variety of fabrication methods usually associated with conventional polymers. These fibres are being extensively studied to meet aesthetic demands and the needs of two key classifications of energy and bionics devices development. CPFs offer high conductivity, rapid charge–discharge rates, relatively inexpensive and simple large scale production, flexible and lightweight, and environmentally friendly devices known to form the next generation of energy suppliers. In energy applications they have been incorporated into devices for a range of purposes from storage to conversion such as electrodes and batteries,92,264–266 chemical sensors,26,238,267 supercapacitors,268–270 smart textiles,177,235,271–273 actuators and artificial muscles.136,250,274 For example, fabrication of polyaniline and polyaniline carbon nanotube composite fibers employing wet spinning as high performance artificial muscles have been reported previously by Spinks et al.250 The fibers have tensile strengths of 255 MPa and operate to stress levels in excess of 100 MPa, three times higher than previously reported for conducting-polymer actuators and 300 times higher than skeletal muscle. Furthermore, a wet spinning process was described to produce fiber capacitor electrodes of PAni–CNT which showed the maximum specific capacitance of 29.7 F cm−2 in 1 M HCl solution.275 Conducting fibres also provide benefits for either their direct use as energy storage devices or to be integrated into fabrics to create multi-functional wearable smart textiles. This trend could facilitate the rapid development of portable and flexible electronic devices. However great efforts have been paid to investigate different aspects of the usage of CPFs in the field of energy, their electrochemical performance as well as mechanical properties are still far from satisfied, when compared to some other kinds of materials, such as CNT fibers. It has also been suggested that they show promise for applications in photovoltaic (solar) cells,83,158 electronic circuits,46 organic light-emitting diodes,78,276 and electrochromic displays.83,240Applications of CPFs in biological field were expanded later on with the discovery that these materials were compatible with many biological molecules in late 1980s.23 Most CPs present a number of important advantages for biomedical applications, including biocompatibility, ability to entrap and controllably release biological molecules, ability to transfer charge from a biochemical reaction, and the potential to alter the properties of the CPs to better suit the nature of the specific application.23 Conducting fibres can provide self-supporting three-dimensional, flexible structures suitable for in vitro and in vivo bionic applications compared to the films. These functional aspects may also require the overlap of certain characteristics for example for uses in implantable batteries and bio-actuators.23,277 In more detail, storage or conversion of energy and provide the required biocompatibility. Today, the major bioapplications of CPFs are generally within the area of electrical stimulation and signal recording,244,278,279 drug-delivery devices,239 tissue-engineering scaffolds,125,132,280 and biosensors.23,281 Recently, there is a growing interest in using conducting fibres for neural tissue engineering applications. These conductive fibrillar pathways may provide appropriate replacements for nerve fibres after injuries. Electrical stimulation has been shown to enhance the nerve regeneration process and this consequently makes the use of electrically conductive polymer fibres very attractive for the construction of scaffolds for nerve tissue engineering. For instance, Li et al. investigated the feasibility to generate novel electrospun PAni–gelatin blended scaffolds as potential scaffolds for neural tissue engineering.170 They reported that as-prepared fibers are biocompatible, supporting attachment, migration, and proliferation of rat cardiac myoblasts. In another study, the feasibility of fabricating a blended fibre of PAni–polypropylene as a conductive pathway was studied for neurobiological applications.280 In addition, production of conducting fibres for controlled drug release applications is currently of particular interest of many research groups. Fabrication of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS–chitosan hybrid fibres was described using a novel wet spinning strategy to achieve a controlled release of an antibiotic drug.239 Still, there remain limitations for use of CPs due to their manufacturing costs, material inconsistencies, poor solubility in solvents and inability to directly melt process. Moreover, oxidative dopants could diminish their solubility in organic solvents and water and hence their processability.
PSS–chitosan hybrid fibres was described using a novel wet spinning strategy to achieve a controlled release of an antibiotic drug.239 Still, there remain limitations for use of CPs due to their manufacturing costs, material inconsistencies, poor solubility in solvents and inability to directly melt process. Moreover, oxidative dopants could diminish their solubility in organic solvents and water and hence their processability.
Despite the above mentioned impressive achievements, further developments are needed for high-performance flexible conductive fibres that can simultaneously ensure high conductivity, excellent mechanical robustness and flexibility as well as high electrochemical performance for practical applications. There also exist potential applications of conducting polymer fibres in electrodes, microelectronics, sensors, actuators and rechargeable batteries outside of those already discussed. Besides, conducting polymer fibres could be considered as candidates for interconnection technology. However, to further improve the field of conducting polymer fibres, the following features could be potentially investigated and improved.
Great efforts have been paid to the field of fabrication of mechanically robust yet flexible robust conducting fibres; however, CPFs still lack desirable mechanical robustness comparing to common traditional textile fibres or some other kinds of polymers. Thus, there's an increasing tendency in recent years toward improving their toughness by producing composite fibres. This trend is expected to remain as the biggest challenge in their further development. There is also a necessity to establish a unified standard method to investigate mechanical flexibility of fibres.
Moreover, the trend for the development of smart textiles is to integrate or embed conducting fibres within common textile structures using facile knitting/braiding techniques to facilitate free and easy access to them while imparting a number of smart functionalities such as signalling, sensing, actuating, energy storage or information processing by creating hybrid systems. Some preliminary works have been carried out to study the incorporation of flexible conducting fibres into common textiles. However, integration of CPFs into the garments for practical applications is still a challenge.
