A “turn-on” fluorescent probe used for the specific recognition of intracellular GSH and its application in bioimaging†
Lanfang Pang,
Yanmei Zhou*,
Enze Wang,
Fang Yu,
Hua Zhou and
Wenli Gao
Institute of Environmental and Analytical Sciences, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, Henan 475004, P. R. China. E-mail: zhouyanmei@henu.edu.cn; Fax: +86-371-23881589; Tel: +86-371-22868833-3422
First published on 13th January 2016
Abstract
We have designed and synthesized a carbazole-based fluorescent probe (CZ-Nm) for the specific recognition of GSH over Cys and Hcy. The probe is almost non-fluorescent owing to the photoinduced electron transfer (PET) process from the carbazole fluorophore to the nitroolefin moiety. Upon treatment with GSH, owing to the Michael addition of GSH to the double bond of the nitroolefin moiety, the probe shows a fluorescence enhancement and absorption change. The CZ-Nm probe displays desirable properties such as acting as a “naked eye” probe, a wide linear range of 0–0.01 M, high sensitivity, and strong anti-jamming capability. More importantly, the probe can also be successfully applied to the detection of intracellular GSH with a bright fluorescence signal, good cell permeability and bio-compatibility.
Introduction
Cysteine (Cys), homocysteine (Hcy), and glutathione (GSH) are important intracellular thiols that play vital roles in physiological and pathological events,1–3 and abnormal levels of these thiols are closely related to certain diseases.4,5 In particular, GSH is the most abundant intracellular thiol (1–10 mM) in mammalian and many prokaryotic cells, functioning as an essential endogenous antioxidant primarily involved in regulating cellular redox activities and preventing cell damage via scavenging free radicals and peroxides.6,7 Aberrant levels of GSH are directly associated with numerous clinical diseases, including cancer, human immunodeficiency virus (HIV), Alzheimer's, liver damage, leukocyte loss, cardiovascular disease and others.8,9 Accordingly, developing efficient techniques for the detection of GSH under physiological conditions is of considerable significance and has attracted a great deal of attention.10,11 Among the various detection methods that have been reported in the past few decades,12 optical imaging through staining with a fluorescent probe is considered to be the most convenient and efficient approach owing to its high sensitivity, good flexibility as well as apparent simplicity.13,14 In particular, the fluorescent probe could be successfully applied to intracellular detection.15,16To date, numerous thiol probes have been designed and synthesized utilizing the strong nucleophilicity of the thiol group.17 Although these probes can selectively detect biothiols from other amino acids, the discriminative detection of certain types of thiols still remains a significant challenge due to their similar structure and reactivity.18 In recent years, pioneered by Strongin's group, the selective detection of Cys/Hcy over GSH by the cyclization of Cys/Hcy with aldehydes was realized.19–22 Since then, the specific detection of Cys from Hcy/GSH and Hcy from GSH/Cys using Michael addition combined with steric and electrostatic interactions,23,24 Cys-induced substitution-rearrangement cascade reaction25,26 or the cyclization of Cys with acrylates27,28 have also been developed. By comparison, the probes capable of detecting GSH from Cys/Hcy have been addressed to a relatively less extent.29,30 To the best of our knowledge, some fluorescent probes based on organic dyes for the highly selective detection of GSH have been designed.31 For example, Chmielewski's group designed a seminal GSH-specific fluorescent probe based on breaking the disulfide bonds followed by intramolecular cyclization and cleavage of a neighboring carbonate bond between a rhodamine 110 derivative and GSH.32 Yang's group prepared a bis-spiropyran sensor based on supramolecular interactions for selectively detecting GSH.33 Taking advantage of the thiol-induced addition reaction, Wang,34 Huo35 and Keillor36 designed innovated chemical sensors to selectively detect GSH. Moreover, Yang,37 Guo38 and Lin39 designed GSH-specific fluorescent probes using a thiol-induced substitution reaction. Therefore, it is of high interest to design GSH-specific fluorescent probes.
