High temperature ammonia modification of rice husk char to enhance CO2 adsorption: influence of pre-deashing
Xiong Zhang,
Shihong Zhang,
Haiping Yang,
Jingai Shao,
Yingquan Chen,
Ye Feng,
Xianhua Wang and
Hanping Chen*
State Key Laboratory of Coal Combustion, Huazhong University of Science and Technology, 1037 Luoyu Road, Wuhan 430074, Hubei Province, P. R. China. E-mail: fbghust@163.com; Fax: +86-27-87545526; Tel: +86-27-87542417
First published on 7th December 2015
Abstract
To enhance the CO2 adsorption capacity of rice husk char, pre-deashing of raw materials and high temperature ammonia treatment were combined to prepare the nitrogen-enriched char in this work. Two different pre-deashing methods were compared: HCl pre-deashing and HF–HCl pre-deashing treatment. The physicochemical properties and CO2 adsorption capacity of the prepared nitrogen-enriched chars were investigated. The experimental results show that the micropore surface area of nitrogen-enriched char derived from HF–HCl deashed rice husk (HF–HCl–N-Char) is 549.61 m2 g−1, much greater than the HCl deashed (HCl–N-Char) of 313.72 m2 g−1 and the non-deashed (N-Char) of 303.10 m2 g−1. The nitrogen content of HF–HCl–N-Char (3.32 wt%) is also the highest among the three types of chars, while that of HCl–N-Char is only 1.01 wt%, even less than the N-Char (1.64 wt%). In addition, at adsorption temperatures of 30 °C and 120 °C, HF–HCl–N-Char demonstrates the largest CO2 adsorption capacity among the three types of chars, which is 85.90 mg g−1 at 30 °C and 19.27 mg g−1 at 120 °C, respectively. However, compared with N-Char, HCl–N-Char exhibits no obvious increase in CO2 adsorption capacity. This indicates that HF–HCl pre-deashing improves the CO2 adsorption capacity of nitrogen-enriched char, but HCl pre-deashing does not.
1. Introduction
Rice husk is a by-product of rice-milling. Annually about 40 millions of tons is produced; a major source of agricultural waste in China.1 It possesses a high content of organic constituents (i.e., cellulose, hemicellulose and lignin), and is a potential source of renewable energy.2 Unfortunately, only a small portion of the rice husk is used to prepare poultry feed. Most of it is used as bedding material for animals or in direct combustion.3 Those disposal methods cause resource wastes and environmental problems.Thus, more eco-friendly and economical uses of rice husk must be identified. Using it as precursor for preparing nitrogen-enriched biochar by high temperature ammonia treatment (hereafter ammonification) is a relatively new developed method for rise husk reuse.4–9 The derived nitrogen-enriched biochar demonstrates both porous structure and good surface chemical properties, making it an alternative material for CO2 adsorption. In addition, waste nitrogen-enriched biochar that has adsorbed CO2 can be used as organic carbon, nitrogen fertilizer, and nutrient for amending soil quality.10 However, the performance improvement from using ammonification on rice husks is less obvious than that of other precursors (i.e., eucalyptus wood,8 almond shells,11 and cotton stalks12). The main reason for the difference in ammonification performance is that rice husk has a high ash content,13–15 whereas eucalyptus wood, almond shells, and cotton stalks have relatively low ash content.
In general, HF leaching is used to remove ash from rice husk, because its main ash component is silica.16,17 However, HF is effective in eliminating Si, but not Mg, Ca, and Fe, and the deashed rice husk still contains considerable amounts of Mg, Ca, and Fe. The likely mechanism of this phenomenon is that compounds containing Mg, Ca, or Fe are inclined to react with HF to form insoluble or just slightly soluble compounds, such as MgF2, CaF2, or FeF3. These insoluble or slightly soluble compounds remain in the precursor and may block the pore structures, degrading the textual characteristics of the derived biochar. Furthermore, Yin et al.18 also reported that Ca, Al, and Fe minerals in active carbon are disadvantageous for CO2 adsorption.
