Three-component reaction between substituted 2-(2-nitrovinyl)-phenols, acetylenedicarboxylate and amines: diversity-oriented synthesis of novel pyrrolo[3,4-c]coumarins†
Shuwen Xuea,
Juan Yaoa,
Jiaming Liua,
Lizhong Wangb,
Xinyu Liua and
Cunde Wang*a
aSchool of Chemistry and Chemical Engineering, Yangzhou University, 180 Siwangting Street, Yangzhou 225002, P. R. China. E-mail: wangcd@yzu.edu.cn; Fax: +86-514-8797-5244; Tel: +86-514-8797-5568
bDepartment of Environmental and Chemical Engineering, Taizhou Polytechnic College, Taizhou 225300, P. R. China
First published on 21st December 2015
Abstract
An efficient and straightforward synthetic protocol has been developed for the preparation of pyrrolo[3,4-c]coumarins via FeCl3-promoted three component reaction between substituted 2-(2-nitrovinyl)phenols, acetylenedicarboxylate and amines for the generation of a wide range of structurally interesting and pharmacologically significant compounds.
The coumarin ring is an important structure in a number of bioactive natural products.1 Coumarins and their annulated derivatives display a wide spectrum of pharmacological profiles,2 including anticancer activity,3 steroid 5α-reductase,4 inhibition of platelet aggregation,5 and HIV-1 protease.6 Coumarins have also been used as insecticides.7 Additionally, many synthetic coumarin compounds with the π-conjugated lactone-(hetero)aryl motifs as the fluorescent core objects have been frequently used in molecular materials such as optical brightening agents,8 dispersed fluorescent and laser dyes.9 Recently, it is noteworthy that when coumarins are fused with other heterocycles possesses interesting properties. Studies have revealed that a number of biologically important properties of the coumarins fused with other heterocycles are dependent upon structural features of other heterocycles. Chemical modification of other heterocycles provides a way to alter the functional groups, sizes, and stereochemistry of the coumarin derivatives, and numerous structure–activity relationships have been established by such synthetic alterations. For example, modified coumarin derivatives have exhibited a broad range of biological activities as potent anti-HIV agents and anticancer agents.10,11 Specifically, those bearing a coumarin–pyrrole skeleton possess interesting properties like the family of lamellarins as potent cytotoxic agent against various tumor cells.12 Similarly, pyrroles and their derivatives represent an important class of nitrogen-containing heterocycles.13 They are core structural skeletons in a variety of natural and synthetic products exhibiting many ubiquitous and varied biological properties.14–18 Additional, some investigations also reported that substituted pyrroles find application as dyes, pigments, and other functional materials.19
A combination of chromene with a pyrrole moiety in a single molecule has also been explored for the identification of promising bioactive molecules.20 Among them, pyrrolo[c]coumarins, polycyclic systems in which a pyrrole ring is fused to the chromene unit, are of particular interest since they exhibit potent biological and pharmacological activity,20e–f as well as their potential applications as blue and green light-emitting fluorophores and electroluminescent materials for OLED devices.21 In the light of the significance of pyrrolo-annulated coumarin systems and their diverse pharmacological properties, there has been a continuous effort to develop new, convenient, and versatile methods for synthesis of this class of compounds. A range of methods has been reported for their synthesis including Cu-mediated/MW-assisted C–O carboxylic lactonization of 2-halophenylindole carboxylic acids.22 In the same way, using 4-(2-chlorophenyl)-1H-pyrrole-3-carboxylate as a starting material, heating a mixture of 4-(2-chlorophenyl)-1H-pyrrole-3-carboxylate and NaOH under refluxing, the following copper(I)-thiophene-2-carboxylate-mediated lactonization at high temperature (160 °C) in DMF also afforded pyrrolo[3,4-c]coumarins.23 Zhou and co-workers described an appealing strategy to directly construct pyrrolo[3,4-c]coumarins by one-pot domino reactions from benzyl halides with pyridine and 3,4-dichlorocoumarin in the presence of potassium carbonate via in situ generated N-ylide intermediate.24 Additionally, the pyrrolo[3,4-c]coumarins also were formed by the Fischer–Fink reaction starting from 4-chloro-3-formylcoumarin and α-aminoester derivatives.25 Using isocyanides as starting materials, Ramazani and co-workers reported a novel procedure for the synthesis of pyrrolo[3,4-c]coumarins via the ring opening of coumarins and subsequent [4 + 1] cycloaddition reaction.26 More recently, Alizadeh and co-workers reported that pyrrolo[3,4-c]chromene derivatives were synthesized starting from p-toluenesulfonylmethyl isocyanide as a synthetic reagent with commercially available salicylaldehydes and β-keto esters via a sequential three-component reaction in a one-step procedure.27
