DOI:
10.1039/C5RA23706H
(Paper)
RSC Adv., 2015,
5, 106860-106867
Acid promoted synthesis of cyclic 1,3-dione fused symmetrical 2,8-dioxabicyclo[3.3.1]nonanes†
Received
10th November 2015
, Accepted 4th December 2015
First published on 7th December 2015
Abstract
A one-pot access to cyclic 1,3-dione fused symmetrical 2,8-dioxabicyclo[3.3.1]nonanes (4) via acetic acid-mediated reaction of β-enamino ketones (1) and 1,3-cyclohexanediones (2) by heating under reflux is reported. The reaction proceeds via intramolecular cyclization of intermediate 2,2′-(3-oxo-3-arylpropane-1,1-diyl)bis(3-hydroxy-5,5-dimethylcyclohex-2-en-1-one) (3) and is highly selective towards the formation of product 4. Unlike similar reactions, the presence of trifluoroacetic acid in a toluene medium affords xanthenes (5) exclusively.
1. Introduction
The 2,8-dioxabicyclo[3.3.1]nonane skeleton is a unique bicyclic framework which is frequently found in natural products and biologically active compounds.1 Development of methodical synthetic protocols to this type of molecule is notable and also challenging owing to their rigid geometry.2 A prominent methylene bridged bicyclic compound such as Troger's base3 and its derivatives have been widely employed as high affinity DNA targeting fluorescent supramolecular scaffolds,4 molecular tweezers5 and ion receptors.6 Similarly, some of the 2,8-dioxabicyclo[3.3.1]nonanes e.g. dracoflavan C, dracoflavan D, and procyanidin A1 an important methylene bridged bicyclic compounds have been found in medicinal plants. In accordance, a great deal of effort has been devoted and a number of protocols have thus appeared on the synthesis of 2,8-dioxabicyclo[3.3.1]nonane frame work with varied structural analogues.7 However, entire reports uses expensive metal catalysts and/or organic solvents and follow the traditional approach. In general traditional organic synthesis require purification processes which is often time consuming and expensive. Further, organic solvents contribute the major share to the pollution problem in practical organic syntheses.8
On this account, it is ideal promoting the use of greener solvents, solvent free reactions or alternatives that can be benign to environment and human health. With this background and also the nowadays keen research interest on synthesis and derivatisation of complex structural motifs with attached bicyclo[3.3.1]nonane frame work, we envisaged the construction of symmetrical methylene bridged [3.3.1] cyclic system by reaction of β-enamino ketones and cyclic 1,3-diones. Before embarking to act on our envision, literature survey was conducted and surprisingly no report was found on the synthesis of bicyclo compounds from enamino ketones and 1,3-diones. Based on the literature revelation and also as a part of our continuous endeavour to synthesize new bio-active molecules9–11 employing green solvents herein report for the first time a green, novel and efficient, acetic acid mediated one pot reaction for synthesis of 1,3-dione fused symmetrical 2,8-dioxabicyclo[3.3.1]nonanes (4) starting from β-enamino ketones (1) and 1,3-cyclohexanediones (2) without employing any extrinsic catalyst.
2. Results and discussions
To begin with, the prerequisite β-enamino ketones viz (E)-3-(dimethylamino)-1-aryl/alkyl-prop-2-en-1-ones (1) were readily prepared by condensation of respective alkyl, aryl and heteroaryl methyl ketones with dimethylformamide dimethylacetal (DMF-DMA) by a reported procedure.12 Then a model reaction was conducted by reacting (E)-3-(dimethylamino)-1-phenylprop-2-en-1-one (1a) with 5,5-dimethyl-1,3-cyclohexanedione (2a) in ethanol at reflux temperature without any added catalyst (Scheme 1). Surprisingly, formation of enolic hydroxy compound 3a was obtained in 70% yields via double Michael addition at a variance to the expected bicyclic product. The structure of 3a was characterized by the spectral data and a representative sample was unambiguously conformed by X-ray crystallography (Fig. 1).13
 |
| | Scheme 1 Reaction of β-enaminoketone with dimedone. | |
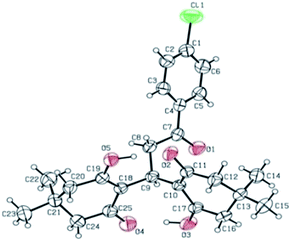 |
| | Fig. 1 ORTEP diagram of compound 3b. | |
Next, the same reaction (Scheme 1) was carried out in water and organic solvents like acetonitrile, toluene, and 1,2-dichloroethane independently at reflux temperature and ended up with 3a. However, in xylene medium, apart from 3a, formation of desired bicyclic product 4a (11% yields) was also observed. Stimulated over the formation of anticipated product 4a, we presumed that an acidic medium may better accelerate the transformation of 3a into a bicyclic product. To imply the validity of this presumption, compound 3a was refluxed in ethanol by employing PTSA (1 eq.) (Scheme 2). Interestingly, this reaction gave 4a in 25% and xanthenes (5a) in 60% yields. Formation of 5a is attributable to the loss of water from 3a. Since the desired product formation is minor under these reaction condition and also the stoichiometry of PTSA requiring one equivalent (otherwise 3a remaining), we have attempted the same reaction in acetic acid under reflux condition.
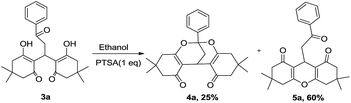 |
| | Scheme 2 Reaction of 3a in ethanol with PTSA. | |
Dramatically, this reaction gave the bicyclic product 4a exclusively in high yields. The structure of 4a was well characterized by the spectral data and also confirmed by X-ray crystallography (Fig. 2).13 Having confirmed the formation of bicyclic product from 4a, the next reaction was carried out by reacting compound 1 with 5,5-dimethyl-1,3-cyclohexanedione (2) in acetic acid at reflux temperature (Scheme 3). To our delight, this reaction resulted in formation of 1,3-dione fused symmetrical 2,8-dioxabicyco[3,3,1]nonane (4) in 84% yields. Further, the same reaction when conducted in ethanol and acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at reflux temperature, gratifyingly obtained 3a and 4a in 1
1) at reflux temperature, gratifyingly obtained 3a and 4a in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Similar results were also observed in water and acetic acid (1
1 ratio. Similar results were also observed in water and acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mediated reaction. Fascinated by the exceptional formation of 4 in acetic acid mediated one pot catalyst free reaction in high yields, the generality and scope of this protocol was demonstrated by synthesizing a series of 1,3-dione fused symmetrical 2,8-dioxabicyco[3,3,1]nonane derivatives (4a–t). A wide range of enamino ketones (alkyl, aromatic and hetero aromatic) and alkyl, aryl substituted 1,3-cyclohexanediones were well tolerated under this set of reaction conditions and furnished 4 in high yields.
1) mediated reaction. Fascinated by the exceptional formation of 4 in acetic acid mediated one pot catalyst free reaction in high yields, the generality and scope of this protocol was demonstrated by synthesizing a series of 1,3-dione fused symmetrical 2,8-dioxabicyco[3,3,1]nonane derivatives (4a–t). A wide range of enamino ketones (alkyl, aromatic and hetero aromatic) and alkyl, aryl substituted 1,3-cyclohexanediones were well tolerated under this set of reaction conditions and furnished 4 in high yields.