Accordingly, in this work, two different pre-deashing treatments for developing efficient CO2 adsorbents were compared: HCl pre-deashing and HF–HCl pre-deashing treatment. The main objective of this work is to optimize the modification performance of high temperature ammonia treatment while eliminating the adverse effects of Ca and Fe. In both cases, the final aim is to enhance CO2 adsorption.
2. Materials and methods
2.1. Materials and treatments
Raw rice husks were ground and sieved, and a particle size between 0.1 and 1 mm was selected for further treatment. The first step in the treatment was acid-washing with HCl or HF–HCl at 60 °C for 24 h (details are shown in Table 1). The deashed rice husk samples were then washed with distilled water until the pH reached 7 and dried at 50 °C for 12 h. Then carbonization and ammonification were carried out at 600 °C in N2 (400 ml min−1) for 30 min and at 700 °C in a mixture of N2 and NH3 (N2 = 400 ml min−1, NH3 = 80 ml min−1) for 30 min (referred to as one-step ammonification). Raw rice husk, and rice husk deashed with HCl or HF–HCl were labeled as RH, HCl-RH, and HF–HCl-RH, respectively. The obtained nitrogen-enriched biochars from RH, HCl-RH, and HF–HCl-RH were labeled as N-Char, HCl–N-Char, and HF–HCl–N-Char, respectively. RH was carbonized without ammonification treatment as a control, and the derived char was labeled as R-Char.| Sample | Treatment | Implementation |
|---|---|---|
| HCl-RH | HCl deashing treatment | First, deashing with HCl at a ratio of RH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl (36–38%) HCl (36–38%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 25 g deionized water = 25 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ml 30 ml![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ml. Second, filtrating and washing with distilled water until pH reaches 7 100 ml. Second, filtrating and washing with distilled water until pH reaches 7 |
| HF–HCl-RH | HF–HCl deashing treatment | First, deashing with HF at a ratio of RH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HF (40%) HF (40%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water = 25 g deionized water = 25 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ml 30 ml![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ml. Second, filtrating and washing with distilled water until pH reaches 7. Third, deashing the derived RH with HCl solution (details refer to HCl deashing treatment). Last, filtrating and washing with distilled water until pH reaches 7 60 ml. Second, filtrating and washing with distilled water until pH reaches 7. Third, deashing the derived RH with HCl solution (details refer to HCl deashing treatment). Last, filtrating and washing with distilled water until pH reaches 7 |
2.2. Characteristic analysis
Proximate analysis was used to determine the ash content. The composition of the ash was analyzed by X-ray Fluorescence (XRF) instrument (EAGLE III, EDAX Inc., USA). Field emission scanning electron microscopy (FESEM, Sirion 200, FEI, Holland) coupled with an energy-dispersive X-ray (EDX, EDAX Inc., USA) was used to analyze the morphology and elemental composition of the RHs and chars. The Brunauer–Emmett–Teller surface area (SBET), micropore surface area (Smic), micropore volume (Vmic) and pore size distribution were determined by automatic adsorption equipment (ASAP2020, Micromeritics, USA). Smic and Vmic were calculated using the Dubinin–Radushkevich (DR) method. The pore size distribution was analyzed by the density function theory (DFT). The surface functional groups of samples were characterized using Fourier Transform Infrared Spectroscopy (FTIR, VERTEX 70, Bruker, Germany). The ultimate analysis of the RHs and chars was performed with a CHNS elementary analyzer (Vario Micro Cube, Germany).2.3. CO2 adsorption
The CO2 adsorption–desorption circle of biochars was evaluated in a fixed bed reactor with a thermogravimetric analyzer (TGA, shown in Fig. 1). During the process of adsorption, CO2 was purged (100 ml min−1) through the thermogravimetric system at the targeted adsorption temperature of 30 °C/120 °C for 1 h. The weight increase when exposed to CO2, was defined as the CO2 adsorption capacity of the samples. Once the desorption process began, the CO2 atmosphere was switched to N2 (100 ml min−1) and the sample was kept at 200 °C for 1 h to achieve regeneration.3. Results and discussion
3.1. Ash content and composition analysis
The ash content of the deashed and raw rice husk samples was evaluated using proximate analysis. Results are presented in Table 2. After HCl pre-deashing, the ash percentage (A) clearly increases, while the volatile content (V) significantly decreases. Moisture content (M) and fixed carbon (FC) show negligible change. This phenomenon is consistent with previous studies which demonstrate that the removal performance of HCl is better for volatile components than for other components.19 In contrast to HCl pre-deashing, HF–HCl pre-deashing treatment leads to an apparent reduction in ash content, but a distinct increase in V and FC. This indicates that the deashing effectiveness varies with different treatments.The chemical composition of the ash was obtained by XRF analysis (shown in Table 3). The HCl deashing treatment removes almost all of the inorganic elements, except Si. By contrast, HF–HCl deashing washes out Si effectively, as well as Mg, Ca, and Fe. The MgF2, CaF2, and FeF3 formed during the HF deashing treatment can be removed by the following HCl deashing treatment. A possible mechanism is illustrated by eqn (1)–(3).20–22 Moreover, HF–HCl deashing leads to an apparent increase in S, which is ascribed to the insolubility of CaSO4 in both HF and HCl solutions.