Among the various organic fluorophores, carbazole dye has been used as the mother molecule due to its widely accepted superiority40,41 (e.g., high fluorescence quantum yield, good water-solubility, good absorption cross-section, high photostability, visible light absorption and a modular nature for facile functionalization). Focusing on advancing new strategies in sensing and signaling, we now construct a nitroethenyl-carbazole conjugate for both colorimetric and fluorescence “turn-on” response to GSH. In the absence of GSH, the nitroolefin moiety serves as an electron acceptor for the photoinduced electron transfer (PET) process, quenching the fluorescence of the carbazole fluorophore (ΦF = 0.006). Upon treatment with GSH, owing to the Michael addition reaction, the double bond between the fluorophore and the quencher is broken, blocking the PET process and leading to the fluorescence recovery (ΦF = 0.30).42,43 Moreover, we conclude that the reaction of GSH with the nitroolefin breaks the conjugated structure of the CZ-Nm probe and inhibits the intramolecular charge transfer (ICT) between the N-ethyl moiety and the nitroolefin moiety, producing a blue shift in the fluorescence spectra, which is accompanied with an obvious colour change in the solution44,45 (Scheme 1).
Experimental
Apparatus
1H and 13C NMR spectra were obtained on a Bruker DMX-300 spectrometer operating at 400 MHz in chloroform-d. MS spectra were obtained on an Agilent 1100. UV-vis absorption spectra were recorded on a U-4100 spectrophotometer. An Edinburgh FS5 spectrofluorometer was used for fluorescence measurements. An Olympus Zeiss 710 laser scanning confocal microscope was used for obtaining fluorescence image of the cells. pH measurements were conducted using a Jingke PHS-3D digital pH-meter.Materials
Carbazole, sodium hydride, nitromethane and amino acids were purchased from Aladdin. Iodoethane was purchased from Energy Chemical. Piperidine was purchased from Sinopharm Chemical Reagent Plant. The solvents were used as received without further purification. Distilled water was used throughout.Synthesis
As shown in Scheme S1,† the key intermediate N-ethylcarbazole-3-carboxaldehyde was synthesized according to a literature procedure. The CZ-Nm probe was further prepared according to the well-known Knoevenagel condensation.46 A solution of N-ethylcarbazole-3-carboxaldehyde (0.50 g, 2.24 mmol), MeNO2 (196 μL, 6.72 mmol), and NH4OAc (0.52 g, 6.72 mmol) in 20 mL of acetic acid was heated at reflux with magnetic stirring for 3 h. After cooling, the resulting solution was concentrated by distillation under reduced pressure, and purified by chromatography on silica gel using DCM/PE (v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as the eluent to obtain an orange–red solid (0.53 g, 90% yield). Mass spectrometry: m/z, calcd: 266.11, found: 267.03 ([M + H]+). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.27 (s, 1H), 8.23 (d, J = 13.5 Hz, 1H), 8.12 (d, J = 7.8, 0.9 Hz, 1H), 7.71 (d, J = 13.5 Hz, 1H), 7.65 (d, J = 8.6 Hz, 1H), 7.54 (t, J = 8.3 Hz, 1H), 7.48–7.42 (m, 2H), 7.32 (t, J = 8.0 Hz, 1H), 4.39 (q, J = 7.2 Hz, 2H), 1.47 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 142.21, 140.93, 140.52, 134.19, 126.87, 126.59, 123.73, 122.96, 122.55, 120.73, 120.67, 120.27, 109.19, 109.37, 37.92, 13.89.
2) as the eluent to obtain an orange–red solid (0.53 g, 90% yield). Mass spectrometry: m/z, calcd: 266.11, found: 267.03 ([M + H]+). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.27 (s, 1H), 8.23 (d, J = 13.5 Hz, 1H), 8.12 (d, J = 7.8, 0.9 Hz, 1H), 7.71 (d, J = 13.5 Hz, 1H), 7.65 (d, J = 8.6 Hz, 1H), 7.54 (t, J = 8.3 Hz, 1H), 7.48–7.42 (m, 2H), 7.32 (t, J = 8.0 Hz, 1H), 4.39 (q, J = 7.2 Hz, 2H), 1.47 (t, J = 7.3 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 142.21, 140.93, 140.52, 134.19, 126.87, 126.59, 123.73, 122.96, 122.55, 120.73, 120.67, 120.27, 109.19, 109.37, 37.92, 13.89.