4. Conclusions
Development of materials and methods for the preparation of conducting polymer fibres is an important enabling step towards their application, particularly in smart textiles. We have summarized the history of emergence of CPs, categories, preparation and spinning methods for the recent development of pristine and composite conducting polymer fibres as well as their current/future of applications. Wet spinning is the preferred method for preparing conducting polymer fibres. Electrospinning was also used widely to produce nanoscale nonwoven fibres. Due to the intractable nature of many conducting polymers, the first step to create fibres is the development of methods for the preparation of conducting polymer solutions. PAc was discovered in 1977 and was at the centre of attention for a time but its poor processability limited further development. PAni was the next conducting polymer of interest and drew much attention from the late 1980s, owing to its unique combination of processability and good electrical conductivity. It is readily soluble in emeraldine base and leucoemeraldine base forms, providing the opportunity to directly spin fibres. However, such fibres displayed suboptimal properties due to their undoped state. This could be rectified by wet spinning the conducting emeraldine salt form from concentrated sulfuric acid. Better results were obtained using large molecule dopants that rendered the emeraldine salt form soluble in organic solvents. The highest conductivity values for PAni fibres were measured to be up to 150 S cm−1. However, this value would be raised up to even 1500 S cm−1 upon stretching or heating.54,95 The Young's moduli of these fibres were also reported to shift in the range of 0.5–13 GPa while stretched.57 Fibres prepared by such methods could be further improved by mechanical drawing and incorporation of CNTs. Similar approaches have been applied in the preparation of PPy fibres, while production of composite fibres have been focused on owing to the poor processability of PPy. The obtained electrical conductivities for polypyrrole fibres were appeared to be much lower compared to that of PAni fibres, up to the highest value of ∼3 S cm−1;61 however, higher Young's moduli was achievable with the maximum value of ∼4.2 GPa.62 Polythiophene is readily available in the water soluble form of PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS, which may be readily wet-spun. PEDOT
PSS, which may be readily wet-spun. PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS fibres indicated a better performance in contrast to the previously stated PAni and PPy fibres in terms of electrical conductivity and mechanical properties with the highest obtained conductivity of ∼470 S cm−1 and Young's moduli in the range of ∼3.3–4.0 GPa.63,64
PSS fibres indicated a better performance in contrast to the previously stated PAni and PPy fibres in terms of electrical conductivity and mechanical properties with the highest obtained conductivity of ∼470 S cm−1 and Young's moduli in the range of ∼3.3–4.0 GPa.63,64
The greatest improvements in conducting polymer fibre mechanical strength and electrical conductivity have been achieved through the incorporation of CNTs. An enhanced electrical conductivity of ∼750 S cm−1 was determined for PAni fibres after addition of 0.3% w/w CNT.249 However, CNTs incorporation leads to relatively brittle fibres, with typical elongation at break values of less than 20%. Such brittleness is in contrast to common textile fibres such as nylons and polyesters, and limits the application of conducting polymer fibres. Therefore, the challenging task of improving the toughness of conducting polymer fibres needs to be a focus of future development. Although recent developments in CPFs appear extremely promising, there still remain challenges to improve their properties and performance to become adequate for practical and commercial applications.
Acknowledgements
This work was supported by funding from the University of Wollongong, Australian Research Council Centre of Excellence and Laureate Fellowships (G. G. Wallace) and the Australian Research Council under Discovery Early Career Researcher award (Javad Foroughi DE12010517). The authors would like to thank Mr Saber Mostafavian for his 3D set-up designs. The authors would also like to appreciate Dr George Tsekouras for his great help with critical revising and also Mr Sayamk Farajikh for his assistance.References
- D. Kumar and R. C. Sharma, Eur. Polym. J., 1998, 34, 1053–1060 CrossRef CAS.
- H. Shirakawa, E. J. Louis, A. G. MacDiarmid, C. K. Chiang and A. J. Heeger, J. Chem. Soc., Chem. Commun., 1977, 16, 578–580 RSC.
- A. G. Macdiarmid, Angew. Chem., Int. Ed., 2001, 40, 2581–2590 CrossRef CAS.
- J. Foroughi, Development of novel nanostructured conducting polypyrrole fibres, PhD thesis, University of Wollongong, 2009, p. 210.
- C. K. Chiang, M. A. Druy, S. C. Gau, A. J. Heeger, E. J. Louis, A. G. MacDiarmid, Y. W. Park and H. Shirakawa, J. Am. Chem. Soc., 1978, 100, 1013–1015 CrossRef CAS.
- C. K. Chiang, C. R. Fincher, Y. W. Park, A. J. Heeger, H. Shirakawa, E. J. Louis, S. C. Gau and A. G. MacDiarmid, Phys. Rev. Lett., 1977, 39, 1098–1101 CrossRef CAS.
- H. Derivatives, J. Louis and A. G. Macdiarmid, J. Chem. Soc., Chem. Commun., 1977, 16, 578–580 Search PubMed.
- T. C. Street and G. B. Clarke, IBM J. Res. Dev., 1981, 25, 51–57 CrossRef.
- G. Clark, Am. Econ. Rev., 2007, 90, 802–828 Search PubMed.
- G. G. Wallace, P. R. Teasdale, G. M. Spinks and L. A. P. Kane-Maguire, Conductive electroactive polymers, 2009 Search PubMed.
- J. E. Fischer, X. Tang, E. M. Scherr, V. B. Cajipe and A. G. MacDiarmid, Synth. Met., 1991, 43, 661–664 CrossRef.
- L. Pan, H. Qiu, C. Dou, Y. Li, L. Pu, J. Xu and Y. Shi, Int. J. Mol. Sci., 2010, 11, 2636–2657 CrossRef CAS PubMed.
- J. Wang, C. Y. Wang, C. O. Too and G. G. Wallace, J. Power Sources, 2006, 161, 1458–1462 CrossRef CAS.
- C. Y. Wang, V. Mottaghitalab, C. O. Too, G. M. Spinks and G. G. Wallace, J. Power Sources, 2007, 163, 1105–1109 CrossRef CAS.
- A. Mazzoldi and D. Rossi, Electro-active polymer fiber actuators polyelectrolyte gel fiber actuators conducting polymer fiber actuators, 2003 Search PubMed.
- S. Kakuda, T. Momma and T. Osaka, J. Electrochem. Soc., 1995, 142, 1–2 CrossRef.
- M. D. Irwin, D. A. Roberson, R. I. Olivas, R. B. Wicker and E. Macdonald, Fibers Polym., 2011, 12, 904–910 CrossRef CAS.
- Y. Li, X. Y. Cheng, M. Y. Leung, J. Tsang, X. M. Tao and M. C. W. Yuen, Synth. Met., 2005, 155, 89–94 CrossRef CAS.
- A. Granero, J. Razal, G. Wallace and M. Panhuis, J. Mater. Chem., 2010, 20, 7953–7956 RSC.
- I. Cucchi, A. Boschi, C. Arosio, F. Bertini, G. Freddi and M. Catellani, Synth. Met., 2009, 159, 246–253 CrossRef CAS.
- S. Karamchandani, H. D. Mustafa, S. N. Merchant and U. B. Desai, Sens. Actuators, B, 2011, 156, 765–772 CrossRef CAS.
- M. A. Mestrovic, Characterisation and Biomedical application of Fabric Sensors, Master's degree thesis, RMIT University, 2007, p. 162.
- N. K. Guimard, N. Gomez and C. E. Schmidt, Prog. Polym. Sci., 2007, 32, 876–921 CrossRef CAS.
- X. Li, X. Hao, H. Yu and H. Na, Mater. Lett., 2008, 62, 1155–1158 CrossRef CAS.
- S. N. Khan, Electrospinning polymer nanofibers-electrical and optical Characterization, PhD thesis, Ohio University, 2007, p. 107.
- T. Ahuja and D. Kumar, Sens. Actuators, B, 2009, 136, 275–286 CrossRef.
- J. R. Cárdenas, M. G. O. De França, E. A. De Vasconcelos, W. M. De Azevedo and E. F. Da Silva, J. Phys. D: Appl. Phys., 2007, 40, 1068–1071 CrossRef.