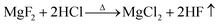 | (1) |
 | (2) |
 | (3) |
| Sample | Composition (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl | K2O | CaO | MnO | Fe2O3 | |
| RH | 0.48 | 1.03 | 91.25 | 0.75 | 1.17 | 0.61 | 2.56 | 1.62 | 0.19 | 0.34 |
| HCl-RH | — | — | 99.96 | — | — | — | — | 0.04 | — | — |
| HF–HCl-RH | — | 1.69 | 11.03 | 3.89 | 50.88 | — | 2.00 | 28.08 | — | 2.43 |
3.2. Textural characterization
| Samples | N2 adsorption | CO2 adsorption | C (wt%) | N (wt%) | |
|---|---|---|---|---|---|
| SBET (m2 g−1) | Smic (m2 g−1) | Vmic (ml g−1) | |||
| RH | 0.79 | 45.93 | 0.018 | 42.04 | 0.38 |
| HCl-RH | 7.67 | 62.61 | 0.025 | 41.52 | 0.22 |
| HF–HCl-RH | 0.76 | 36.85 | 0.015 | 52.81 | 0.41 |
| R-Char | 243.06 | 296.69 | 0.119 | 52.62 | 0.52 |
| N-Char | 231.27 | 303.10 | 0.121 | 53.36 | 1.64 |
| HCl–N-Char | 254.95 | 313.72 | 0.126 | 47.74 | 1.01 |
| HF–HCl–N-Char | 420.01 | 549.61 | 0.220 | 91.86 | 3.32 |
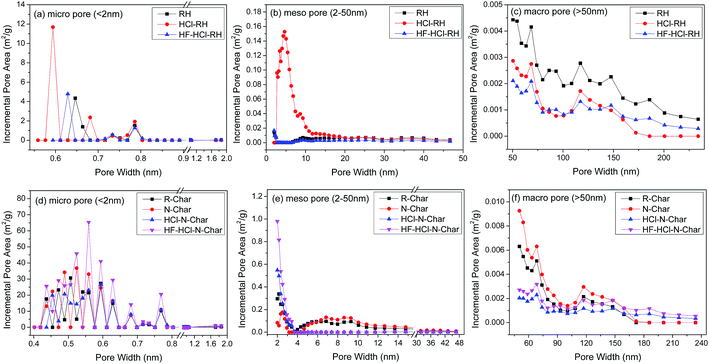
Fig. 3a–c present the DFT pore size distributions of RHs. The narrow micropore (<0.7 nm) and mesopore of HCl-RH are significantly better than those of RH, but the macropore structure is poorer. This indicates that the HCl deashing pretreatment improves the narrow micropore and mesopore of the precursor, but damages the macropore. This phenomenon may be attributed to the cleanup of all inorganic minerals except Si by HCl deashing. Unlike HCl-RH, though the HF–HCl deashing treatment removes most of the minerals, including Si, there is a decreasing trend in the pore structure of HF–HCl-RH. It may be attributed to the destruction of the Si-rich protective layer. The pore size distributions of nitrogen-enriched biochars are illustrated in Fig. 3d–f. The HF–HCl–N-Char exhibits the best pore structure among all chars, whereas the pore structure of the HCl–N-Char has not been improved, and even shows a reducing trend in the narrow mesoporous pores (2 to 4 nm). It can be concluded that although HF–HCl deashing treatment deteriorates the pore structure of the precursor, it is beneficial for the pore structure development of its nitrogen-enriched biochar. Conversely, HCl deashing improves the pore structure of the precursor, but does not facilitate the pore structure development of its nitrogen-enriched biochar.