General spectral measurements
A 1.0 × 10−3 M stock solution of the CZ-Nm probe was prepared in DMF. Stock solutions of the biologically relevant analytes (i.e. GSH, Cys, Hcy, Thr, Ser, Glu, Lys, Phe, His, Gly, Ala, Val, and Tyr) were prepared in distilled water with a concentration of 4.0 × 10−2 M for the UV/vis absorption and fluorescence spectroscopy. In a typical experiment, test solutions were prepared by placing 40 μL of the probe stock solution into a solution of 4 mL buffered (0.1 M PBS, pH 7.4) aqueous DMF solution (H2O/DMF = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The fluorescence spectra were obtained after the addition of the analytes for 30 min at room temperature (λex = 360 nm).
1, v/v). The fluorescence spectra were obtained after the addition of the analytes for 30 min at room temperature (λex = 360 nm).
Cell cultures
PC12 cells were seeded in a glass bottom culture dishes and grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2.5% fetal bovine serum (FBS) and 15% horse serum at 37 °C under a 5% CO2 atmosphere until harvesting for the future experiments. When harvesting, the DMEM was drawn out from the culture dishes, the dishes were rinsed three times with 10 mM phosphate buffered saline (PBS) and then treated with 4 mL trypsinase solution containing 0.25% EDTA for 3 min in the incubator.Results and discussion
Conditional experiments
During the initial attempts, the CZ-Nm probe exhibited a response to GSH. Then, we investigated the influence of the fraction of water on the interaction of the CZ-Nm probe with GSH. Among various fractions of water, a combination of 0.1 M PBS buffer–DMF (pH 7.4, v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) proved to be highly efficient in the sensing process (Fig. S1†). Therefore, we chose 0.1 M PBS buffer–DMF (pH 7.4, v/v, 4
1) proved to be highly efficient in the sensing process (Fig. S1†). Therefore, we chose 0.1 M PBS buffer–DMF (pH 7.4, v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as our test system.
1) as our test system.
To test the effect of pH on the fluorescence intensity of the CZ-Nm probe in the absence and presence of GSH, GSH was prepared at different pH values (from 3.0 to 11.0 using NaOH and HCl). Fig. S2† shows the relationship between the pH and the fluorescence intensity at 420 nm. As can be seen, the CZ-Nm probe exhibited an obvious response in the range of 7.0–11.0. For the detection of the GSH in vivo, PBS buffer at pH 7.4 was selected as the general measurement condition owing to the fact that the biologically relevant pH range is 5.8–8.0, and the CZ-Nm probe could be used to detect intracellular GSH without interference.
The time dependent fluorescent responses of the CZ-Nm probe to thiols were monitored at 420 nm under optimal conditions (0.1 M PBS buffer–DMF, pH 7.4, v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Fig. S3† shows the fluorescence intensity reached its maximum value at about 25 min. Therefore, a 25 min reaction time and a medium of 0.1 M PBS buffer–DMF (pH 7.4, v/v, 4
1). Fig. S3† shows the fluorescence intensity reached its maximum value at about 25 min. Therefore, a 25 min reaction time and a medium of 0.1 M PBS buffer–DMF (pH 7.4, v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution were selected in subsequent experiments to facilitate the reaction of the CZ-Nm probe with GSH.
1) solution were selected in subsequent experiments to facilitate the reaction of the CZ-Nm probe with GSH.
UV-vis absorption and fluorescence spectra of CZ-Nm in response to GSH
The absorption spectra and fluorescence spectra of the CZ-Nm probe (10 μM) were explored in PBS buffer–DMF (0.1 M, pH 7.4, v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution in the presence of different amino acids and thiol compounds. As shown in Fig. 1, owing to the influence of the PET and ICT effect between the fluorophore and nitroolefin moiety, the CZ-Nm probe has a very weak fluorescence (ΦF = 0.006) under a 365 nm UV lamp, which can be barely observed with the naked eye. The addition of GSH induced a variation in the solution from no fluorescence to a strong blue fluorescence (ΦF = 0.30), and the maximum fluorescence emission peak exhibited a blue shift from 480 nm to 420 nm. However, biologically relevant analytes, such as Cys, Hcy, Thr, Ser, Glu, Lys, Phe, His, Gly, Ala, Val and Tyr, showed almost no changes in the fluorescence spectra, which indicated that the CZ-Nm probe selectively responded to GSH.