- C. Merlini, B. S. Rosa, D. Müller, L. G. Ecco, S. D. A. S. Ramôa and G. M. O. Barra, Polym. Test., 2012, 31, 971–977 CrossRef CAS.
- P. Chandrasekhar, Conducting Polymers: Fundamentals and Applications, Kluwer Academic Publishers, 1999 Search PubMed.
- G. G. Wallace and L. A. P. Kane-maguire, Adv. Mater., 2002, 14, 953–960 CrossRef CAS.
- A. F. Diaz, K. K. Kanazawa and G. P. Gardini, J. Chem. Soc., Chem. Commun., 1979, 635–636 RSC.
- G. M. Spinks, B. Xi, V. Truong and G. G. Wallace, Synth. Met., 2005, 151, 85–91 CrossRef CAS.
- J. Wang, C. O. Too, D. Zhou and G. G. Wallace, J. Power Sources, 2005, 140, 162–167 CrossRef CAS.
- Y. Lin and G. G. Wallace, J. Controlled Release, 1994, 30, 137–142 CrossRef CAS.
- J. N. Barisci, R. Stella, G. M. Spinks and G. G. Wallace, Synth. Met., 2001, 124, 407–414 CrossRef CAS.
- L. A. Kane-Maguire, I. D. Norris and G. G. Wallace, Synth. Met., 1999, 101, 817–818 CrossRef CAS.
- L. A. P. Kane-maguire, A. G. Macdiarmid, I. D. Norris and G. G. Wallace, Synth. Met., 1999, 106, 171–176 CrossRef CAS.
- C. Wang, A. Ballantyne, S. Hall, C. Too, D. Officer and G. Wallace, J. Power Sources, 2006, 156, 610–614 CrossRef CAS.
- C. Liu, C. Lin, C. Kuo, S. Lin and W. Chen, Prog. Polym. Sci., 2011, 36, 603–637 CrossRef CAS.
- C. Li, H. Bai and G. Shi, Chem. Soc. Rev., 2009, 38, 2149–2496 RSC.
- M. Wan, Macromol. Rapid Commun., 2009, 30, 963–975 CrossRef CAS PubMed.
- J. Stejskal, I. Sapurina and M. Trchová, Prog. Polym. Sci., 2010, 35, 1420–1481 CrossRef CAS.
- C. Laslau, Z. Zujovic and J. Travas-sejdic, Prog. Polym. Sci., 2010, 35, 1403–1419 CrossRef CAS.
- A. J. Heeger, Synth. Met., 2002, 125, 23–42 CrossRef CAS.
- B. Semire and O. A. Odunola, Aust. J. Basic Appl. Sci., 2011, 5, 354–359 CAS.
- B. Weng, R. L. Shepherd, K. Crowley, A. J. Killardb and G. G. Wallace, Analyst, 2010, 135, 2779–2789 RSC.
- H. H. Kipphan, Handbook of print media, Springer Science & Business Media, 2001 Search PubMed.
- A. Earls and V. Baya, Disruptive Manuf. Eff. 3D Print., 2014, p. 14 Search PubMed.
- T. C. Gomes, C. J. L. Constantino, E. M. Lopes, A. E. Job and N. Alves, Thin Solid Films, 2012, 520, 7200–7204 CrossRef CAS.
- M. V. Kulkarni, S. K. Apte, S. D. Naik, J. D. Ambekar and B. B. Kale, Sens. Actuators, B, 2013, 178, 140–143 CrossRef CAS.
- B. Weng, A. Morrin, R. Shepherd, K. Crowley, A. J. Killard, P. C. Innis and G. G. Wallace, J. Mater. Chem. B, 2014, 2, 793–799 RSC.
- M. F. Mabrook, C. Pearson and M. C. Petty, Sens. Actuators, B, 2006, 115, 547–551 CrossRef CAS.
- I. Zergioti, M. Makrygianni, P. Dimitrakis, P. Normand and S. Chatzandroulis, Appl. Surf. Sci., 2011, 257, 5148–5151 CrossRef CAS.
- N. Farra, Development and Characterization of Conductive Polyaniline Fibre Actuators, Bachelor's degree thesis, University of Toronto, 2008, p. 40.
- A. D. Jannakoudakis, P. D. Jannakoudakis, N. Pagalos and E. Theodoridou, Electrochim. Acta, 1993, 38, 1559–1566 CrossRef CAS.
- K. T. Tzou and R. V. Gregory, Synth. Met., 1995, 69, 109–112 CrossRef CAS.
- B. R. Mattes, H. L. Wang and D. Yang, Synth. Met., 1997, 84, 45–49 CrossRef CAS.
- C. Woodings, Regenerated Cellulose Fibres, CRC Press, 2001 Search PubMed.
- M. N. Gupta, A. K. Sengupta and V. Kothari, Manufactured Fibre Technology, Springer Science & Business Media, 1997 Search PubMed.
- S. J. Pomfret, P. N. Adams, N. P. Comfort and A. P. Monkman, Polym. J., 2000, 41, 2265–2269 CAS.
- J. Foroughi, G. M. Spinks, G. G. Wallace and P. G. Whitten, Synth. Met., 2008, 158, 104–107 CrossRef CAS.
- J. Foroughi, G. M. Spinks and G. G. Wallace, Synth. Met., 2009, 159, 1837–1843 CrossRef CAS.
- R. Jalili, J. M. Razal, P. C. Innis and G. G. Wallace, Adv. Funct. Mater., 2011, 21, 3363–3370 CrossRef CAS.
- H. Okuzaki, Y. Harashina and H. H. Yan, Eur. Polym. J., 2009, 45, 256–261 CrossRef CAS.
- D. Zhang, Advances in Filament Yarn Spinning of Textiles and Polymers, Woodhead Publishing, 2014 Search PubMed.
- H. Wieden, J. Romatowski, F. Moosmueller and H. Lenz, Polyurethane spinning solutions containing ethylene diamine and bis-(4-aminophenyl)-alkane polyurethanes, US pat. 3485800 A, Bayer AG, Dec 23rd, 1969.
- R. W. Moncrieff, Man-Made Fibres, Wiley, illustrate., 1970 Search PubMed.
- T. Huang, L. R. Marshall, J. E. Armantrout, S. Yembrick, W. H. Dunn, J. M. Oconnor, T. Mueller, M. Avgousti and M. D. Wetzel, Production of nanofibers by melt spinning, US pat. 8277711 B2, E I Du Pont De Nemours And Company, 2 Oct 2012.
- J. Jia, MELT spinning of continuous filaments by cold air attenuation, PhD degree, Georgia Institute of Technology, 2010, p. 168.
- B. Kim, V. Koncar, E. Devaux, C. Dufour and P. Viallier, Synth. Met., 2004, 146, 167–174 CrossRef CAS.
- D. H. Reneker and I. Chun, Nanotechnology, 1996, 7, 216–223 CrossRef CAS.