Multicomponent reactions as an efficient synthetic strategy have drawn considerable attention over the past decades, because complex products are formed in a one-pot reaction and diversity can be simply attained by relatively simple starting materials. However there are only a few one-pot multicomponent reactions for the construction of structurally and stereochemically diverse pyrrolo[3,4-c]chromenes. The varied biological activity of the coumarins fused with other heterocycles, has encouraged research with regard to the procedures and substrates, which due to their versatility, allow the easy preparation of broad families of these compounds. Our continued interest in the synthesis of the coumarins fused with other heterocycles28 invokes us to search a novel route for fused pyrrolochromenes. Accordingly, we decided to investigate the three-component reaction of substituted 2-(2-nitrovinyl)phenols, acetylenedicarboxylate and amines in the synthesis of new coumarin derivatives.
Based on our previous results,29 a basic reagent was usually used as a promoter for the addition reaction of electron-deficient β-nitrostyrenes and acetylenedicarboxylates, thus, our initial experiments focused on the identification of an appropriate basic agent. A generally organic base Et3N was chosen as a promoter, using 2-(2-nitrovinyl)phenol (1a), dimethyl acetylenedicarboxylate (2) and benzylamine (3a) as the model substrate and methanol as the solvent, the reaction was carried out at rt for 10 h and under refluxing for 10 h, but the desired product was not obtained (Table 1). Next the replacement of MeOH by EtOH, CH3CN or PhCH3 respectively also did not give the desired product (Table 1, entries 2–4). Then the Et3N was replaced with a strong base NaOH for the reaction under the same condition, no reaction still occurred (Table 1, entry 5). The above results indicted a large effect on the nature of the catalyst or promoter on this process. Further, we used p-methylbenzenesulfonic acid (PTSA) for this reaction at room temperature, no desired product was formed (Table 1, entry 6). However, under the same reaction conditions, by replacing PTSA with Lewis acid FeCl3 (0.05 equiv.), the reaction afforded expected product in ca. 35% yield within 20 h of reaction time (Table 1, entry 7).
| Entry | Additive (equiv.) | Solvent | T (°C) | t (h) | Yield (%) (4a)a |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | Et3N(3.0) | MeOH | rt-ref | 20 | 0 |
| 2 | Et3N(3.0) | EtOH | rt-ref | 20 | 0 |
| 3 | Et3N(3.0) | CH3CN | rt-ref | 20 | 0 |
| 4 | Et3N(3.0) | PhMe | rt-ref | 20 | 0 |
| 5 | NaOH(1.0) | PhMe | rt-ref | 20 | 0 |
| 6 | PTSA(0.3) | PhMe | rt | 20 | 0 |
| 7 | FeCl3(0.05) | PhMe | rt | 20 | 35 |
| 8 | FeCl3(0.1) | PhMe | rt | 20 | 38 |
| 9 | FeCl3(0.2) | PhMe | rt | 20 | 50 |
| 10 | FeCl3(0.3) | PhMe | rt | 16 | 78 |
| 11 | FeCl3(0.4) | PhMe | rt | 10 | 90 |
| 12 | FeCl3(0.5) | PhMe | rt | 10 | 90 |
| 13 | FeCl3(0.4) | PhMe | 40 | 10 | 83 |
| 14 | FeCl3(0.4) | DMF | rt | 10 | 75 |
| 15 | FeCl3(0.4) | Dioxane | rt | 10 | 71 |
Then our efforts further focused on the amount of Lewis acid FeCl3, the yield was increased slightly when the amount of FeCl3 was changed from 0.05 equiv. to 0.1 equiv. (Table 1, entries 7–8). When the amount of Lewis acid FeCl3 was increased further to between 0.2 and 0.5 equiv., the yield of the desired product 4a was obviously increased (entries 9–12). In the presence of 0.2, 0.3, 0.4, and 0.5 equiv. of FeCl3, the yield of product 4a obtained was 50, 78, 90, and 90%, respectively. Using 0.4 equiv. FeCl3, the reaction was complete after 10 h and the isolated yield was the best (entry 11). Moreover, raising reaction temperature to 40 °C can not improve the reaction (entry 13). Pyrrolo[3,4-c]coumarin 4a was produced in slightly lower yield when the reaction was performed in DMF, or dioxane (entries 14–15).