1) solution in the presence of different amino acids and thiol compounds. As shown in Fig. 1, owing to the influence of the PET and ICT effect between the fluorophore and nitroolefin moiety, the CZ-Nm probe has a very weak fluorescence (ΦF = 0.006) under a 365 nm UV lamp, which can be barely observed with the naked eye. The addition of GSH induced a variation in the solution from no fluorescence to a strong blue fluorescence (ΦF = 0.30), and the maximum fluorescence emission peak exhibited a blue shift from 480 nm to 420 nm. However, biologically relevant analytes, such as Cys, Hcy, Thr, Ser, Glu, Lys, Phe, His, Gly, Ala, Val and Tyr, showed almost no changes in the fluorescence spectra, which indicated that the CZ-Nm probe selectively responded to GSH.
Similarly, the UV-vis spectra also exhibited the response of the CZ-Nm probe to GSH. Upon the addition of GSH, the absorption peak at 420 nm decreased and a new peak appeared at 267 nm, accompanied with a colour change from yellow to colourless (Fig. 2), implying the break in the intramolecular conjugated structure of CZ-Nm. However, the absorption spectra did not change significantly in the presence of different amino acids. As shown in Fig. 3, when the concentration of GSH was increased, the absorption intensity at 420 nm was gradually decreased, and the new absorption peak at 267 nm was gradually increased. Under the same conditions, the CZ-Nm probe also showed similar responses to other thiol-containing compounds, namely, Cys and Hcy. However, owing to the response of the CZ-Nm probe to GSH (3 seconds) being much faster than that found for Cys and Hcy (5 min), exquisite selectivity for GSH can be obtained by controlling the reaction time.
 | ||
Fig. 3 Absorbance spectra of the reaction solution of CZ-Nm (10 μM) in 0.1 M PBS buffer–DMF (pH 7.20; v/v, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution with different concentrations of GSH. 1) solution with different concentrations of GSH. | ||
Linearity
Under the optimized conditions, the responsive properties of the CZ-Nm probe to GSH were further investigated using fluorescence titration studies. As shown in Fig. 4, the fluorescence intensity increased up to 7-fold upon increasing the GSH concentration (0–500 equivalents). As envisioned, a good linear increase in fluorescence intensity could be observed upon increasing the concentration of GSH over a wide linear range (0–0.01 M) (Fig. 5). The regression equation is Y = 123116 + 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972.9X (R = 0.9987). Then, we obtained a low detection limit of 6.4 μM based on 3 × δblank/k (where δblank is the standard deviation of the blank solution and k is the slope of the calibration plot). The relative fluorescence quantum yields were determined to be 0.30 using quinine sulfate dehydrate in 0.1 M H2SO4 as a standard. The corrected emission spectra were obtained for the quinine sulfate dehydrate standard (λex = 360 nm; A (absorbance) < 0.01; ΦF = 0.56) and calculated using the following equation.47
972.9X (R = 0.9987). Then, we obtained a low detection limit of 6.4 μM based on 3 × δblank/k (where δblank is the standard deviation of the blank solution and k is the slope of the calibration plot). The relative fluorescence quantum yields were determined to be 0.30 using quinine sulfate dehydrate in 0.1 M H2SO4 as a standard. The corrected emission spectra were obtained for the quinine sulfate dehydrate standard (λex = 360 nm; A (absorbance) < 0.01; ΦF = 0.56) and calculated using the following equation.47where the subscripts x and s refer to the unknown and the standard, Φ stands for quantum yield, F represents the integrated area under the emission curve, A is the absorbance intensity at the excitation wavelength, λex denotes the excitation wavelength, and n is index of refraction of the solution.