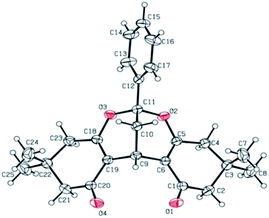 |
| | Fig. 2 ORTEP diagram of compound 4a. | |
 |
| | Scheme 3 Synthesis of symmetrical 2,8-dioxabicyclo[3.3.1]nonanes(4). aReaction conditions: β-enamino ketone (1, 1.14 mmol), cyclic 1,3-dione (2, 2.28 mmol) in acetic acid (10 mL) at reflux temperature. Yields refer to isolated pure products. | |
Additionally, change in substitution such as electron withdrawing and electron releasing groups on 1 also were well tolerated. However, the yields are varied. Compounds bearing electron withdrawing groups gave the better yields compared to the electron donating groups. Similarly, reaction of (E)-3-ethoxy-1-phenylprop-2-ene-1-one14 (1aa) with 5,5-dimethyl-1,3-cyclohaxanedione (2a) under identical reaction conditions also gave the 2,8-dioxabicyco[3,3,1]nonane compound 4 (Scheme 4). Pure products were obtained by simple filtration and wash with hexanes. Reaction of compound 1a with other 1,3-diketo compounds such as 4-hydroxycoumarine, 2-hydroxy-1,4-napthaquinone, 1,3-indanedione were not successful. Whereas with acetyl acetone, formation of tribenzoyl product was obtained as reported in literature.15
 |
| | Scheme 4 Synthesis of 4a from (E)-3-ethoxy-1-phenylprop-2-ene-1-one (1aa). aReaction conditions: 1aa (1.14 mmol), dimedone (2, 2.28 mmol) in acetic acid (10 mL) at reflux temperature. Yields refer to isolated pure products. | |
At this juncture, studies were directed to check the exclusive formation of xanthenes (5). On this account, it is notable to mention that, exclusive formation of (3,3,6,6-tetramethyl-9-(2-oxo-2-phenylethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione) (5a) in 80% yields was accomplished from 3a in toluene by adding catalytic amount of trifluoroacetic acid. Subsequently, compound 5 was also obtained by the sequential addition of trifluoroacetic acid (catalytic amount) to the one pot reaction of 1 and 2 in toluene without isolating 3 (Scheme 5). Structure of 5 was well characterized by NMR, IR and mass spectrometry. After achieving the exclusive formation of 5 successfully, the scope of this protocol was generalised by synthesizing a number of xanthene derivatives by varying the substitution on 1. Electron withdrawing groups (–NO2, –Cl, –Br) and electron releasing groups (–OMe, Me, methylene dioxy) on 1 were well tolerated. However, the yields varied. Compounds bearing electron withdrawing groups gave the better yields and the compounds with electron releasing groups could not make much impact when compared with simple aryl groups.
 |
| | Scheme 5 Synthesis of (3,3,6,6-tetramethyl-9-(2-oxo-2-phenylethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione) (5). aReaction conditions: β-enamino ketone (1, 1.14 mmol), cyclic 1,3-dione (2, 2.28 mmol) in toluene at reflux temperature for 5 h followed by addition CF3COOH (0.5 mmol) and stirred at RT for 1 h. Yields refer to pure products after column chromatography. | |
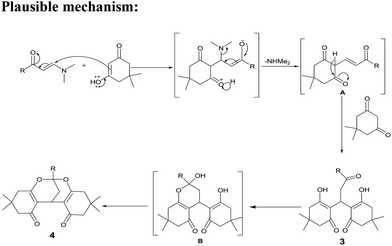
A plausible mechanism was proposed for the formation of 4 where the active methylene functional of diketone attack on β-carbon of enamino ketone in Michael fashion to give A.16 Subsequent attack of second molecule of diketone on β-carbon of A lead to enolic dihydroxy compound 3 (isolated). Next, acetic acid probably playing a dual role as a solvent and also as a catalyst leading to the formation of six member ring B which on subsequent dehydration gives the (3,3,9,9-tetramethyl-6-phenyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione) (4).
3. Conclusions
In conclusion, we have developed a novel protocol to construct the cyclic 1,3-dione fused symmetrical 2,8-dioxabicyco[3,3,1]nonanes (4) for the first time which involves no extrinsic catalyst and no column chromatography. Further, this protocol has high compatibility to broad range of functional group. We believe that this operationally simple protocol may find an application in organic and medicinal chemistry. Further, just by altering the reaction condition, we also developed an efficient procedure for synthesis of xanthenes (5).
4. Experimental
4.1 General
Melting points were measured by CINTEX programmable melting point apparatus and are uncorrected. 1H and 13C NMR spectra of samples in CDCl3 and DMSO-d6 were recorded on AVANCE-300 MHz, 400 MHz and 500 MHz spectrometers. Chemical shifts (δ) are reported relative to TMS (δ = 0.0) as the internal standard. Mass spectra were recorded in ESI spectrometers. All high resolution mass spectra were recorded on QSTAR XL hybrid ms/ms system (Applied Bio systems/MDS sciex, foster city, USA), equipped with an ESI source (IICT, Hyderabad). IR was recorded on Thermo Nicolet nexus-670 spectrometer with reference to KBr. TLC was performed on Merck 60 F-254 silica gel plates. The chemicals used in this work were obtained from commercial channels and were used without purification.
4.2. General procedure for the synthesis of 2,2′-(3-oxo-3-arylpropane-1,1-diyl)bis(3-hydroxy-5,5-dimethylcyclohex-2-enone) (3a and 3b)
A mixture of β-keto enaminone viz (E)-3-(dimethylamino)-1-aryl-prop-2-en-1-one derivatives (1, 1.14 mmol) and cyclic 1,3-diones (2, 2.28 mmol) was heated to reflux in toluene (10 mL) while stirring in a round bottomed flask fitted with reflux condenser and stopper. Up on heating, the reaction mixture became homogenous and turned into yellowish color. After completion of the reaction as indicated by TLC (in 4 to 5 hours), the homogenous mass was allowed to come down to room temperature and evaporated solvent and residual mass was passed on to column chromatography 15–20% ethyl acetate and hexane to get the pure compounds.
4.3. General procedure for the synthesis of symmetrical 2,8 dioxabicyclo[3.3.1]nonane derivatives (4)
A mixture of β-keto enaminone viz (E)-3-(dimethylamino)-1-aryl/alkyl-prop-2-en-1-one derivatives (1, 1.14 mmol) and cyclic 1,3-diones (2, 2.28 mmol) was heated to reflux in acetic acid (10 mL) while stirring in a round bottomed flask fitted with reflux condenser and stopper. Up on heating, the reaction mixture became homogenous and turned into brownish color. After completion of the reaction as indicated by TLC, the homogenous mass was allowed to come down to room temperature and transferred on to crushed ice. The separated solid was filtered, washed with water (2 × 10 mL) followed by hexane (2 × 10 mL) and dried in woven to get the pure compounds.