- Z. Huang, Y.-Z. Zhang, M. Kotaki, S. Ramakrishna, S. R. Zheng-Ming Huang, Y.-Z. Zhang and M. Kotaki, Compos. Sci. Technol., 2003, 63, 2223–2253 CrossRef CAS.
- A. Ziebicki, Fundamentals of Fibre Formation, Wiley, London, 2010 Search PubMed.
- Y. Srivastava, M. Marquez and T. Thorsen, Biomicrofluidics, 2009, 3, 12801 CrossRef PubMed.
- J. Kameoka, R. Orth, Y. Yang, D. Czaplewski, R. Mathers, G. W. Coates and H. G. Craighead, Nanotechnology, 2003, 1124, 1124–1129 CrossRef.
- T. S. Kang, S. W. Lee, J. Joo and J. Y. Lee, Synth. Met., 2005, 153, 61–64 CrossRef CAS.
- L. Dai, in Intelligent Macromolecules for Smart Devices, Springer Science & Business Media, 1975, vol. 1980, pp. 41–80 Search PubMed.
- A. Farcas and C. I. Simionescu, Rev. Roum. Chim., 2006, 51, 1153–1156 CAS.
- K. Akagi, G. Piao, S. Kaneko and K. Sakamaki, Science, 1998, 282, 1683–1686 CrossRef CAS PubMed.
- A. Pron, P. Rannou and Â. Synthe, Prog. Polym. Sci., 2002, 27, 135–190 CrossRef CAS.
- D. E. Sliva, W. G. Selley, Continuous method for making spinnable polyacetylene solutions convertible to high strength carbon fiber, US pat. 3928516, General Electric Company, Schenectady, NY, Dec. 23rd, 1975.
- G. T. Kim, M. Burghard, D. S. Suh, K. Liu, J. G. Park, S. Roth and Y. W. Park, Synth. Met., 1999, 105, 207–210 CrossRef CAS.
- N. Gospodinova and L. Terlemezyan, Prog. Polym. Sci., 1998, 23, 1443–1484 CrossRef CAS.
- B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef.
- A. A. Syed and M. K. Dinesan, Talanta, 1991, 38, 815–837 CrossRef CAS PubMed.
- S. Ramakrishnan, Resonance, 2011, 2, 48–58 CrossRef.
- H. S. Kolla, S. P. Surwade, X. Zhang, A. G. Macdiarmid and S. K. Manohar, J. Am. Chem. Soc., 2005, 127, 16770–16771 CrossRef CAS PubMed.
- E. Peter Maziarz III, S. A. Lorenz, T. P. White and T. D. Wood, J. Am. Soc. Mass Spectrom., 2000, 11, 659–663 CrossRef.
- S. Virji, J. Huang, R. B. Kaner and B. H. Weiller, Nano Lett., 2004, 4, 491–496 CrossRef CAS.
- C. He, Y. Tan and Y. Li, J. Appl. Polym. Sci., 2003, 87, 1–4 CrossRef.
- X. Zhang, R. Chan-Yu-King, A. Jose and S. K. Manohar, Synth. Met., 2004, 145, 23–29 CrossRef CAS.
- R. Pauliukaite, C. M. A. Brett and A. P. Monkman, Electrochim. Acta, 2004, 50, 159–167 CAS.
- E. Smela and B. R. Mattes, Synth. Met., 2005, 151, 43–48 CrossRef CAS.
- A. Mazzoldi, C. Degl'Innocenti, M. Michelucci and D. De Rossi, Mater. Sci. Eng., C, 1998, 6, 65–72 CrossRef.
- S. J. Pomfret, P. N. Adams, N. P. Comfort and A. P. Monkman, Synth. Met., 1999, 101, 724–725 CrossRef CAS.
- D. Yang, A. Fadeev, P. N. Adams and B. R. Mattes, in Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices, 2001, vol. 4329, pp. 59–71 Search PubMed.
- W. Lu and B. R. Mattes, Synth. Met., 2005, 152, 53–56 CrossRef CAS.
- X. Wang, J. Liu, X. Huang and L. Men, Polym. Bull., 2008, 60, 1–6 CrossRef CAS.
- N. C. S. U. Tushar K. Ghosh, T. Ghosh and M. Ramasubramanian, Muscle Like Extruded Fiber Actuators, 2009 Search PubMed.
- A. Andreatta, C. Yong, J. C. Chiang, A. J. Heeger and P. Smith, Synth. Met., 1988, 26, 383–389 CrossRef CAS.
- C. H. Hsu, J. D. Cohen and R. F. Tietz, Synth. Met., 1993, 59, 37–41 CrossRef CAS.
- C. C. Han and R. L. Elsenbaumer, International Patent, WG92/11695, 1990.
- S. Metals, Synth. Met., 1991, 41–43, 849–854 Search PubMed.
- B. R. Mattes, H.-L. Wang, Stable, concentrated solutions of high molecular weight polyaniline and articles therefrom, is patented in both US pat. (US6099907 A) and Europe (EP1008148 A1) invented by assigned to The Regents Of The University Of California, published on 16th Jun 1999 and May 29, 1997, respectively.
- P. N. Adams, P. Devasagayam, S. J. Pomfret, L. Abell and A. P. Monkman, J. Phys.: Condens. Matter, 1998, 10, 8293–8303 CrossRef CAS.
- Y. Chen, H. Zhao and B. Han, Russ. J. Phys. Chem. A, 2014, 88, 2314–2317 CrossRef CAS.
- J. Huang, Pure Appl. Chem., 2006, 78, 15–27 CrossRef CAS.
- N. J. Pinto, A. T. Johnson, A. G. MacDiarmid, C. H. Mueller, N. Theofylaktos, D. C. Robinson and F. A. Miranda, Appl. Phys. Lett., 2003, 83, 4244 CrossRef CAS.
- N. Ayu and K. Umiati, in ICITACEE, 2014, pp. 79–82 Search PubMed.
- C. M. A. Brett, R. Pauliukaite and A. P. Monkman, Polyaniline Fibres as Substrates for Microsensors, 2002, vol. 539 Search PubMed.
- A. Macagnano, E. Zampetti, S. Pantalei, M. Italia, C. Spinella and A. Bearzotti, in CP1137, Olfaction and Electronic Nose: Proceedings of the 13th International Symposium, 2009, pp. 373–377 Search PubMed.
- Y. Zhang, J. J. Kim, D. Chen, H. L. Tuller and G. C. Rutledge, Adv. Funct. Mater., 2014, 24, 4005–4014 CrossRef CAS.
- G. Jin, J. Norrish, C. Too and G. Wallace, Curr. Appl. Phys., 2004, 4, 366–369 CrossRef.
- F. D. Blum, S. K. Pillalamarri, L. K. Werake and J. G. Story, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 2006, 47, 2966 Search PubMed.