 | ||
Fig. 4 Fluorescence emission spectra of the CZ-Nm probe (10 μM and λex = 360 nm) upon increased concentrations of GSH (0–1000 equivalents) in PBS buffer–DMF (0.1 M, pH 7.4, and v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
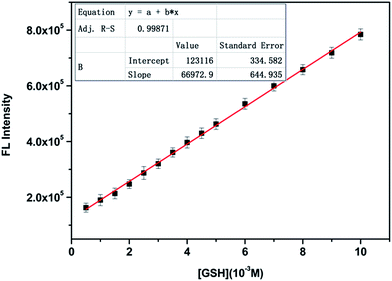 | ||
Fig. 5 Changes in the fluorescence intensity of the CZ-Nm probe (10 μM and λex = 360 nm) against various concentrations of GSH (0–0.01 M) in PBS buffer–DMF (0.1 M, pH 7.4, and v/v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
Tolerance of CZ-Nm to GSH over other interferents
A competitive experiment was implemented to analyze the influence of other amino acids and thiol compounds on the reaction of the CZ-Nm probe with GSH. As shown in Fig. 6, the change in the fluorescence emission intensity caused by GSH in the presence of background species, such as Cys, Hcy, Thr, Ser, Glu, Lys, Phe, His, Gly, Ala, Val and Tyr, was similar to that caused by GSH alone. The results indicated that the recognition of GSH using the CZ-Nm probe was barely affected by other amino acids and thiol compounds.Proposed mechanism
According to the literature,48 we speculate the Michael addition reaction between the CZ-Nm probe and GSH is likely to be responsible for the fluorescence enhancement and changes in the UV-vis spectra. To investigate this mechanism, the stoichiometry of a binding event between GSH and the CZ-Nm probe was first determined. The results obtained from the Job's plot showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between GSH and CZ-Nm probe (Fig. S4†). Mass spectrometry also provided evidence for the reaction of GSH with nitroolefin. As shown in Fig. 7, the peak at m/z = 267.03 corresponded to CZ-Nm. And the peak at m/z = 572.16 corresponded to the reaction product between GSH and the CZ-Nm probe. Overall, all the measurements proved the occurrence of Michael addition between the CZ-Nm probe and GSH.
1 stoichiometry between GSH and CZ-Nm probe (Fig. S4†). Mass spectrometry also provided evidence for the reaction of GSH with nitroolefin. As shown in Fig. 7, the peak at m/z = 267.03 corresponded to CZ-Nm. And the peak at m/z = 572.16 corresponded to the reaction product between GSH and the CZ-Nm probe. Overall, all the measurements proved the occurrence of Michael addition between the CZ-Nm probe and GSH.
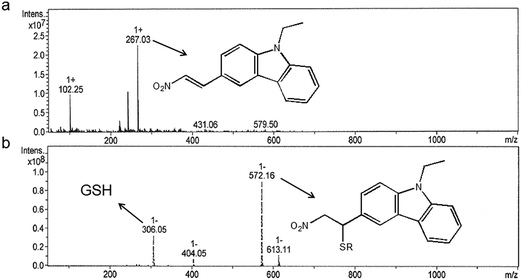 | ||
| Fig. 7 Mass spectrometry monitoring the Michael addition between the CZ-Nm probe and GSH: (a) the mass spectrum of the CZ-Nm probe and (b) the mass spectrum of CZ-Nm + GSH. | ||
Laser scanning confocal microscopy experiments with PC12
To further demonstrate that the permeability and the monitoring of intracellular GSH, fluorescence microscopy experiments were carried out using PC12 cells. As shown in Fig. 8b, the living PC12 cells exhibited almost no fluorescence signal without the incubation of the CZ-Nm probe in the control group with excitation at 405 nm. However, when the PC-12 cells were incubated with CZ-Nm (10 μM) for 30 min at 37 °C, a strong blue fluorescence was exhibited inside the cells (Fig. 8e). This indicated the cell permeability of the probe (transduction the cellular membrane). In another control group, the PC12 cells were pretreated with 0.5 mM thiol reactive N-ethylmaleimide (NEM, a trapping reagent of thiol species) for 30 min to consume all the free thiols in the PC12 cells, followed by treatment with the CZ-Nm probe for 30 min. The confocal microscopic images did not show a remarkable fluorescence signal (Fig. 8h). In addition, if the PC12 cells were pretreated with 2 mM GSH for 30 min followed by incubation with the CZ-Nm probe for another 30 min, a brighter fluorescence signal (Fig. 8k) can be observed than that shown in Fig. 8e. This demonstrated that the fluorescence change was dependent on the concentration of the intracellular GSH and not on that of the other related species. Thus, the CZ-Nm probe might provide a simple way to detect the intracellular GSH from Cys and Hcy, or other common amino acids.Conclusions
In summary, we have developed a novel “turn-on” carbazole-based fluorescent probe (CZ-Nm) for the specific detection of GSH from Cys and Hcy, or other natural amino acids in a PBS buffer–DMF (pH 7.4, v/v, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. The abovementioned results show that the CZ-Nm probe with GSH has a wide linear range and low detection limit under physiological conditions. Furthermore, the confocal fluorescence microscopy imaging of PC12 cells was carried out successfully, which not only indicated the cell permeability and bio-compatibility of the CZ-Nm probe, but also demonstrated that this probe can be applied to monitor GSH in living cells.