4.4. General procedure for the synthesis of (3,3,6,6-tetramethyl-9-(2-oxo-2-phenylethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione) derivatives (5)
To a round bottomed flask equipped with a reflux condenser and stopper was added β-keto enaminone viz (E)-3-(dimethylamino)-1-aryl/alkyl-prop-2-en-1-one derivatives (1, 1.14 mmol), cyclic 1,3-diones (2, 2.28 mmol), toluene (10 mL) and was heated to reflux while stirring. After 5 to 6 hours of reflection, the homogenous mass was allowed to come down to room temperature and to this was added CF3COOH (0.5 mmol) and continued the stirring at RT for 1 hour. After completion of the reaction as indicated by TLC, solvent was removed by rotary evaporator under reduced pressure, added water (10 to 15 mL) and extracted with ethyl acetate. Organic layer was dried over anhydrous Na2SO4, evaporated under reduced pressure and the residual mass was passed through flash column chromatography by eluting with 20–25% (ethyl acetate–hexane) to furnish the pure compounds.
4.4.1 2,2′-(3-Oxo-3-phenylpropane-1,1-diyl)bis(3-hydroxy-5,5-dimethylcyclohex-2-enone) (3a). Isolated as a solid; yield 79%; mp. 132–133 °C; IR (KBr): 2961, 2874, 2631, 1690, 1599, 1448, 1368, 1247, 1151, 909, 762 cm−1; 1H NMR (300 MHz, CDCl3): δ 12.51 (br s, 1H), 7.4 (d, J = 7.5 Hz, 2H), 7.55 (t, J = 7.1 Hz, 1H), 7.44 (t, J = 7.7 Hz, 2H), 4.81 (t, J = 7.1 Hz, 1H), 3.76 (d, J = 6.9 Hz, 2H), 2.50–2.08 (m, 8H), 1.16–0.92 (m, 12H) ppm; 13C NMR (125 MHz, CDCl3): δ 198.0, 190.3, 189.3, 136.7, 132.9, 128.4, 127.9, 116.3, 46.8, 46.0, 38.5, 31.0, 29.7, 26.2, 24.2 ppm; ESI-MS: m/z 411 [M + H]+; HRMS (ESI) anal. calcd for C25H30O5Na m/z 433.1985 [M + H]+, found 433.1969.
4.4.2 2,2′-(3-(4-Chlorophenyl)-3-oxopropane-1,1-diyl)bis(3-hydroxy-5,5-dimethylcyclohex-2-enone) (3b). Isolated as a solid; yield 79%; mp. 158–159 °C; IR (KBr): 2963, 2886, 2631, 1688, 1593, 1451, 1399, 1248, 1147, 1088, 907, 767 cm−1; 1H NMR (300 MHz, CDCl3): δ 12.47 (br s, 1H), 7.88 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 4.79 (t, J = 7.0 Hz, 1H), 3.73 (d, J = 7.0 Hz, 2H), 2.39–2.19 (m, 8H), 1.01 (d, J = 32.5 Hz, 12H) ppm; 13C NMR (125 MHz, CDCl3): δ 196.9, 190.4, 189.3, 139.4, 135.1, 129.3, 128.8, 116.2, 46.8, 46.0, 38.4, 31.0, 29.7, 26.2, 24.3 ppm; ESI-MS: m/z 445 [M + H]+; HRMS (ESI) anal. calcd for C25H30ClO5 m/z 445.1776 [M + H]+, found 445.1766.
4.4.3 3,3,9,9-Tetramethyl-6-phenyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4a). Isolated as a solid; yield 84%; mp. 179–180 °C; IR (KBr): 2956, 2868, 1662, 1635, 1498, 1456, 1385, 1222, 1110, 1032, 966, 849, 766 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.57–7.52 (m, 2H), 7.48–7.40 (m, 3H), 4.42 (s, 1H), 2.46–2.36 (m, 4H), 2.30–2.21 (m, 4H), 2.05 (d, J = 2.8 Hz, 2H), 1.08 (s, 6H), 1.01 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.9, 138.8, 129.2, 128.3, 125.2, 115.9, 99.8, 50.4, 41.5, 33.1, 32.0, 28.4, 27.8, 16.9 ppm; ESI-MS: m/z 393 [M + H]+; HRMS (ESI) anal. calcd for C25H29O4 m/z 393.2060 [M + H]+, found 393.2043.
4.4.4 3,3,9,9-Tetramethyl-6-(4-nitrophenyl)-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4b). Isolated as a solid; yield 86%; mp. 176–177 °C; IR (KBr): 2959, 2872, 1672, 1644, 1518, 1468, 1386, 1325, 1221, 1107, 1036, 967, 878, 753 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.31 (d, J = 8.8 Hz, 2H), 7.75 (d, J = 8.8 Hz, 2H), 4.45 (s, 1H), 2.50–2.35 (m, 4H), 2.34–2.19 (m, 4H), 2.06 (d, J = 2.8 Hz, 2H), 1.10 (s, 6H), 1.02 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.5, 166.6, 148.2, 145.2, 126.7, 123.6, 115.8, 99.0, 50.2, 41.3, 32.7, 32.0, 28.4, 27.7, 16.6 ppm; ESI-MS: m/z 438 [M + H]+; HRMS (ESI) anal. calcd for C25H28NO6 m/z 438.1911 [M + H]+, found 438.1885.
4.4.5 6-(4-Bromophenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4c). Isolated as a solid; yield 84%; mp. 142–144 °C; IR (KBr): 2956, 1669, 1647, 1489, 1378, 1316, 1220, 1106, 1034, 953, 876, 758 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.57 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.3 Hz, 2H), 4.41 (s, 1H), 2.44–2.33 (m, 4H), 2.31–2.20 (m, 4H), 2.01 (d, J = 2.7 Hz, 2H), 1.08 (s, 6H), 1.01 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.7, 137.8, 131.4, 127.0, 123.4, 115.8, 99.4, 50.2, 41.3, 32.8, 32.0, 28.4, 27.7, 16.7 ppm; ESI-MS: m/z 471 [M + H]+; HRMS (ESI) anal. calcd for C25H28BrO4 m/z 471.1165 [M + H]+, found 471.1141.
4.4.6 6-(4-Chlorophenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4d). Isolated as a solid; yield 85%; mp. 209–210 °C; IR (KBr): 2956, 2871, 1672, 1641, 1492, 1386, 1325, 1224, 1108, 1032, 954, 868, 746 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.48 (d, J = 8.5 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 4.41 (s, 1H), 2.44–2.34 (m, 4H), 2.32–2.21 (m, 4H), 2.02 (d, J = 3.0 Hz, 2H), 1.08 (s, 6H), 1.01 (s, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 194.4, 166.7, 137.3, 135.1, 128.5, 126.8, 115.8, 99.3, 50.2, 41.3, 32.9, 32.0, 28.4, 27.7, 16.7 ppm; ESI-MS: m/z 427 [M + H]+; HRMS (ESI) anal. calcd for C25H28ClO4 m/z 427.1670 [M + H]+, found 427.1647.
4.4.7 6-(4-Fluorophenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4e). Isolated as a solid; yield 85%; mp. 184–185 °C; IR (KBr): 2960, 2924, 1670, 1640, 1515, 1389, 1219, 1112, 1091, 957, 841, 795, 635 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.62–7.47 (m, 2H), 7.13 (t, J = 7.6 Hz, 2H), 4.41 (s, 1H), 2.48–2.34 (m, 4H), 2.33–2.13 (m, 4H), 2.03 (d, J = 2.2 Hz, 2H), 1.08 (s, 6H), 1.10 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.7, 162.9 (d, J = 248.8 Hz), 134.7, 127.3 (d, J = 8.1 Hz), 115.8, 115.3, 115.1, 99.4, 50.3, 41.3, 33.0, 32.0, 28.4, 27.7, 16.8 ppm; ESI-MS: m/z 411 [M + H]+; HRMS (ESI) anal. calcd for C25H28FO4 m/z 411.1966 [M + H]+, found 411.1944.