- L. K. Werake, J. G. Story, M. F. Bertino, S. K. Pillalamarri and F. D. Blum, Nanomedicine, 2005, 16, 2833–2837 CAS.
- X. Zhang and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 12714–12715 CrossRef CAS PubMed.
- J. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851–855 CrossRef CAS PubMed.
- U. Tamer, Ç. Kanbeş, H. Torul and N. Ertaş, React. Funct. Polym., 2011, 71, 933–937 CrossRef CAS.
- D. Li, J. Huang and R. B. Kaner, Acc. Chem. Res., 2009, 42, 135–145 CrossRef CAS PubMed.
- X. Zhang, W. Goux and S. Manohar, J. Am. Chem. Soc., 2004, 126, 4502–4503 CrossRef CAS PubMed.
- A. Abdolahi, E. Hamzah, Z. Ibrahim and S. Hashim, Materials, 2012, 5, 1487–1494 CrossRef CAS.
- S. Xing, C. Zhao, S. Jing and Z. Wang, Polym. J., 2006, 47, 2305–2313 CAS.
- D. Han, H. J. Lee and S. Park, Electrochim. Acta, 2005, 50, 3085–3092 CrossRef CAS.
- T. V. Vernitskaya and O. N. Efimov, Russ. Chem. Rev., 1997, 443, 443–457 CrossRef.
- A. Dall'olio, G. Dascola, V. Vacara and V. Bocchi, Resonance paramagnetique electronique et conductivite ́d'un noir d'oxypyrrol electrolytique, CR Acad. Sci., 1968, 267, 433–435 Search PubMed.
- J. Wu and J. Pawliszyn, J. Chromatogr. A, 2001, 909, 37–52 CrossRef CAS PubMed.
- D. Kim and Y. D. Kim, J. Ind. Eng. Chem., 2007, 13, 879–894 CAS.
- C. J. Cui, G. M. Wu, H. Y. Yang, S. F. She, J. Shen, B. Zhou and Z. H. Zhang, Electrochim. Acta, 2010, 55, 8870–8875 CrossRef CAS.
- X. Yuan, X. Ding, C. Wang and Z. Ma, Energy Environ. Sci., 2013, 1105–1124 CAS.
- A. Mirabedini, J. Foroughi, T. Romeo and G. G. Wallace, Macromol. Mater. Eng., 2015, 300, 1217–1225 CrossRef CAS.
- E. W. H. Jager, C. Immerstrand, K. Magnusson, O. Inganas and I. Lundstrom, in Annual International IEEE-EMBS Special Topic Conference on Microtechnologies in Medicine & Biology, 2000, vol. c, pp. 58–61 Search PubMed.
- Z. B. Huang, G. F. Yin, X. M. Liao and J. W. Gu, Front. Mater. Sci., 2014, 8, 39–45 CrossRef.
- H. Qin, A. Kulkarni, H. Zhang, H. Kim, D. Jiang and T. Kim, Sens. Actuators, B, 2011, 158, 223–228 CrossRef CAS.
- S. Hamilton, M. J. Hepher and J. Sommerville, Sens. Actuators, B, 2005, 107, 424–432 CrossRef CAS.
- B. E. Smela, Adv. Mater., 2003, 15, 481–494 CrossRef.
- J. Foroughi, G. Spinks and G. Wallace, Sens. Actuators, B, 2011, 155, 278–284 CrossRef CAS.
- P. Saville, Polypyrrole, Formation and Use, 2005 Search PubMed.
- N. M. Rowley and R. J. Mortimer, Sci. Prog., 2002, 85, 243–262 CrossRef CAS PubMed.
- C. Y. Kim, J. Y. Lee, D. Y. Kim, Soluble, Electroconductive polypyrrole and method for preparing the same, US pat. 005795953A, Korea Institute of Science and Technology. Seoul. Rep. of Korea, Aug 18, 1998.
- G. J. Lee, S. H. Lee, K. S. Ahn and K. H. Kim, J. Appl. Polym. Sci., 2002, 84, 2583–2590 CrossRef CAS.
- E. J. Oh and K. S. Jang, Synth. Met., 2001, 119, 109–110 CrossRef CAS.
- Z. Qi and P. G. Pickup, Chem. Mater., 1997, 9, 2934–2939 CrossRef CAS.
- I. S. Chronakis, S. Grapenson and A. Jakob, Polym. J., 2006, 47, 1597–1603 CAS.
- T. Tian, J. Deng, Z. Xie, Y. Zhao, Z. Feng, X. Kang and Z. Gu, Analyst, 2012, 137, 1846–1852 RSC.
- E. M. Thurman and M. S. Mills, Rapid Commun. Mass Spectrom., 1998, 12, 988 CrossRef.
- B. S. Li, C. W. Macosko and H. S. White, Adv. Mater., 1993, 5, 575–576 CrossRef.
- J. Foroughi, S. R. Ghorbani, G. Peleckis, G. M. Spinks, G. G. Wallace, X. L. Wang and S. X. Dou, J. Appl. Phys., 2010, 107, 103712 CrossRef.
- M. A. De'Paoli, R. C. D. Peres and J. M. Pernaut, J. Braz. Chem. Soc., 1990, 1, 50–52 CrossRef.
- J. Roncali, Chem. Rev., 1992, 92, 711–738 CrossRef CAS.
- T. Yamamoto, K. Sanechika and A. Yamamoto, J. Polym. Sci., 1980, 18, 9–12 CAS.
- L. P. Dudek and J. W. P. Lin, J. Polym. Sci., 1980, 18, 2869–2873 Search PubMed.
- S. M. Unni, V. M. Dhavale, V. K. Pillai and S. Kurungot, J. Phys. Chem. C, 2010, 114, 14654–14661 CAS.
- A. M. Dadras and A. Entezami, Iran. Polym. J., 1993, 3, 2–12 Search PubMed.
- X. Zhang, S. P. Surwade, V. Dua, R. Bouldin, N. Manohar and S. K. Manohar, Chem. Lett., 2008, 37, 526–527 CrossRef CAS.
- X. Zhang, A. G. Macdiarmid and S. K. Manohar, Chem. Commun., 2005, 5328–5330 RSC.
- H. Park, S. Ko, J. Park, J. Y. Kim and H. Song, Sci. Rep., 2013, 3, 1–6 Search PubMed.
- M. Akerfeldt, Electrically conductive textile coatings with PEDOT:PSS, University of Boras, Faculty of Textiles, Engineering and Business, 2015, p. 46 Search PubMed.
- E. Environ, D. Alemu, H. Wei, K. Ho and C. Chu, Energy Environ. Sci., 2012, 5, 9662–9671 Search PubMed.
- X. Guo, X. Liu, F. Lin, H. Li, Y. Fan and N. Zhang, Sci. Rep., 2015, 5, 1–9 Search PubMed.