1) solution. The abovementioned results show that the CZ-Nm probe with GSH has a wide linear range and low detection limit under physiological conditions. Furthermore, the confocal fluorescence microscopy imaging of PC12 cells was carried out successfully, which not only indicated the cell permeability and bio-compatibility of the CZ-Nm probe, but also demonstrated that this probe can be applied to monitor GSH in living cells.
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (21576071) and the International Science and Technology Cooperation Project of Henan Province (152102410023).Notes and references
- F. Wang, L. Zhou, C. Zhao, R. Wang, Q. Fei, S. Luo, Z. Guo, H. Tian and W. H. Zhu, Chem. Sci., 2015, 6, 2584–2589 RSC.
- H. M. Meng, Z. Jin, Y. Lv, C. Yang, X. B. Zhang, W. Tan and R. Q. Yu, Anal. Chem., 2014, 86, 12321–12326 CrossRef CAS PubMed.
- C. Yin, F. Huo, J. Zhang, R. Martinez-Manez, Y. Yang, H. Lv and S. Li, Chem. Soc. Rev., 2013, 42, 6032–6059 RSC.
- Y. H. Lee, W. X. Ren, J. Han, K. Sunwoo, J.-Y. Lim, J.-H. Kim and J. S. Kim, Chem. Commun., 2015, 51, 14401–14404 RSC.
- X. Xiong, L. Zheng, J. Yan, F. Ye, Y. Qian and F. Song, RSC Adv., 2015, 5, 53660–53664 RSC.
- N. Fahimi-Kashani, P. Shadabipour and M. R. Hormozi-Nezhad, RSC Adv., 2015, 5, 82906–82915 RSC.
- Q. Miao, Q. Li, Q. Yuan, L. Li, Z. Hai, S. Liu and G. Liang, Anal. Chem., 2015, 87, 3460–3466 CrossRef CAS PubMed.
- Y. Zhang, Y. Tang, X. Liu, L. Zhang and Y. Lv, Sens. Actuators, B, 2013, 185, 363–369 CrossRef CAS.
- Q. Y. Cai, J. Li, J. Ge, L. Zhang, Y. L. Hu, Z. H. Li and L. B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef CAS PubMed.
- L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed.
- D. Su, C. L. Teoh, S. Sahu, R. K. Das and Y.-T. Chang, Biomaterials, 2014, 35, 6078–6085 CrossRef CAS PubMed.
- R. Peng, L. Lin, X. Wu, X. Liu and X. Feng, J. Org. Chem., 2013, 78, 11602–11605 CrossRef CAS PubMed.
- L. Song, H. Tian, X. Pei, Z. Zhang, W. Zhang and J. Qian, RSC Adv., 2015, 5, 59056–59061 RSC.
- D. Zhang, Z. Yang, H. Li, Z. Pei, S. Sun and Y. Xu, Chem. Commun., 2016, 52, 749–752 RSC.
- X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120–2135 RSC.
- C. Han, H. Yang, M. Chen, Q. Su, W. Feng and F. Li, ACS Appl. Mater. Interfaces, 2015, 7, 27968–27975 CAS.
- Y. Yang, F. Huo, C. Yin, A. Zheng, J. Chao, Y. Li, Z. Nie, R. Martinez-Manez and D. Liu, Biosens. Bioelectron., 2013, 47, 300–306 CrossRef CAS PubMed.
- J. Liu, Y. Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574–577 CrossRef CAS PubMed.
- O. Rusin, N. N. S. Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438–439 CrossRef CAS PubMed.
- M. Zhang, M. X. Yu, F. Y. Li, M. W. Zhu, M. Y. Li, Y. H. Gao, L. Li, Z. Q. Liu, J. P. Zhang, D. Q. Zhang, T. Yi and C. H. Huang, J. Am. Chem. Soc., 2007, 129, 10322–10323 CrossRef CAS PubMed.
- W. Lin, L. Long, L. Yuan, Z. Cao, B. Chen and W. Tan, Org. Lett., 2008, 10, 5577–5580 CrossRef CAS PubMed.
- S. Madhu, R. Gonnade and M. Ravikanth, J. Org. Chem., 2013, 78, 5056–5060 CrossRef CAS PubMed.
- S. Lim, J. O. Escobedo, M. Lowry, X. Xu and R. Strongin, Chem. Commun., 2010, 46, 5707–5709 RSC.