4.4.8 6-(3,4-Dimethoxyphenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4f). Isolated as a solid; yield 74%; mp. 145–147 °C; IR (KBr): 2957, 1666, 1637, 1516, 1386, 1267, 1105, 1026, 967, 861, 757 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.13–7.05 (m, 2H), 6.94–6.86 (m, 1H), 4.41 (t, J = 2.5 Hz, 1H), 3.93 (s, 3H), 3.91 (s, 3H), 2.46–2.34 (m, 4H), 2.31–2.19 (m, 4H), 2.08 (d, J = 3.0 Hz, 2H), 1.08 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.6, 167.1, 149.8, 148.7, 131.3, 117.8, 115.9, 110.6, 108.8, 99.8, 55.98, 55.93, 50.4, 41.5, 32.8, 32.1, 28.6, 27.7, 16.9 ppm; ESI-MS: m/z 453 [M + H]+; HRMS (ESI) anal. calcd for C27H33O6 m/z 453.2271 [M + H]+, found 453.2248.
4.4.9 3,3,9,9-Tetramethyl-6-(3,4,5-trimethoxyphenyl)-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4g). Isolated as a solid; yield 72%; mp. 159–160 °C; IR (KBr): 2956, 2870, 1663, 1636, 1512, 1468, 1348, 1238, 1130, 1031, 966, 874, 766 cm−1; 1H NMR (500 MHz, CDCl3): δ 6.77 (s, 2H), 4.41 (t, J = 2.7 Hz, 1H), 3.91 (s, 6H), 3.88 (s, 3H), 2.44–2.33 (m, 4H), 2.31–2.21 (m, 4H), 2.09 (d, J = 3.0 Hz, 2H), 1.09 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.5, 166.9, 153.0, 138.7, 134.2, 115.9, 102.8, 99.7, 60.7, 56.1, 50.3, 41.4, 32.6, 32.0, 28.6, 27.6, 16.8 ppm; ESI-MS: m/z 483 [M + H]+; HRMS (ESI) anal. calcd for C28H35O7 m/z 483.2377 [M + H]+, found 483.2344.
4.4.10 6-(4-(Benzyloxy)phenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4h). Isolated as a solid; yield 73%; mp. 131–133 °C; IR (KBr): 2956, 2922, 1670, 1513, 1462, 1384, 1221, 1101, 1028, 832, 739 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.50–7.30 (m, 7H), 7.02 (d, J = 8.6 Hz, 2H), 5.10 (s, 2H), 4.40 (s, 1H), 2.43–2.31 (m, 4H), 2.30–2.17 (m, 4H), 2.03 (d, J = 2.1 Hz, 2H), 1.07 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 194.4, 166.9, 159.0, 136.2, 130.9, 128.2, 127.7, 127.0, 126.4, 115.5, 114.3, 99.6, 69.6, 50.1, 41.2, 32.7, 31.8, 28.2, 27.5, 16.7 ppm; ESI-MS: m/z 499 [M + H]+; HRMS (ESI) anal. calcd for C32H35O5 m/z 499.2479 [M + H]+, found 499.2459.
4.4.11 6-(4-Methoxyphenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4i). Isolated as a solid; yield 77%; mp. 140–141 °C; IR (KBr): 2949, 2870, 1662, 1636, 1516, 1468, 1386, 1253, 1105, 1029, 962, 864, 744 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.47 (d, J = 8.5 Hz, 2H), 6.95 (d, J = 8.5 Hz, 2H), 4.40 (s, 1H), 3.85 (s, 3H), 2.43–2.32 (m, 4H), 2.31–2.19 (m, 4H), 2.04 (d, J = 2.5 Hz, 2H), 1.08 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 194.4, 166.9, 160.0, 130.9, 126.6, 115.7, 113.5, 99.8, 55.2, 50.3, 41.4, 32.9, 32.0, 28.4, 27.7, 16.8 ppm; ESI-MS: m/z 423 [M + H]+; HRMS (ESI) anal. calcd for C26H31O5 m/z 423.2166 [M + H]+, found 423.2140.
4.4.12 3,3,9,9-Tetramethyl-6-(p-tolyl)-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4j). Isolated as a solid; yield 78%; mp. 126–128 °C; IR (KBr): 2958, 1666, 1638, 1514, 1464, 1380, 1319, 1224, 1105, 1032, 963, 872, 757 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.47–7.39 (d, J = 8.2 Hz, 2H), 7.28–7.21 (m, 2H), 4.40 (s, 1H), 2.43–2.34 (m, 7H), 2.30–2.20 (m, 4H), 2.04 (d, J = 2.8 Hz, 2H), 1.07 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.5, 167.0, 139.2, 135.9, 129.0, 125.2, 115.9, 99.9, 50.4, 41.5, 33.0, 32.1, 28.5, 27.8, 21.1, 16.9 ppm; ESI-MS: m/z 407 [M + H]+; HRMS (ESI) anal. calcd for C26H31O4 m/z 407.2216 [M + H]+, found 407.2196.
4.4.13 6-(4-Ethoxyphenyl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4k). Isolated as a solid; yield 77%; mp. 89–90 °C; IR (KBr): 2950, 1664, 1514, 1473, 1386, 1246, 1102, 1037, 866, 649 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.45 (d, J = 8.4 Hz, 2H), 6.93 (d, J = 8.1 Hz, 2H), 4.39 (s, 1H), 4.07 (q, J = 6.7 Hz, 2H), 2.49–2.15 (m, 8H), 2.05 (m, 2H), 1.43 (t, J = 6.7 Hz, 3H), 1.04 (d, J = 22.2 Hz, 12H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.9, 159.3, 130.5, 126.4, 115.6, 113.9, 99.7, 63.2, 50.2, 41.3, 32.8, 31.8, 28.3, 27.6, 16.8, 14.4 ppm; ESI-MS: m/z 437 [M + H]+; HRMS (ESI) anal. calcd for C27H33O5 m/z 437.2322 [M + H]+, found 437.2306.
4.4.14 6-(Benzo[d][1,3]dioxol-5-yl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4l). Isolated as a solid; yield 79%; mp. 144–146 °C; IR (KBr): 3055, 2956, 1662, 1636, 1492, 1384, 1234, 1108, 1034, 969, 865, 820 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.11–6.97 (m, 2H), 6.84 (d, J = 8.1 Hz, 1H), 6.02 (s, 2H), 4.39 (s, 1H), 2.45–2.32 (m, 4H), 2.31–2.17 (m, 4H), 2.01 (d, J = 2.8 Hz, 2H), 1.07 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.8, 148.1, 147.5, 132.6, 118.9, 115.7, 107.7, 106.0, 101.3, 99.6, 50.2, 41.3, 33.0, 31.9, 28.3, 27.7, 16.8 ppm; ESI-MS: m/z 437 [M + H]+; HRMS (ESI) anal. calcd for C26H29O6 m/z 437.1958 [M + H]+, found 437.1931.