- M. M. Islam, A. T. Chidembo, S. H. Aboutalebi, D. Cardillo, H. K. Liu and E. Al, Front. Mater. Sci., 2014, 2, 1–21 CAS.
- Y. Zhang and K. S. Suslick, Chem. Mater., 2015, 27, 7559–7563 CrossRef CAS.
- M. J. Sailor and C. L. Curtis, Adv. Mater., 1994, 6, 688–692 CrossRef CAS.
- H. Okuzaki and M. Ishihara, Macromol. Rapid Commun., 2003, 24, 261–264 CrossRef CAS.
- W. Baik, W. Luan, R. H. Zhao, S. Koo and K. Kim, Synth. Met., 2009, 159, 1244–1246 CrossRef CAS.
- T. Takahashi, M. Ishihara and H. Okuzaki, Synth. Met., 2005, 152, 73–76 CrossRef CAS.
- A. G. MacDiarmid, Curr. Appl. Phys., 2001, 1, 11–22 CrossRef.
- P. V. Segonds and A. J. Epstein, Synth. Met., 1991, 43, 1005–1008 Search PubMed.
- A. Mirmohseni, D. Salari and R. Nabavi, Iran. Polym. J., 2006, 15, 259–264 CAS.
- R. H. Cruz-estrada and M. J. Folkes, J. Mater. Sci., 2000, 35, 5065–5069 CrossRef CAS.
- M. Li, Y. Guo, Y. Wei, A. G. Macdiarmid and P. I. Lelkes, Biomaterials, 2006, 27, 2705–2715 CrossRef CAS PubMed.
- I. D. Norris, M. M. Shaker, F. K. Ko and A. G. Macdiarmid, Synth. Met., 2000, 114, 109–114 CrossRef CAS.
- Y. Zhang and G. C. Rutledge, Macromolecules, 2012, 45, 4238–4246 CrossRef CAS.
- M. Gizdavic-Nikolaidis, S. Ray, J. R. Bennett, A. J. Easteal and R. P. Cooney, Macromol. Biosci., 2010, 10, 1424–1431 CrossRef CAS PubMed.
- S. H. Bhang, S. I. Jeong, T. Lee, I. Jun, Y. B. Lee, B. Kim and H. Shin, Macromol. Biosci., 2012, 12, 402–411 CrossRef CAS PubMed.
- Q. Yu, M. Wang, H. Chen and Z. Dai, Mater. Chem. Phys., 2011, 129, 666–672 CrossRef CAS.
- R. Fryczkowski, M. Rom and B. Fryczkowska, Fibres Text. East. Eur., 2005, 13, 141–143 CAS.
- E. Devaux, V. Koncar, B. Kim, M. Rochery, C. Campagne and D. Saihi, Trans. Inst. Meas. Control, 2007, 29, 355–376 CrossRef.
- Y. Wang, Y. Li, J. Feng and C. Sun, Anal. Chim. Acta, 2008, 619, 202–208 CrossRef CAS PubMed.
- X. Jin, C. Xiao and W. Wang, Synth. Met., 2010, 160, 368–372 CrossRef CAS.
- J. Molina, M. F. Esteves, J. Fernández, J. Bonastre and F. Cases, Eur. Polym. J., 2011, 47, 2003–2015 CrossRef CAS.
- S. Chandran and S. K. Narayanankutty, Polym.-Plast. Technol. Eng., 2011, 50, 37–41 Search PubMed.
- Q. Fan, X. Zhang and Z. Qin, J. Macromol. Sci., Part B: Phys., 2012, 51, 736–746 CrossRef CAS.
- S. Izwan, W. A. Wan, S. Hashim and M. Y. Yahya, Compos. Interfaces, 2012, 19, 356–376 Search PubMed.
- X. Liu, W. Zhou, X. Qian, J. Shen and X. An, Carbohydr. Polym., 2013, 92, 659–661 CrossRef CAS PubMed.
- T. Takano, M. Tagaya and T. Kobayashi, Polym. Bull., 2013, 70, 3019–3030 CrossRef CAS.
- X. Chen, H. Cai, Q. Tang, D. Liang, M. Wang and B. He, Polym. Compos., 2013, 35, 235–262 Search PubMed.
- D. Passeri, A. Biagioni, M. Rossi, E. Tamburri and M. L. Terranova, Eur. Polym. J., 2013, 49, 991–998 CrossRef CAS.
- P. Schmitz, C. Merlini, G. M. O. Barra, A. Silveira, T. Medeiros, S. D. A. S. Ram and A. Pegoretti, Polym. Test., 2014, 38, 18–25 CrossRef.
- S. Poyraz, I. Cerkez, T. S. Huang, Z. Liu, L. Kang, J. Luo and X. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 20025–20034 CAS.
- C. Hsu, H. Shih, S. Subramoney and A. J. Epstein, Synth. Met., 1999, 101, 677–680 CrossRef CAS.
- Q. H. Zhang, Z. C. Sun, J. Li, X. H. Wang, H. F. Jin, X. B. Jing and F. S. Wang, Synth. Met., 1999, 102, 1198–1199 CrossRef CAS.
- Y. A. Ismail, S. R. Shin, K. M. Shin, S. G. Yoon, K. Shon, S. I. Kim and S. J. Kim, Sens. Actuators, B, 2008, 129, 834–840 CrossRef CAS.
- A. Soroudi and M. Skrifvars, Preparation of melt spun conductive polypropylene/polyaniline fibres for smart textile applications, 2008 Search PubMed.
- A. Soroudi and M. Skrifvars, Polym. Eng. Sci., 2012, 1606–1612 CAS.
- S. Mondal, U. Rana and S. Malik, J. Appl. Mater. Interfaces, 2015, 7, 10457–10465 CrossRef CAS PubMed.
- J. J. Alcaraz-espinoza, A. E. Cha, J. C. Medina-llamas, A. S. Andrade and C. P. De Melo, ACS Appl. Mater. Interfaces, 2015, 7, 7231–7240 CAS.
- L. A. Mccullough, B. Dufour and K. Matyjaszewski, Macromolecules, 2009, 42, 8129–8137 CrossRef CAS.
- S. Xing and G. Zhao, J. Appl. Polym. Sci., 2007, 104, 1987–1996 CrossRef CAS.
- B. Grunden and J. O. Iroh, Polym. J., 1995, 36, 559–563 CAS.
- O. Flores, A. Romo-Uribe, M. E. Romero-Guzman, A. E. Gonzalez, R. Cruz-Silva and B. Campillo, J. Appl. Polym. Sci., 2009, 112, 934–941 CrossRef CAS.
- F. Granato, A. Bianco, C. Bertarelli and G. Zerbi, Macromol. Rapid Commun., 2009, 30, 453–458 CrossRef CAS PubMed.