3.3. Chemical characterization
| Sample | Element (wt%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cs | Ns | Os | Mgs | Als | Sis | Ps | Ss | Cls | Ks | Cas | Mns | |
| a sSurface element. | ||||||||||||
| RH | 18.80 | 2.28 | 33.86 | 0.05 | — | 43.71 | 1.30 | — | — | — | — | — |
| HCl-RH | 18.56 | 3.11 | 39.66 | 0.1 | 0.24 | 37.18 | 0.07 | 0.19 | 0.15 | 0.08 | 0.18 | 0.48 |
| HF–HCl-RH | 56.86 | 1.37 | 40.74 | 0.1 | — | — | 0.1 | 0.15 | 0.12 | 0.15 | 0.12 | 0.29 |
| HCl–N-Char | 35.26 | 2.04 | 17.91 | — | — | 44.57 | 0.05 | 0.03 | — | — | — | 0.12 |
| HF–HCl–N-Char | 90.51 | 5.17 | 3.81 | 0.08 | 0.06 | 0.07 | — | — | — | — | 0.09 | 0.22 |
| Sample | Ca (at%) | Na (at%) | Oa (at%) | Sia (at%) | Na/Ca | Oa/Ca | Sia/Ca |
|---|---|---|---|---|---|---|---|
| a aSurface atomic. | |||||||
| RH | 28.75 | 2.99 | 38.87 | 28.58 | 0.10 | 1.35 | 0.99 |
| HCl-RH | 27.54 | 3.95 | 44.18 | 23.6 | 0.14 | 1.60 | 0.87 |
| HF–HCl-RH | 63.93 | 1.32 | 34.38 | — | 0.02 | 0.54 | — |
| HCl–N-Char | 50.66 | 2.51 | 19.3 | 27.44 | 0.05 | 0.38 | 0.54 |
| HF–HCl–N-Char | 92.38 | 4.52 | 2.92 | 0.03 | 0.05 | 0.03 | 0.0003 |
Tables 5 and 6 also summarize the surface elemental analysis and atomic ratio of HCl–N-Char and HF–HCl–N-Char. Although HCl deashing increases the nitrogen and oxygen contents of the precursor, the surface nitrogen weight and atomic ratio of HCl–N-Char were only 2.04 wt% and 2.51 at%, much less than those of HF–HCl–N-Char. Furthermore, its nitrogen content (1.01 wt%) is the lowest among all nitrogen-enriched biochars (Table 4), even less than N-Char (1.64 wt%). Unlike HCl–N-Char, HF–HCl–N-Char shows greater surface nitrogen weight and atomic ratio, 5.17 wt% and 4.52 at% respectively, and its nitrogen content is also the highest, 3.32 wt%. This indicates that HF–HCl pre-deashing treatment increases the nitrogen content of nitrogen-enriched biochar, and promotes the performance of ammonification, even though it reduces the surface nitrogen and oxygen of the precursor.
Shafeeyan et al.26 suggest that surface oxygen functional groups are important active sites for the introduction of nitrogen functional groups during high temperature ammonia treatment. However, in this work, though HCl deashing increases the surface oxygen functional groups of the precursor, it fails to promote the introduction of nitrogen functional groups. By contrast, HF–HCl deashing treatment decreases the surface oxygen functional groups, but enhances the introduction of nitrogen functional groups. This indicates that although surface oxygen functional groups can provide active sites for the introduction of nitrogen functional groups during the ammonification process, not all of them form these active sites. Further, HF–HCl deashing treatment may increase the effective active oxygen functional groups for the introduction of nitrogen functional groups.