4.4.15 3,3,9,9-Tetramethyl-6-(naphthalen-2-yl)-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4m). Isolated as a solid; yield 81%; mp. 157–158 °C; IR (KBr): 2957, 2927, 1664, 1637, 1509, 1467, 1384, 1323, 1224, 1105, 1032, 967, 871, 750 cm−1; 1H NMR (300 MHz, CDCl3): δ 8.05 (s, 1H), 7.97–7.84 (m, 3H), 7.64–7.50 (m, 3H), 4.46 (s, 1H), 2.54–2.37 (m, 4H), 2.36–2.21 (m, 4H), 2.15 (d, J = 2.8 Hz, 2H), 1.11 (s, 6H), 1.03 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.5, 167.0, 135.9, 133.3, 132.5, 128.3, 127.5, 126.8, 126.5, 124.6, 122.8, 115.9, 100.0, 50.4, 41.5, 32.9, 32.1, 28.5, 27.8, 16.9 ppm; ESI-MS: m/z 443 [M + H]+; HRMS (ESI) anal. calcd for C29H31O4 m/z 443.2192 [M + H]+, found 443.2186.
4.4.16 3,3,9,9-Tetramethyl-6-(thiophen-2-yl)-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4n). Isolated as a solid; yield 79%; mp. 182–184 °C; IR (KBr): 2957, 1662, 1634, 1460, 1384, 1321, 1219, 1108, 1030, 963, 839, 731 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.39 (d, J = 4.8 Hz, 1H), 7.21 (d, J = 3.5 Hz, 1H), 7.05 (t, J = 4.8 Hz, 1H), 4.43 (s, 1H), 2.42–2.32 (m, 4H), 2.30–2.22 (m, 4H), 2.20 (d, J = 2.8 Hz, 2H), 1.07 (s, 6H), 0.99 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.5, 142.0, 126.7, 126.4, 125.1, 115.7, 98.5, 50.3, 41.3, 33.0, 32.0, 28.4, 27.7, 16.8 ppm; ESI-MS: m/z 399 [M + H]+; HRMS (ESI) anal. calcd for C23H27O4S m/z 399.1624 [M + H]+, found 399.1607.
4.4.17 6-(Furan-2-yl)-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4o). Isolated as a solid; yield 77%; mp. 205–207 °C; IR (KBr): 3134, 2961, 1663, 1500, 1464, 1380, 1222, 1109, 1026, 966, 853, 763 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.48 (s, 1H), 6.61 (d, J = 3.9 Hz, 1H), 6.47–6.42 (m, 1H), 4.43 (s, 1H), 2.35 (s, 4H), 2.28–2.18 (m, 4H), 2.17 (d, J = 3.0 Hz, 2H), 1.05 (s, 6H), 0.98 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.4, 166.4, 150.3, 143.1, 115.9, 110.4, 107.9, 95.9, 50.3, 41.3, 32.0, 29.8, 28.4, 27.8, 15.9 ppm; ESI-MS: m/z 383 [M + H]+; HRMS (ESI) anal. calcd for C23H27O5 m/z 383.1853 [M + H]+, found 383.1838.
4.4.18 6-Isobutyl-3,3,9,9-tetramethyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4p). Isolated as a solid; yield 69%; mp. 108–109 °C; IR (KBr): 2960, 1668, 1636, 1464, 1388, 1325, 1260, 1139, 1025, 964, 844, 796 cm−1; 1H NMR (500 MHz, CDCl3): δ 4.23 (s, 1H), 2.26–2.06 (m, 8H), 1.93–1.79 (m, 3H), 1.78–1.66 (m, 2H), 1.05–0.84 (m, 18H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.5, 166.9, 115.7, 101.1, 50.3, 46.9, 41.5, 32.0, 30.0, 28.4, 27.8, 23.7, 23.6, 16.2 ppm; ESI-MS: m/z 373 [M + H]+; HRMS (ESI) anal. calcd for C23H33O4 m/z 373.2373 [M + H]+, found 373.2358.
4.4.19 6-Phenyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4q). Isolated as a solid; yield 73%; mp. 157–158 °C; IR (KBr): 3058, 2926, 1664, 1498, 1382, 1270, 1185, 1111, 1023, 917, 830, 766 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.61–7.52 (m, 2H), 7.49–7.39 (m, 3H), 4.44 (t, J = 2.5 Hz, 1H), 2.59–2.39 (m, 6H), 2.38–2.30 (m, 2H), 2.05–1.91 (m, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 195.1, 168.6, 138.7, 129.2, 128.3, 125.2, 117.1, 99.5, 36.4, 33.0, 27.6, 20.3, 16.9 ppm; ESI-MS: m/z 337 [M + H]+; HRMS (ESI) anal. calcd for C21H21O4 m/z 337.1434 [M + H]+, found 337.1417.
4.4.20 6-(4-Chlorophenyl)-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4r). Isolated as a solid; yield 75%; mp. 168–169 °C; IR (KBr): 2928, 2868, 1671, 1636, 1492, 1383, 1347, 1269, 1183, 1116, 1088, 986, 813, 737 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.49 (d, J = 8.6 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 4.44 (t, J = 2.5 Hz, 1H), 2.59–2.39 (m, 6H), 2.38–2.30 (m, 2H), 2.03–1.88 (m, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.9, 168.3, 137.3, 135.1, 128.5, 126.8, 117.1, 99.1, 36.3, 32.8, 27.6, 20.3, 16.8 ppm; ESI-MS: m/z 371 [M + H]+; HRMS (ESI) anal. calcd for C21H20ClO4 m/z 371.1044 [M + H]+, found 371.1030.
4.4.21 3,6,9-Triphenyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4s). Isolated as solid and mixture of diastereomers; yield 80%; mp. 188–190 °C; IR (KBr): 3027, 2925, 1667, 1636, 1495, 1386, 1318, 1211, 1110, 1029, 917, 872, 760 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.64–7.51 (m, 2H), 7.49–7.41 (m, 2H), 7.40–7.30 (m, 4H), 7.29–7.18 (m, 7H), 4.57–4.48 (m, 1H), 3.51–3.22 (m, 2H), 2.92–2.51 (m, 8H), 2.21–2.01 (m, 2H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.2, 194.0, 193.9, 193.5, 168.5, 168.1, 167.3, 167.1, 142.3, 138.5, 138.4, 129.3, 128.67, 128.61, 128.4, 126.9, 126.57, 126.51, 125.28, 116.96, 116.93, 100.13, 100.10, 43.7, 43.6, 43.5, 43.4, 39.2, 39.0, 38.1, 38.0, 35.2, 35.0, 33.08, 33.01, 32.6, 29.5, 17.3, 17.2, 17.0 ppm; ESI-MS: m/z 489 [M + H]+; HRMS (ESI) anal. calcd for C33H29O4 m/z 489.2060 [M + H]+, found 489.2030.