- S. Nair, S. Natarajan and S. Kim, Macromol. Rapid Commun., 2005, 26, 1599–1603 CrossRef CAS.
- H. Wang, N. Leaukosol and Z. He, Cellulose, 2013, 20, 1587–1601 CrossRef CAS.
- H. H. Kuhn, W. C. Kimbrell, J. E. Fowler and C. N. Barry, Synth. Met., 1993, 57, 3707–3712 CrossRef CAS.
- C. Forder, S. P. Armes, A. W. Simpson, C. Maggiore and M. Hawley, J. Mater. Chem., 1993, 3, 563–569 RSC.
- N. Balci, L. Topparet and E. Bayramlit, Composites, 1995, 26, 229–231 CrossRef CAS.
- T. Qiu, H. Xie and J. Zhang, J. Nanopart. Res., 2011, 13, 1175–1182 CrossRef CAS.
- X. Jin, W. Wang, L. Bian, C. Xiao, G. Zheng and C. Zhou, Synth. Met., 2011, 161, 984–989 CrossRef CAS.
- K. Firoz Babu, P. Dhandapani, S. Maruthamuthu and M. Anbu Kulandainathan, Carbohydr. Polym., 2012, 90, 1557–1563 CrossRef CAS PubMed.
- S. Maity and A. Chatterjee, J. Text. Sci. Eng., 2014, 4, 4–7 Search PubMed.
- D. S. Pramila Devi, P. K. Bipinbal, T. Jabin and S. K. N. Kutty, Mater. Des., 2013, 43, 337–347 CrossRef CAS.
- C. Merlini, S. Ramoa and G. M. O. Barra, Polym. Compos., 2013, 537–543 CrossRef CAS.
- J. Foroughi, G. M. Spinks and G. G. Wallace, J. Mater. Chem., 2011, 21, 6421 RSC.
- Y. Wang, K. Bian, C. Hu, Z. Zhang, N. Chen, H. Zhang and L. Qu, Electrochem. Commun., 2013, 35, 49–52 CrossRef CAS.
- K. S. U. Schirmer, D. Esrafilzadeh, B. C. Thompson, A. F. Quigley, R. M. I. Kapsa and G. G. Wallace, J. Mater. Chem. B, 2016, 4, 42–47 RSC.
- C. Xu, P. Wang and X. Bi, J. Appl. Polym. Sci., 1995, 58, 2155–2159 CrossRef CAS.
- J. W. Cho and H. Jung, J. Mater. Sci., 1997, 32, 5371–5376 CrossRef CAS.
- A. Esfandiari, World Appl. Sci. J., 2008, 3, 470–475 Search PubMed.
- M. B. Gholivand, M. M. Abolghasemi and P. Fattahpour, Anal. Chim. Acta, 2011, 704, 174–179 CrossRef CAS PubMed.
- A. Maziz, A. Khaldi, N. Persson and E. W. H. Jager, in Proc. of SPIE, 2015, vol. 9430, pp. 1–6 Search PubMed.
- Y. Srivastava, I. Loscertales, M. Marquez and T. Thorsen, Microfluid. Nanofluid., 2007, 4, 245–250 CrossRef.
- S. Sen, F. J. Davis, G. R. Mitchell and E. Robinson, J. Phys.: Conf. Ser., 2009, 183, 12–20 CrossRef.
- S. Maity and A. Chatterjee, J. Compos. Mater., 2015, 2015, 1–6 Search PubMed.
- M. J. Hearn, I. W. Fletcher, S. P. Church and S. P. Armes, Polym. J., 1993, 34, 262–266 CAS.
- S. N. Bhadani, M. Kumari, S. K. Sen Gupta and G. C. Sahu, J. Appl. Phys., 1998, 64, 1073–1077 Search PubMed.
- X. Lu, Q. Zhao, X. Liu, D. Wang, W. Zhang, C. Wang and Y. Wei, Macromol. Rapid Commun., 2006, 27, 430–434 CrossRef CAS.
- A. Mollahosseini and E. Noroozian, Anal. Chim. Acta, 2009, 638, 169–174 CrossRef CAS PubMed.
- A. D. Kammerich, Y. Srivastava, K. N. Hargett, V. S. R. Harrison and J. F. Rubinson, J. Electrochem. Soc., 2009, 156, P68 CrossRef CAS.
- P. Olszowy, M. Szultka, T. Ligor, J. Nowaczyk and B. Buszewski, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 2226–2234 CrossRef CAS PubMed.
- S. Yow, T. Lim, E. Yim, C. Lim and K. Leong, Polymers, 2011, 3, 527–544 CrossRef CAS.
- D. Zhang, L. Luo, Q. Liao, H. Wang, H. Fu and J. Yao, J. Phys. Chem. C, 2011, 115, 2360–2365 CAS.
- J. Wang, H. Naguib and A. Bazylak, Behavior and mechanics of multifunctional materials and composites, 2012, vol. 8342, pp. 1–13 Search PubMed.
- Y. Yang, J. Wen, J. Wei, R. Xiong, J. Shi and C. Pan, Appl. Mater. Interfaces, 2013, 5, 6201–6207 CrossRef CAS PubMed.
- A. Malik, R. Abbasi and J. Militky, J. Chem. Eng., 2013, 7, 256–259 Search PubMed.
- S. Maity, A. Chatterjee, B. Singh and A. P. Singh, J. Text. Inst., 2015, 5000, 887–893 Search PubMed.
- S. Lee, B.-J. Yi, K.-Y. Chun, J. Lee, Y. T. Kim, E.-J. Cha and S. J. Kim, J. Nanosci. Nanotechnol., 2015, 15, 2537–2541 CrossRef CAS PubMed.
- I. F. Perepichka, M. Besbes, E. Levillain, M. Salle and J. Roncali, Chem. Mater., 2002, 14, 449–457 CrossRef CAS.
- E. Zampetti, S. Pantalei, A. Muzyczuk, A. Bearzotti, F. D. Cesare, C. Spinella and A. Macagnano, Sens. Actuators, B, 2013, 176, 390–398 CrossRef CAS.
- D. Esrafilzadeh, J. Razal, S. Moulton, E. Stewart and G. Wallace, J. Controlled Release, 2013, 169, 313–320 CrossRef CAS PubMed.
- Y. Ding, M. A. Invernale and G. A. Sotzing, ACS Appl. Mater. Interfaces, 2010, 2, 1588–1593 CAS.
- M. Stra and A. Maria, Text. Res. J., 2013, 83, 618–627 CrossRef.
- Y. Liu, X. Li and J. C. Lu, J. Appl. Polym. Sci., 2013, 130, 370–374 CrossRef CAS.
- M. Z. Seyedin, J. M. Razal, P. C. Innis and G. G. Wallace, Adv. Funct. Mater., 2014, 24, 2957–2966 CrossRef CAS.