A series of experiments revealed that the optimal results were obtained when the reaction of 2-(2-nitrovinyl)phenol (1a), dimethyl acetylenedicarboxylate (2) and benzylamine (3a) together with 0.4 equiv. FeCl3 was carried out in toluene, the resultant mixture was stirred for 10 h at room temperature, whereby the yields of 4a reached 90% (Table 1, entry 11).
Under the optimized conditions as described in entry 11, Table 1, the generality of the reaction was examined. The reaction tolerates different substituents on the amines and substituted 2-(2-nitrovinyl)phenols, generally, amines with a range of substitutents such as benzyl, chlorobenzyl, fluorobenzyl, aryl, and aliphatic groups all worked well to give pyrrolo[3,4-c]coumarin derivatives. Substrate benzyl (chlorobenzyl, fluorobenzyl) amines gave the products in higher yields than aryl amines and general aliphatic amines. The electronic properties of the substituents on the benzene ring of benzylamines had a slight effect on the reaction. The introduction of an electron-withdrawing group such as chloro or fluoro speeded down the reaction and slightly decreased the yield of product. Additionally, 2-(2-nitrovinyl) phenol with substitutents such as fluoro, chloro, bromo, and methyl also worked well to give the corresponding products. However, we found substituted positions of phenyl groups of 2-(2-nitrovinyl)phenol mainly affected the reaction, the substitutents at C4 of 2-(2-nitrovinyl)phenol were propitious to the reaction compared with the substitutent at C6 (Table 2, entries 3 and 13). The molecular structures of all pyrrolo[3,4-c]coumarins 4a–q were elucidated from their spectroscopic analyses as described herein for 4a. In the IR spectrum of 4a, two sharp absorption bands at 1745 and 1689 cm−1, three bands at 1580, 1530, and 1497 cm−1, and two absorption bands at 1289 and 1167 cm−1 could be related to ArOCO and CO2Me, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–O stretching frequencies. The mass spectrum of 4a displayed the molecular ion peak at m/z = 334.4 (M + 1), which is in good agreement with the proposed structure. The 1H NMR spectrum of 4a exhibited three sharp singlet signals at 7.16 ppm (s, 1H), 5.59 ppm (s, 2H), 3.95 ppm (s, 3H) for C1–H, PhCH2 and CO2CH3, respectively. Characteristic 1H chemical shift of C1–H, PhCH2 and CO2CH3 unequivocally indicated the exclusive chemical environment of pyrrolo[3,4-c]coumarins 4a protons. The 1H-decoupled 13C NMR spectrum of 4a showed 18 distinct signals in agreement with the suggested structure. The important peaks were related to the ArO
C, and C–O stretching frequencies. The mass spectrum of 4a displayed the molecular ion peak at m/z = 334.4 (M + 1), which is in good agreement with the proposed structure. The 1H NMR spectrum of 4a exhibited three sharp singlet signals at 7.16 ppm (s, 1H), 5.59 ppm (s, 2H), 3.95 ppm (s, 3H) for C1–H, PhCH2 and CO2CH3, respectively. Characteristic 1H chemical shift of C1–H, PhCH2 and CO2CH3 unequivocally indicated the exclusive chemical environment of pyrrolo[3,4-c]coumarins 4a protons. The 1H-decoupled 13C NMR spectrum of 4a showed 18 distinct signals in agreement with the suggested structure. The important peaks were related to the ArO![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) O,
O, ![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) O2Me, Ph
O2Me, Ph![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2 and CO2
H2 and CO2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3 groups which appeared at δ = 169.1, 161.0, 53.4, and 52.7 ppm (see ESI†). Final confirmation for the formation of the reaction products was obtained by X-ray crystal structure analysis of compounds 4a and 4o. The structures of 4a and 4o was unambiguously solved by X-ray crystallography (Fig. 1).30 X-ray crystallographic analysis determined that products 4a and 4o possess an alkyl and an ester contiguous substituents at N(2) and C(3) of pyrrolo[3,4-c]coumarin core.