4.4.22 6-(4-Chlorophenyl)-3,9-diphenyl-3,4,8,9,10,12-hexahydro-1H-6,12-methanodibenzo[d,g][1,3]dioxocine-1,11(2H)-dione (4t). Isolated as solid and mixture of diasteromers; yield 81%; mp. 113–115 °C; IR (KBr): 3025, 2944, 1665, 1637, 1494, 1392, 1323, 1211, 1138, 1093, 949, 870, 749 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.55–7.47 (m, 2H), 7.44–7.39 (m, 2H), 7.38–7.30 (m, 4H), 7.29–7.19 (m, 6H), 4.56–4.49 (m, 1H), 3.52–3.19 (m, 2H), 2.85–2.63 (m, 6H), 2.62–2.50 (m, 1H), 2.17–2.00 (m, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 194.1, 193.9, 193.8, 193.4, 168.3, 167.8, 167.0, 166.8, 142.2, 137.1, 137.0, 135.3, 131.3, 128.69, 128.63, 126.9, 126.8, 126.56, 126.50, 116.97, 116.94, 99.7, 99.6, 43.68, 43.62, 43.49, 43.45, 39.1, 39.0, 38.1, 38.0, 35.2, 34.9, 32.97, 32.90, 32.57, 29.5, 17.2, 17.1, 16.9 ppm; ESI-MS: m/z 523 [M + H]+; HRMS (ESI) anal. calcd for C33H28ClO4 m/z 523.1670 [M + H]+, found 523.1639.
4.4.23 3,3,6,6-Tetramethyl-9-(2-oxo-2-phenylethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5a). Isolated as a solid; yield 72%; mp. 141–142 °C; IR (KBr): 3085, 2953, 1682, 1652, 1464, 1378, 1326, 1248, 1024, 914, 740 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.90 (d, J = 7.0 Hz, 2H), 7.51 (t, J = 7.3 Hz, 1H), 7.42 (t, J = 7.9 Hz, 2H), 4.02 (t, J = 4.2 Hz, 1H), 3.39 (d, J = 4.4 Hz, 2H), 2.43–2.32 (m, 4H), 2.28–2.16 (m, 4H), 1.08 (s, 6H), 1.01 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 199.9, 197.3, 164.7, 137.4, 132.6, 128.3, 127.9, 113.4, 50.7, 40.7, 40.0, 31.9, 29.1, 27.0, 24.0 ppm; ESI-MS: m/z 393 [M + H]+; HRMS (ESI) anal. calcd for C25H28O4Na m/z 415.1879 [M + H]+, found 415.1868.
4.4.24 3,3,6,6-Tetramethyl-9-(2-(4-nitrophenyl)-2-oxoethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5b). Isolated as a solid; yield 73%; mp. 107–109 °C; IR (KBr): 3105, 2961, 1692, 1656, 1602, 1521, 1466, 1383, 1269, 1198, 938, 853 cm−1; 1H NMR (500 MHz, CDCl3): δ 8.31 (d, J = 8.8 Hz, 2H), 8.22 (d, J = 8.8 Hz, 2H), 4.04 (t, J = 5.3 Hz, 1H), 3.28 (d, J = 5.3 Hz, 2H), 2.47–2.37 (m, 4H), 2.30–2.20 (m, 4H), 1.11 (s, 6H), 1.06 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 197.6, 197.4, 164.8, 150.0, 141.5, 129.3, 123.7, 113.4, 50.6, 43.4, 40.8, 32.0, 29.2, 27.0, 24.3 ppm; ESI-MS: m/z 438 [M + H]+; HRMS (ESI) anal. calcd for C25H27NO6Na m/z 460.1730 [M + Na]+, found 460.1710.
4.4.25 9-(2-(4-Chlorophenyl)-2-oxoethyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5c). Isolated as a solid; yield 73%; mp. 142–143 °C; IR (KBr): 3095, 2962, 1684, 1651, 1619, 1585, 1467, 1380, 1206, 1141, 1005, 937, 836 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.90 (d, J = 8.3 Hz, 2H), 7.40 (d, J = 8.0 Hz, 2H), 4.01 (t, J = 4.4 Hz, 1H), 3.29 (d, J = 4.7 Hz, 2H), 2.45–2.34 (m, 4H), 2.28–2.18 (m, 4H), 1.09 (s, 6H), 1.02 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 198.4, 197.3, 164.7, 139.0, 135.6, 129.5, 128.6, 113.4, 50.6, 41.4, 40.7, 31.9, 29.2, 26.9, 24.1 ppm; ESI-MS: m/z 427 [M + H]+; HRMS (ESI) anal. calcd for C25H27ClO4Na m/z 449.1490 [M + Na]+, found 449.1482.
4.4.26 9-(2-(4-Bromophenyl)-2-oxoethyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5d). Isolated as a solid; yield 72%; mp. 135–136 °C; IR (KBr): 2958, 2873, 1687, 1656, 1581, 1466, 1385, 1274, 1199, 1004, 968, 818 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.83 (d, J = 8.4 Hz, 2H), 7.57 (d, J = 8.4 Hz, 2H), 4.01 (t, J = 4.5 Hz, 1H), 3.29 (d, J = 4.9 Hz, 2H), 2.45–2.32 (m, 4H), 2.30–2.15 (m, 4H), 1.09 (s, 6H), 1.03 (s, 6H) ppm; 13C NMR (75 MHz, CDCl3): δ 198.4, 197.2, 164.6, 135.9, 131.5, 129.5, 127.7, 113.3, 50.5, 41.1, 40.6, 31.8, 29.1, 26.9, 24.1 ppm; ESI-MS: m/z 471 [M + H]+; HRMS (ESI) anal. calcd for C25H28O4Br m/z 471.1165 [M + H]+, found 471.1157.
4.4.27 9-(2-(4-Methoxyphenyl)-2-oxoethyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5e). Isolated as a solid; yield 69%; mp. 139–140 °C; IR (KBr): 2959, 1654, 1599, 1507, 1464, 1382, 1253, 1168, 1019, 969, 835, 752 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.96–7.83 (m, 2H), 6.89 (d, J = 8.8 Hz, 2H), 4.01 (s, 1H), 3.85 (s, 3H), 3.31 (d, J = 4.5 Hz, 2H), 2.49–2.31 (m, 4H), 2.30–2.13 (m, 4H), 1.08 (s, 6H), 1.01 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 198.3, 197.3, 163.1, 132.5, 130.2, 113.8, 113.4, 113.5, 55.3, 50.7, 40.7, 39.8, 31.9, 29.1, 27.0, 24.1 ppm; ESI-MS: m/z 423 [M + H]+; HRMS (ESI) anal. calcd for C26H30O5Na m/z 445.1985 [M + Na]+, found 445.1976.
4.4.28 3,3,6,6-Tetramethyl-9-(2-oxo-2-p-tolylethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5f). Isolated as a solid; yield 70%; mp. 117–118 °C; IR (KBr): 3025, 2955, 1684, 1654, 1621, 1466, 1380, 1273, 1169, 968, 811, 745 cm−1; 1H NMR (400 MHz, CDCl3): δ 7.80 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 7.9 Hz, 2H), 4.01 (t, J = 4.1 Hz, 1H), 3.34 (d, J = 4.5 Hz, 2H), 2.44–2.31 (m, 7H), 2.28–2.14 (m, 4H), 1.07 (s, 6H), 1.00 (s, 6H) ppm; 13C NMR (125 MHz, CDCl3): δ 199.4, 197.2, 164.5, 143.2, 135.0, 128.9, 127.9, 113.4, 50.6, 40.6, 39.9, 31.8, 29.1, 26.9, 23.9, 21.4 ppm; ESI-MS: m/z 429 [M + Na]+; HRMS (ESI) anal. calcd for C26H30O4Na m/z 429.2036 [M + Na]+, found 429.2020.