- S. Tsukada, H. Nakashima and K. Torimitsu, PLoS One, 2012, 7, 1–10 Search PubMed.
- H. Miura, K. Morohoshi, J. Okada, B. Lin and M. Kimura, Cellul. Ind., 2010, 66, 280–283 CAS.
- R. Y. Suckeveriene, E. Zelikman, M. Narkis and L. Nicolais, in Wiley Encyclopedia of Composites, John Wiley & Sons, Inc., 2011 Search PubMed.
- V. Mottaghitalab, B. Xi, G. M. Spinks, G. G. Wallace and G. G. Wallace, Synth. Met., 2006, 156, 796–803 CrossRef CAS.
- V. Mottaghitalab, G. M. Spinks and G. G. Wallace, Synth. Met., 2005, 152, 77–80 CrossRef CAS.
- V. Mottaghitalab, G. M. Spinks and G. G. Wallace, Polym. J., 2006, 47, 4996–5002 CAS.
- G. M. Spinks, V. Mottaghitalab, M. Bahrami-samani, P. G. Whitten, G. G. Wallace, B. G. M. Spinks, V. Mottaghitalab, M. Bahrami-samani, P. G. Whitten and G. G. Wallace, Adv. Mater., 2006, 2522, 637–640 CrossRef.
- G. M. Spinks, S. R. Shin, G. G. Wallace, P. G. Whitten, I. Y. Kim, S. I. Kim, S. J. Kim and S. Jeong, Sens. Actuators, B, 2007, 121, 616–621 CrossRef CAS.
- Y. J. Kim, M. K. Shin, S. J. Kim, S.-K. Kim, H. Lee, J.-S. Park and S. I. Kim, J. Nanosci. Nanotechnol., 2007, 7, 4185–4189 CrossRef CAS PubMed.
- Y. Lin and T. Wu, J. Appl. Polym. Sci., 2012, 126, E123–E129 CrossRef CAS.
- Z. Zhang, F. Zhang, X. Jiang, Y. Liu, Z. Guo and J. Leng, Fibres Polym., 2014, 15, 2290–2296 CrossRef CAS.
- J. Fan, M. Wan, D. Zhu, B. Chang, Z. Pan and S. Xie, Synth. Met., 1999, 102, 1266–1267 CrossRef CAS.
- J. Fan, M. Wan, D. Zhu, B. Chang, Z. Pan and S. Xie, J. Appl. Polym. Sci., 1999, 74, 2605–2610 CrossRef CAS.
- Y. Ju, G. Choi, H. Jung and W. Lee, Electrochim. Acta, 2008, 53, 5796–5803 CrossRef CAS.
- J. Foroughi, G. M. Spinks, S. R. Ghorbani, M. E. Kozlov, F. Safaei, G. Peleckis, G. G. Wallace and R. H. Baughman, Nanoscale, 2012, 4, 940–945 RSC.
- R. Jalili, J. M. Razal and G. G. Wallace, Nature, 2013, 3, 1–7 Search PubMed.
- M. S. S. Dorraji, I. Ahadzadeh and M. H. Rasoulifard, Int. J. Hydrogen Energy, 2014, 39, 9350–9355 CrossRef.
- M. K. Shin, Y. J. Kim, S. I. Kim, S. Kim and H. Lee, Sens. Actuators, B, 2008, 134, 122–126 CrossRef CAS.
- B. Qiu, C. Xu, D. Sun, H. Wei, X. Zhang, J. Guo and Q. Wang, RSC Adv., 2014, 4, 29855–29865 RSC.
- H. Chen, X. Cai and X. Bi, J. Thermoplast. Compos. Mater., 2014, 27, 1065–1073 CrossRef CAS.
- D. Wei, D. Cotton and T. Ryhänen, Nanomaterials, 2012, 2, 268–274 CrossRef CAS.
- R. Pauliukaite, C. M. A. Brett and A. P. Monkman, Electrochim. Acta, 2004, 50, 159–167 CAS.
- A. Mirabedini, J. Foroughi, B. Thompson and G. G. Wallace, Adv. Eng. Mater., 2015, 18, 284–293 CrossRef.
- S. Seyedin, J. M. Razal, P. C. Innis, A. Jeiranikhameneh, S. Beirne and G. G. Wallace, ACS Appl. Mater. Interfaces, 2015, 7, 21150–21158 CAS.
- J. M. Chem, K. Jost and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 10776–10787 Search PubMed.
- L. Kou, T. Huang, B. Zheng, Y. Han, X. Zhao, K. Gopalsamy, H. Sun and C. Gao, Nat. Commun., 2014, 5, 1–10 Search PubMed.
- D. Yu, K. Goh, H. Wang, L. Wei, W. Jiang, Q. Zhang, L. Dai and Y. Chen, Nat. Nanotechnol., 2014, 9, 555–562 CrossRef CAS PubMed.
- Z. Xu and C. Gao, Acc. Chem. Res., 2014, 47, 1267–1276 CrossRef CAS PubMed.
- Z. Li, Z. Liu, H. Sun and C. Gao, Chem. Rev., 2015, 115, 7046–7117 CrossRef CAS PubMed.
- B. Fang, L. Peng, Z. Xu and C. Gao, ACS Nano, 2015, 9, 5214–5222 CrossRef CAS PubMed.
- T. F. Otero and J. M. Sansinena, Adv. Mater., 1998, 10, 491–494 CrossRef CAS PubMed.
- A. Mirmohseni, M. S. S. Dorraji and M. G. Hosseini, Electrochim. Acta, 2012, 70, 182–192 CrossRef CAS.
- S. J. Wang, Y. Geng, Q. Zheng and J. Kim, Carbon, 2010, 8, 1815–1823 CrossRef.
- S. Li, K. Shu, C. Zhao, C. Wang, Z. Guo, G. Wallace and H. K. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 16679–16686 CAS.
- B. A. F. Quigley, J. M. Razal, B. C. Thompson, S. E. Moulton, M. Kita, E. L. Kennedy, G. M. Clark, G. G. Wallace and R. M. I. Kapsa, Adv. Mater., 2009, 21, 1–5 Search PubMed.
- G. G. Wallace, G. M. Spinks, A. P. Leon and P. R. Teasdale, Conductive Electroactive Intelligent Materials Systems, 2003 Search PubMed.
- D. K. Cullen, A. R. Patel, J. F. Doorish, D. H. Smith and B. J. Pfister, J. Neural. Eng., 2008, 5, 374–384 CrossRef PubMed.
- L. Xia, Z. Wei and M. Wan, J. Colloid Interface Sci., 2010, 341, 1–11 CrossRef CAS PubMed.
Footnote |
| † Dedicated to Prof. Mojtaba Mirabedini for all his help and support during the whole period of my academic life. |
| This journal is © The Royal Society of Chemistry 2016 |