H3 groups which appeared at δ = 169.1, 161.0, 53.4, and 52.7 ppm (see ESI†). Final confirmation for the formation of the reaction products was obtained by X-ray crystal structure analysis of compounds 4a and 4o. The structures of 4a and 4o was unambiguously solved by X-ray crystallography (Fig. 1).30 X-ray crystallographic analysis determined that products 4a and 4o possess an alkyl and an ester contiguous substituents at N(2) and C(3) of pyrrolo[3,4-c]coumarin core.
| Entry | R1 | R2 | R3 | Yieldb |
|---|---|---|---|---|
| a Reaction conditions: substituted 2-(2-nitrovinyl)-phenol 1a–e (1 mmol), acetylenedicarboxylate (213 mg, 1.5 mmol), amines 3a–g (1 mmol) and FeCl3 (65 mg, 0.4 mmol), toluene (5 mL), 110 °C, 6 h.b Isolated yield. | ||||
| 1 | H | H | C6H5CH2 | 90 (4a) |
| 2 | Br | H | C6H5CH2 | 88 (4b) |
| 3 | H | CH3 | C6H5CH2 | 62 (4c) |
| 4 | Cl | H | C6H5CH2 | 92 (4d) |
| 5 | F | H | C6H5CH2 | 80 (4e) |
| 6 | Br | H | p-FC6H4CH2 | 78 (4f) |
| 7 | H | H | p-FC6H4CH2 | 86 (4g) |
| 8 | H | H | p-CH3OC6H4 | 75 (4h) |
| 9 | Cl | H | p-CH3OC6H4 | 74 (4i) |
| 10 | Br | H | m-CH3C6H4 | 65 (4j) |
| 11 | Br | H | n-C8H17 | 55 (4k) |
| 12 | H | H | n-C8H17 | 58 (4l) |
| 13 | H | CH3 | p-ClC6H4CH2 | 72 (4m) |
| 14 | H | H | p-O2NC6H4 | 70 (4n) |
| 15 | H | H | p-ClC6H4CH2 | 89 (4o) |
| 16 | Br | H | p-ClC6H4CH2 | 84 (4p) |
| 17 | F | H | p-ClC6H4CH2 | 86 (4q) |
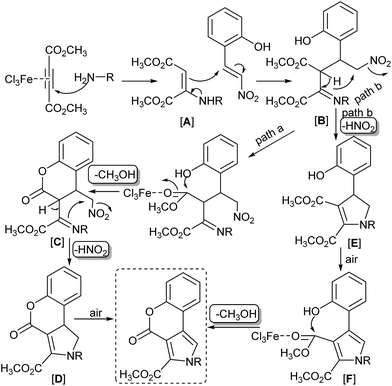
For the readership understanding, we performed some control experiments. Under the optimized conditions, the model reaction of 2-(2-nitrovinyl)phenol (1a), dimethyl acetylenedicarboxylate (2) and benzylamine (3a) was carried out at rt for half of the optimized reaction time (5 h) aimed at yielding some key intermediates and supporting the mechanistic proposal, but the reaction system was very complicated after workup, the desired intermediates were not isolated by flash chromatography. The results indicated there are some paths that lead to the final products. Based upon the above observation, we proposed a plausible reaction mechanism in Scheme 1. The key steps involved the generation of an aminobutene [A] via the nucleophilic addition of acetylenedicarboxylate with an amine in the presence of FeCl3,31 then the nucleophilic addition of [A] to 2-(2-nitrovinyl)phenol to give an intermediate imine [B]. The following FeCl3-mediated intramolecular transesterification formed a coumarin core by path a, the removal of methanol gave a coumarin [C], the subsequent intramolecular nucleophilic substitution formed the dihydropyrrole intermediate [D] by the elimination of the nitro group.32 Then, the air-mediated oxidative dehydrogenation of the dihydropyrrole intermediate [D] resulted in the formation of pyrrolo[3,4-c]coumarins.33 We proposed another plausible reaction mechanism in path b. Initially, an intermediate imine [B] reacted through intramolecular nucleophilic substitution pathway to form the dihydropyrrole intermediate [E] by the elimination of the nitro group. Then, aerobic oxidation of the intermediate [E] yielded the pyrrole [F]. Finally, FeCl3-mediated intramolecular transesterification formed a pyrrolo[3,4-c]coumarin.