4.4.29 9-(2-(Benzo[d][1,3]dioxol-5-yl)-2-oxoethyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5g). Isolated as a solid; yield 69%; mp. 140–141 °C; IR (KBr): 3078, 2961, 1662, 1622, 1499, 1449, 1377, 1289, 1193, 1034, 999, 884, 751 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.57 (d, J = 7.9 Hz, 1H), 7.39 (s, 1H), 6.82 (d, J = 8.3 Hz, 1H), 6.00 (s, 2H), 4.00 (s, 1H), 3.26 (d, J = 4.5 Hz, 2H), 2.50–2.29 (m, 4H), 2.28–2.14 (m, 4H), 1.05 (d, J = 18.0 Hz, 12H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.6, 197.3, 164.6, 151.3, 147.8, 132.3, 124.4, 113.5, 109.4, 107.6, 101.5, 50.6, 40.7, 40.3, 31.9, 29.1, 26.9, 24.1 ppm; ESI-MS: m/z 459 [M + Na]+; HRMS (ESI) anal. calcd for C26H28O6Na m/z 459.1778 [M + Na]+, found 459.1760.
4.4.30 9-(2-(Furan-2-yl)-2-oxoethyl)-3,3,6,6-tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5h). Isolated as a solid; yield 71%; mp. 146–147 °C; IR (KBr): 3132, 2965, 1665, 1645, 1562, 1466, 1379, 1284, 1134, 1010, 951, 885 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.53 (s, 1H), 7.22–7.13 (m, 1H), 6.54–6.41 (m, 1H), 4.00 (t, J = 3.5 Hz, 1H), 3.18 (d, J = 4.3 Hz, 2H), 2.44–2.28 (m, 4H), 2.27–2.14 (m, 4H), 1.08 (s, 6H), 1.01 (s, 6H) ppm; 13C NMR (100 MHz, CDCl3): δ 197.2, 188.2, 164.5, 152.6, 146.3, 117.9, 113.0, 111.8, 50.6, 40.6, 39.7, 31.8, 29.1, 26.9, 24.3 ppm; ESI-MS: m/z 405 [M + Na]+; HRMS (ESI) anal. calcd for C23H26O5Na m/z 405.1672 [M + Na]+, found 405.1663.
4.4.31 9-(2-Oxo-2-phenylethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5i). Isolated as a solid; yield 70%; mp. 131–133 °C; IR (KBr): 3057, 2954, 1669, 1617, 1595, 1447, 1379, 1284, 1175, 1009, 956, 883, 852 cm−1; 1H NMR (500 MHz, CDCl3): δ 7.88 (d, J = 7.3 Hz, 2H), 7.52 (t, J = 7.6 Hz, 1H), 7.42 (t, J = 7.4 Hz, 2H), 4.07 (s, 1H), 3.26 (d, J = 3.8 Hz, 2H), 2.55–2.25 (m, 8H), 2.03–1.78 (m, 4H) ppm; 13C NMR (100 MHz, CDCl3): δ 199.7, 197.1, 165.8, 137.6, 132.5, 128.2, 127.8, 114.2, 40.4, 36.6, 26.8, 24.1, 19.9 ppm; ESI-MS: m/z 359 [M + Na]+; HRMS (ESI) anal. calcd for C21H20O4Na m/z 359.1253 [M + Na]+, found 359.1248.
4.4.32 9-(2-(4-Chlorophenyl)-2-oxoethyl)-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione (5j). Isolated as a solid; yield 72%; mp. 146–147 °C; IR (KBr): 3033, 2946, 2879, 1662, 1621, 1584, 1489, 1379, 1287, 1175, 1133, 1087, 1009, 959, 824, 883 cm−1; 1H NMR (300 MHz, CDCl3): δ 7.90 (d, J = 8.4 Hz, 2H), 7.41 (d, J = 8.6 Hz, 2H), 4.06 (t, J = 4.7 Hz, 1H), 3.18 (d, J = 4.9 Hz, 2H), 2.62–2.22 (m, 8H), 2.11–1.79 (m, 4H) ppm; 13C NMR (125 MHz, CDCl3): δ 198.3, 197.3, 166.0, 139.0, 135.8, 129.5, 128.6, 114.5, 41.5, 36.7, 27.0, 24.3, 20.1 ppm; ESI-MS: m/z 371 [M + H]+; HRMS (ESI) anal. calcd for C21H20ClO4 m/z 371.1044 [M + H]+, found 371.1046.
Acknowledgements
We are grateful to SERB-DST New Delhi for financial support (grant no. SB/EMEQ-075/2013). We thank Head Crop Protection Chemicals Division and Director CSIR-IICT for support. BCJ is thankful to UGC, New Delhi for SRF.
Notes and references
-
(a) M. Ruiz, P. Lopez-Alvarado, G. Giorgi and J. C. Menendez, Chem. Soc. Rev., 2011, 40, 3445 RSC;
(b) J. T. Njardarson, Tetrahedron, 2011, 67, 7631 CrossRef CAS PubMed;
(c) W.-Y. Zhao, Chem. Rev., 2010, 110, 1706 CrossRef PubMed;
(d) E. Butkus, Synlett, 2001, 12, 1827 CrossRef;
(e) M. Presset, Y. Coquerel and J. Rodriguez, Chem. Rev., 2013, 113, 525 CrossRef CAS PubMed.
-
(a) F. M. Moghaddam, Z. Mirjafary, H. Saeidian, S. Taheri, M. Doulabi and M. Kiamehr, Tetrahedron, 2010, 66, 134 CrossRef CAS;
(b) Y.-F. Wang and S. Chiba, J. Am. Chem. Soc., 2009, 131, 12570 CrossRef CAS PubMed;
(c) E. Butkus, U. Berg, J. Malinauskienė and J. Sandström, J. Org. Chem., 2000, 65, 1353 CrossRef CAS PubMed;
(d) C. J. Wallentin, T. Wixe, O. F. Wendt, K. E Bergquist and K. Wärnmark, Chem.–Eur. J., 2010, 16, 3994 CrossRef CAS PubMed;
(e) A. Mendoza, P. Pardo, F. Rodríguez and F. J. Fañanás, Chem.–Eur. J., 2010, 16, 9758 CrossRef CAS PubMed;
(f) T. Enomoto, Y. Yasuia and Y. Takemoto, J. Org. Chem., 2010, 75, 4876 CrossRef CAS PubMed;
(g) K. Vervisch, M. D'hooghe, K. W. Törnroos and N. D. Kimpe, J. Org. Chem., 2010, 75, 7734 CrossRef CAS PubMed;
(h) I. N. Michaelides, B. Darses and D. J. Dixon, Org. Lett., 2011, 13, 664 CrossRef CAS PubMed;
(i) F. M. Moghaddam, S. Taheri, Z. Mirjafary and H. Saeidian, Synlett, 2010, 123 CrossRef CAS;
(j) T. M. Teng, A. Das, D. B. Huple and R.-S. Liu, J. Am. Chem. Soc., 2010, 132, 12565 CrossRef CAS PubMed;
(k) Y. Kuninobu, J. Morita, M. Nishi, A. Kawata and K. Takai, Org. Lett., 2009, 11, 2535 CrossRef CAS PubMed;
(l) C.-L. Cao, X.-L. Sun, Y.-B. Kang and Y. Tang, Org. Lett., 2007, 9, 4151 CrossRef CAS PubMed;
(m) J. M. Aurrecoechea, J. M. Gorgojo and C. Saornil, J. Org. Chem., 2005, 70, 9640 CrossRef CAS PubMed;
(n) N. R. Norcross, J. P. Melbardis, M. Ferris-Solera, M. A. Sephton, C. Kilner, L. N. Zakharov, P. C. Astles, S. L. Warriner and P. R. Blakemore, J. Org. Chem., 2008, 73, 7939 CrossRef CAS PubMed;
(o) P. R. Blakemore, C. Kilner, N. R. Norcross and P. C. Astles, Org. Lett., 2005, 7, 4721 CrossRef CAS;
(p) N. K. Garg, D. D. Caspi and B. M Stoltz, J. Am. Chem. Soc., 2004, 126, 9552 CrossRef CAS PubMed;
(q) L. N. Mander and M. M. McLachlan, J. Am. Chem. Soc., 2003, 125, 2400 CrossRef CAS PubMed;
(r) Y.-Q. Sun, B.-X. Yu, X.-L. Wang, S.-B. Tang, X.-G. She and X.-F. Pan, J. Org. Chem., 2010, 75, 4224 CrossRef CAS PubMed;
(s) D. Solé, E. Peidró and J. Bonjoch, Org. Lett., 2000, 2, 2225 CrossRef.