- H. S. Jung, T. Pradhan, J. H. Han, K. J. Heo, J. H. Lee, C. Kang and J. S. Kim, Biomaterials, 2012, 33, 8495–8502 CrossRef CAS PubMed.
- L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Commun., 2013, 49, 1294–1296 RSC.
- X. Gao, X. Li, L. Li, J. Zhou and H. Ma, Chem. Commun., 2015, 51, 9388–9390 RSC.
- J. Zhang, J. Wang, J. Liu, L. Ning, X. Zhu, B. Yu, X. Liu, X. Yao and H. Zhang, Anal. Chem., 2015, 87, 4856–4863 CrossRef CAS PubMed.
- B. Liu, J. Wang, G. Zhang, R. Bai and Y. Pang, ACS Appl. Mater. Interfaces, 2014, 6, 4402–4407 CAS.
- X. Hou, X. Guo, B. Chen, C. Liu, F. Gao, J. Zhao and J. Wang, Sens. Actuators, B, 2015, 209, 838–845 CrossRef CAS.
- C. Xu, H. Li and B. Yin, Biosens. Bioelectron., 2015, 72, 275–281 CrossRef CAS PubMed.
- J. Yin, Y. Kwon, D. Kim, D. Lee, G. Kim, Y. Hu, J. H. Ryu and J. Yoon, J. Am. Chem. Soc., 2014, 136, 5351–5358 CrossRef CAS PubMed.
- M. M. Pires and J. Chmielewski, Org. Lett., 2008, 10, 837–840 CrossRef CAS PubMed.
- N. Shao, J. Y. Jin, H. Wang, J. Zheng, R. H. Yang, W. H. Chan and Z. Abliz, J. Am. Chem. Soc., 2010, 132, 725–736 CrossRef CAS PubMed.
- J. Chen, X. Jiang, S. L. Carroll, J. Huang and J. Wang, Org. Lett., 2015, 17, 5978–5981 CrossRef CAS PubMed.
- F. Huo, J. Kang, C. Yin, Y. Zhang and J. Chao, Sens. Actuators, B, 2015, 207, 139–143 CrossRef CAS.
- Y. Chen, K. Tsao, É. D. Francesco and J. W. Keillor, J. Org. Chem., 2015, 80, 12182–12192 CrossRef CAS PubMed.
- L. Y. Niu, Y. S. Guan, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed.
- J. Liu, Y. Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574–577 CrossRef CAS PubMed.
- L. He, Q. Xu, Y. Liu, H. Wei, Y. Tang and W. Lin, ACS Appl. Mater. Interfaces, 2015, 7, 12809–12813 CAS.
- L. Li, Y. Q. Wu, Q. L. Zhou and C. Y. He, J. Phys. Org. Chem., 2012, 25, 362–372 CrossRef CAS.
- K. Wang, T. Zhang, Y. H. Hu, W. G. Yang and Y. Shi, Electrochim. Acta, 2014, 130, 46–51 CrossRef CAS.
- X. Guo, X. Zhang, S. Wang, S. Li, R. Hu, Y. Li and G. Yang, Anal. Chim. Acta, 2015, 869, 81–88 CrossRef CAS PubMed.
- M. Lan, J. Wu, W. Liu, H. Zhang, W. Zhang, X. Zhuang and P. Wang, Sens. Actuators, B, 2011, 156, 332–337 CrossRef CAS.
- M. Isik, R. Guliyev, S. Kolemen, Y. Altay, B. Senturk, T. Tekinay and E. U. Akkaya, Org. Lett., 2014, 16, 3260–3263 CrossRef CAS PubMed.
- Y. Liu, K. Li, M. Y. Wu, Y. H. Liu, Y. M. Xie and X. Q. Yu, Chem. Commun., 2015, 51, 10236–10239 RSC.
- L. G. Voskressensky, A. A. Festa and A. V. Varlamov, Tetrahedron, 2014, 70, 551–572 CrossRef CAS.
- R. R. Zhang, J. F. Zhang, S. Q. Wang, Y. L. Cheng, J. Y. Miao and B. X. Zhao, Spectrochim. Acta, Part A, 2015, 137, 450–455 CrossRef CAS PubMed.
- C. Chen, W. Liu, C. Xu and W. Liu, Biosens. Bioelectron., 2015, 71, 68–74 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra25204k |
| This journal is © The Royal Society of Chemistry 2016 |