Conclusions
In conclusion, we have developed a straightforward and efficient FeCl3-promoted three component reaction between substituted 2-(2-nitrovinyl)-phenols, acetylenedicarboxylate and amines for the synthesis of novel pyrrolo[3,4-c]coumarins. This reaction involved the sequential FeCl3-mediated nucleophilic addition of acetylenedicarboxylates, amines, and 2-(2-nitrovinyl)phenols, the following intramolecular transesterification to form a coumarin core. The subsequent intramolecular nucleophilic addition reaction gave the corresponding α-nitrousdihydropyrrole intermediates for the formation of tricyclic pyrrolo[3,4-c]coumarin core, the final removal of nitrous group promoted by FeCl3 formed the products. The development of this strategy offered a complementary approach to substituted pyrrolo[3,4-c]coumarin compounds with advantages that included a variety of cheap and readily available reactants and a wide range of substrates with dense or flexible substitution patterns.Acknowledgements
Financial support of this research by the National Natural Science Foundation of China (NNSFC 21173181) is gratefully acknowledged by authors. A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- R. D. H. Murray, J. Mendey and S. A. Brown, The Natural Coumarins, Wiley, New York, 1982 Search PubMed.
- R. D. H. Murray, in Progress in the Chemistry of Organic Natural Products, ed. W. Herz, H. Falk, G. W. Kirby and R. E. Moore, Springer, Wien, 2002, vol. 83 Search PubMed.
- (a) H. Fan, J. Peng, M. T. Hamann and J. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed; (b) C. J. Wang, Y. J. Hsieh, C. Y. Chu, Y. L. Lin and T. H. Tseng, Cancer Lett., 2002, 183, 163 CrossRef CAS PubMed; (c) I. Kostova, Curr. Med. Chem.: Anti-Cancer Agents, 2005, 5, 29 CrossRef CAS PubMed; (d) K. C. Fylaktakidou, D. J. Hadjipavlou-Litina, K. E. Litinas and D. N. Nicolaides, Curr. Pharm. Des., 2004, 10, 3813 CrossRef CAS PubMed.
- G. J. Fan, W. Mar, M. K. Park, E. Wook Choi, K. Kim and S. Kim, Bioorg. Med. Chem. Lett., 2001, 11, 2361 CrossRef CAS PubMed.
- G. Cravotto, G. M. Nano, G. Palmisano and S. Tagliapietra, Tetrahedron: Asymmetry, 2001, 12, 707 CrossRef CAS.
- S. Kirkiacharian, D. T. Thuy, S. Sicsic, R. Bakhchinian, R. Kurkjian and T. Tonnaire, Farmaco, 2002, 57, 703 CrossRef CAS PubMed.
- R. O. Kennedy and R. D. Zhorenes, Coumarins: Biology, Applications and Mode of Action, John Wiley and Sons, Chichester, 1997 Search PubMed.
- R. O. Kennedy and R. D. Tharnes, Coumarins: Biology, Application and Mode of Action, Wiley, Chichester, 1997 Search PubMed.
- (a) M. Zahradink, The Production and Application of Fluorescent Brightening Agents, Wiley, New York, 1992 Search PubMed; (b) M. Maeda, Laser Dyes, Academic Press, New York, 1984 Search PubMed.
- (a) S. Thaisrivongs, M. N. Janakiraman, K. T. Chong, P. K. Tomich, L. A. Dolack, S. R. Turner, J. W. Strohbach, J. C. Lynn, M. M. Horng, R. R. Hinshaw and K. D. Watenpugh, J. Med. Chem., 1996, 39, 2400 CrossRef CAS PubMed; (b) G. Rappa, K. Shyam, A. Lorico, O. Fodstad and A. C. Sartorelli, Oncol. Res., 2000, 12, 113 CrossRef CAS PubMed; (c) E. D. Yang, Y. N. Zhao, K. Zhang and P. Mack, Biochem. Biophys. Res. Commun., 1999, 260, 682 CrossRef CAS PubMed.