- J. Troger, J. Prakt. Chem., 1887, 36, 225 CrossRef.
-
(a) E. B. Veale, D. O. Frimannsson, M. Lawler and T. Gunnlaugsson, Org. Lett., 2009, 11, 4040 CrossRef CAS PubMed;
(b) E. B. Veale and T. Gunnlaugsson, J. Org. Chem., 2010, 75, 5513 CrossRef CAS PubMed;
(c) Q. Xin, X. T. Tao, F. Z. Wang, J. L. Sun, D. C. Zou, F. J. Wang, H. J. Liu, Z. Liu, Y. Ren and M. H. Jiang, Org. Electron., 2008, 9, 1076 CrossRef CAS.
-
(a) C. Pardo, E. Sesmilo, E. Gutiérrez-Puebla, A. Monge, J. Elguero and A. Fruchier, J. Org. Chem., 2001, 66, 1607 CrossRef CAS;
(b) M. Harmata and T. Murray, J. Org. Chem., 1989, 54, 3761 CrossRef CAS;
(c) C. J. Wallentin, T. Wixe, O. F. Wendt, K. E. Bergquist and K. Warnmark, Chem.–Eur. J., 2010, 16, 3994 CrossRef CAS;
(d) S. Stoncius, E. Butkus, A. Zilinskas, K. Larsson, L. Ohrstrom, U. Berg and K. Warnmark, J. Org. Chem., 2004, 69, 5196 CrossRef CAS PubMed.
- K. Naemura, R. Fukunaga, M. Komatsu, M. Yamanaka and H. Chikamatsu, Bull. Chem. Soc. Jpn., 1989, 62, 83 CrossRef CAS.
-
(a) V. Srinivas and M. Koketsu, J. Org. Chem., 2013, 78, 11612 CrossRef CAS PubMed;
(b) G. Yin, T. Ren, Y. Rao, Y. Zhou, Z. Li, W. Shu and A. Wu, J. Org. Chem., 2013, 78, 3132 CrossRef CAS PubMed;
(c) F. Wang, F. Chen, M. Qu, T. Li, Y. Liu and M. Shi, Chem. Commun., 2013, 49, 3360 RSC;
(d) Y. Rao and G. Yin, Org. Biomol. Chem., 2013, 11, 6029 RSC;
(e) X. Jiang, Z. Song, C. Xu, Q. Yao and A. Zhang, Eur. J. Org. Chem., 2014, 2, 418 CrossRef;
(f) C. Guha, S. Samanta, N. Sepay and A. K. Mallik, Tetrahedron Lett., 2015, 56, 4954 CrossRef CAS.
-
(a) S. Das and A. J. Thakur, Eur. J. Org. Chem., 2011, 12, 2301 CrossRef;
(b) C. J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC.
- B. Chiranjeevi, E. Narender Reddy, C. Madhu, N. Jagadeesh Babu and A. Krishnaiah, RSC Adv., 2014, 4, 35009 RSC.
-
(a) B. Chiranjeevi, E. Narender Reddy, C. Madhu, Y. Poornachandra, C. Ganesh Kumar, N. Jagadeesh Babu and A. Krishnaiah, Bioorg. Med. Chem. Lett., 2015, 25, 1915 CrossRef PubMed;
(b) E. Narender Reddy, A. Krishnaiah, C. G. Kumar, P. Sujitha and N. Jagadeesh Babu, Bioorg. Med. Chem. Lett., 2012, 22, 7261 CrossRef PubMed;
(c) E. Narender Reddy, A. Krishnaiah, C. Madhu and N. J. Babu, RSC Adv., 2014, 4, 1450 Search PubMed.
- C. Madhu, E. Narender Reddy, B. Chiranjeevi, N. Jagadeesh Babu and A. Krishnaiah, Green Chem., 2014, 16, 3237 RSC.
- K. Srinivas, M. VenuChary and V. N. Vuppalapati Srinivasu, Tetrahedron, 2007, 63, 13024 CrossRef.
- ESI†.
- S. Eric, G. Muccioli Giulio, B. Barbara, H. Laurie, M. Regis, H. Jacques Poupaert, H. Jean-Pierre, D. Patrick, J.-F. Goossens and M. Lambert Didier, J. Med. Chem., 2007, 50, 5471 CrossRef PubMed.
- I. Elghamry, Synthesis, 2003, 15, 230 Search PubMed.
- A. Satyapati Devi, S. Kaping and J. N. Vishwakarma, Heterocycl. Lett., 2014, 4, 203 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1056215 and 1056216. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra23706h |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at reflux temperature, gratifyingly obtained 3a and 4a in 1
1) at reflux temperature, gratifyingly obtained 3a and 4a in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Similar results were also observed in water and acetic acid (1
1 ratio. Similar results were also observed in water and acetic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mediated reaction. Fascinated by the exceptional formation of 4 in acetic acid mediated one pot catalyst free reaction in high yields, the generality and scope of this protocol was demonstrated by synthesizing a series of 1,3-dione fused symmetrical 2,8-dioxabicyco[3,3,1]nonane derivatives (4a–t). A wide range of enamino ketones (alkyl, aromatic and hetero aromatic) and alkyl, aryl substituted 1,3-cyclohexanediones were well tolerated under this set of reaction conditions and furnished 4 in high yields.
1) mediated reaction. Fascinated by the exceptional formation of 4 in acetic acid mediated one pot catalyst free reaction in high yields, the generality and scope of this protocol was demonstrated by synthesizing a series of 1,3-dione fused symmetrical 2,8-dioxabicyco[3,3,1]nonane derivatives (4a–t). A wide range of enamino ketones (alkyl, aromatic and hetero aromatic) and alkyl, aryl substituted 1,3-cyclohexanediones were well tolerated under this set of reaction conditions and furnished 4 in high yields.