- R. G. Harvey, C. Cortez, T. P. Ananthanarayan and S. Schmolka, J. Org. Chem., 1988, 53, 3936 CrossRef CAS.
- (a) R. J. Andersen, D. J. Faulkner, G. D. van Duyne and J. Clardy, J. Am. Chem. Soc., 1985, 107, 5492 CrossRef CAS; (b) C. Bailly, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 363 CrossRef CAS PubMed; (c) M. Facompre, C. Tardy, C. Bal-Mahieu, P. Colson, C. Perez, I. Manzanares, C. Cuevas and C. Bailly, Cancer Res., 2003, 63, 7392 CAS; (d) M. Vanhuyse, J. Kluza, C. Tardy, G. Otero, C. Cuevas, C. Bailly and A. Lansiaux, Cancer Lett., 2005, 221, 165 CrossRef CAS PubMed; (e) B. L. Staker, K. Hjerrild, M. D. Feese, C. A. Behnke, A. B. Burgin Jr and L. Stewart, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15387 CrossRef CAS PubMed; (f) F. Ishibashi, S. Tanabe, T. Oda and M. Iwao, J. Nat. Prod., 2002, 65, 500 CrossRef CAS PubMed.
- (a) D. Blank, in Science of Synthesis, ed. M. C. Bagley and D. StC. Black, George-Thieme-Stuttgart, 2006 Search PubMed; (b) E. Ghabraie, S. Balalaie, M. Bararjanian, H. R. Bijanzadeh and F. Rominger, Tetrahedron, 2011, 67, 5415 CrossRef CAS.
- G. Daidone, B. Maggio and D. Schillari, Pharmazie, 1990, 45, 441 CAS.
- D. G. Kaiser and E. M. Glenn, J. Pharm. Sci., 1972, 61, 1908 CrossRef CAS PubMed.
- H. M. Meshram, B. R. V. Prasad and D. A. Kumar, Tetrahedron Lett., 2010, 51, 3477 CrossRef CAS.
- F. A. Davis, K. A. Bowen, H. Xu and V. Velvadapu, Tetrahedron, 2008, 64, 4174 CrossRef CAS PubMed.
- A. S. Demir, I. M. Akhmedov and O. Sesenoglu, Tetrahedron, 2002, 58, 9793 CrossRef CAS.
- (a) P. A. Gale, Acc. Chem. Res., 2006, 39, 465 CrossRef CAS PubMed; (b) M. Takase, N. Yoshida, T. Narita, T. Fujio, T. Nishinaga and M. Iyoda, RSC Adv., 2011, 2, 3221 RSC; (c) V. Blangy, C. Heiss, V. Khlebnikov, C. Letondor, H. S. Evans and R. Neier, Angew. Chem., Int. Ed., 2009, 48, 1688 CrossRef CAS PubMed; (d) M. M. Wienk, M. Turbiez, J. Gilot and R. A. Janssen, J. Adv. Mater., 2008, 20, 2556 CrossRef CAS; (e) D. Wu, A. B. Descalzo, F. Weik, F. Emmerling, Z. Shen, X. Z. You and K. Rurack, Angew. Chem., Int. Ed., 2007, 47, 193 CrossRef PubMed.
- (a) Q. Li, J. Jiang, A. Fan, Y. Cui and Y. Jia, Org. Lett., 2011, 13, 312 CrossRef CAS PubMed; (b) D. Pla, F. Albericio and M. Alvarez, Anti-Cancer Agents Med. Chem., 2008, 8, 746 CrossRef CAS; (c) S. T. Handy and Y. Zhang, Org. Prep. Proced. Int., 2005, 37, 411 CrossRef CAS; (d) C. Bailly, Curr. Med. Chem.: Anti-Cancer Agents, 2004, 4, 363 CrossRef CAS PubMed; (e) D. Pla, A. Marchal, C. A. Olsen, A. Francesh, C. Cuevas, F. Albericio and M. Alvarez, J. Med. Chem., 2006, 49, 3257 CrossRef CAS PubMed; (f) E. Marco, W. Laine, C. Tardy, A. Lansiaux, M. Iwao, F. Ishibashi, C. Bailly and F. Gago, J. Med. Chem., 2005, 48, 3796 CrossRef CAS PubMed.
- Y. M. Shen, G. Grampp, N. Leesakul, H. W. Hu and J. H. Xu, Eur. J. Org. Chem., 2007, 3718 CrossRef CAS.
- N. Thasana, R. Worayuthakarn, P. Kradanrat, E. Hohn, L. Young and S. Ruchirawat, J. Org. Chem., 2007, 72, 9379 CrossRef CAS PubMed.
- J. H. Kim, S. Y. Choi, J. Bouffard and S. Lee, J. Org. Chem., 2014, 79, 9253 CrossRef CAS PubMed.
- H. Hu, K. Shi, R. Hou, Z. Zhang, Y. Zhu and J. Zhou, Synthesis, 2010, 42, 4007 CrossRef.
- A. Alberola, L. Calvo, A. González-Ortega, A. P. Encabo and M. Carmen Sañudo, Synthesis, 2001, 33, 1941 CrossRef.
- M. Khoobi, A. Ramazani, M. Mahdavi, A. Foroumadi, S. Emami, S. W. Joo, K. Slepokura, T. Lis and A. Shafiee, Helv. Chim. Acta, 2014, 97, 847 CrossRef CAS.
- A. Alizadeh, R. Ghanbaripour and L. G. Zhu, Synlett, 2013, 24, 2124 CrossRef CAS.
- Z. Zhou, H. Liu, Y. Li, J. Liu, Y. Li, J. Liu, J. Yao and C. Wang, ACS Comb. Sci., 2013, 15, 363 CrossRef CAS PubMed.
- (a) H. Liu, Z. Zhou, Q. Sun, Y. Li, Y. Li, J. Liu, P. Yan, D. Wang and C. Wang, ACS Comb. Sci., 2012, 14, 366 CrossRef CAS PubMed; (b) Z. Zhou, H. Liu, Q. Sun, Y. Li, J. Yang, J. Liu, P. Yan and C. Wang, Synlett, 2012, 23, 2255 CrossRef CAS; (c) J. Zhang, J. Yao, J. Liu, S. Xue, Y. Li and C. Wang, RSC Adv., 2015, 5, 48580 RSC.
- ESI†.
- (a) N. Kawahara, T. Shimamori, T. Itoh and H. Ogura, Heterocycles, 1986, 24, 2803 CrossRef CAS; (b) I. Yavari, A. Shaabani, H. Soliemani, F. Nourmohammadian and H. Bijanzadeh, Magn. Reson. Chem., 1996, 34, 1003 CrossRef CAS; (c) I. Yavari, A. Mirzaie and L. Moradi, Helv. Chim. Acta, 2006, 89, 2825 CrossRef CAS; (d) A. Alizadeh, F. Movahedi and A. A. Esmaili, Tetrahedron Lett., 2006, 47, 4469 CrossRef CAS.
- (a) E. Ghabraie, S. Balalaie, M. Bararjanian, H. R. Bijanzadeh and F. Rominger, Tetrahedron, 2011, 67, 5415 CrossRef CAS; (b) I. Yavari, M. Ghazvini and A. Aminkhani, J. Chem. Res., 2011, 3, 558 CrossRef; (c) T. Sanaeishoar, S. Adibi-Sedeh and S. Karimian, Comb. Chem. High Throughput Screening, 2014, 17, 157 CrossRef CAS; (d) M. Ghosh, S. Mishra and A. Hajra, J. Org. Chem., 2015, 80, 5364 CrossRef CAS PubMed; (e) J. M. Lopchuk, R. P. Hughes and G. W. Gribble, Org. Lett., 2013, 15, 5218 CrossRef CAS PubMed.
- (a) L. Moradi, M. Piltan and H. Rostami, Chin. Chem. Lett., 2014, 25, 123 CrossRef CAS; (b) L. Moradi, M. Piltan, H. Rostami and G. Abasi, Chin. Chem. Lett., 2013, 24, 740 CrossRef CAS; (c) C. Ravi, D. C. Mohan, N. N. K. Reddy and S. Adimurthy, RSC Adv., 2015, 5, 42961 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Reactions conditions and spectra. CCDC 1406217 and 1431800. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra23392e |
| This journal is © The Royal Society of Chemistry 2016 |