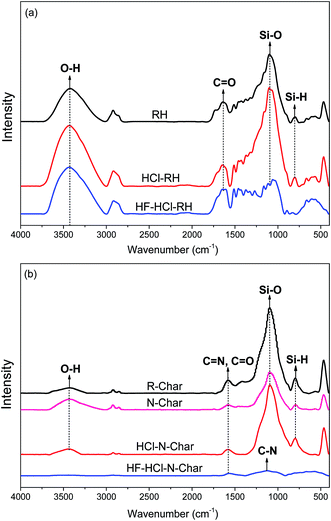
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in ketones and carboxylic acids.5 At the same time, the spectrums of all RHs exhibit differences. For instance, peaks at 1089 cm−1 and 802 cm−1, which can be ascribed to Si–O and Si–H stretching vibrations,28 appear in RH and HCl-RH, but not in HF–HCl-RH. This is because HF deashing can break Si–O and Si–H bonds, but HCl deashing cannot.
O stretching in ketones and carboxylic acids.5 At the same time, the spectrums of all RHs exhibit differences. For instance, peaks at 1089 cm−1 and 802 cm−1, which can be ascribed to Si–O and Si–H stretching vibrations,28 appear in RH and HCl-RH, but not in HF–HCl-RH. This is because HF deashing can break Si–O and Si–H bonds, but HCl deashing cannot.
Fig. 4b depicted the FTIR spectrums of R-Char and nitrogen-enriched biochars. The spectrums of R-Char, N-Char, and HCl–N-Char are almost similar. Peaks at 1100 cm−1 and 802 cm−1 which may be attributed to Si–O and Si–H observed in the precursors may still be observed.29 This indicates that the high temperature ammonia treatment cannot decompose Si–O and Si–H bonds. However, the silicon functional groups hinder the introduction of nitrogen functional groups in ammonification. This can be verified by the fact that the HF–HCl–N-Char contained more nitrogen than the N-Char (Table 4) because of its high efficiency in removing Si, while HCl deashing failed to enhance the introduction of nitrogen functional groups because it failed to remove Si. The spectrum of the HF–HCl–N-Char also demonstrates that Si functional groups obstruct the introduction of nitrogen functional groups. When HF–HCl pre-deashing removes the Si functional groups in the rice husk, O–H (at 3430 cm−1) and aliphatic C–H (at 2920 and 2857 cm−1) of HF–HCl–N-Char break down during ammonification, while the peak at 1126 cm−1, which can be assigned to C–N vibration, becomes stronger.30 In addition, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
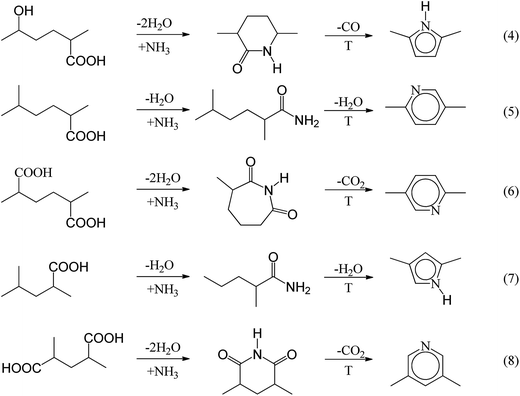
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups (at 1580 cm−1) of HF–HCl–N-Char are slightly reduced compared with the R-Char.31 This is probably because the reactions of ammonia with hydroxyl and carboxylic acids lead to pyrroles and pyridines.32 The reaction mechanism is shown in Fig. 5.
N groups (at 1580 cm−1) of HF–HCl–N-Char are slightly reduced compared with the R-Char.31 This is probably because the reactions of ammonia with hydroxyl and carboxylic acids lead to pyrroles and pyridines.32 The reaction mechanism is shown in Fig. 5.
3.4. CO2 adsorption
| qt = k0t½ + C | (9) |
The Bangham model34 is given as eqn (10):
 | (10) |
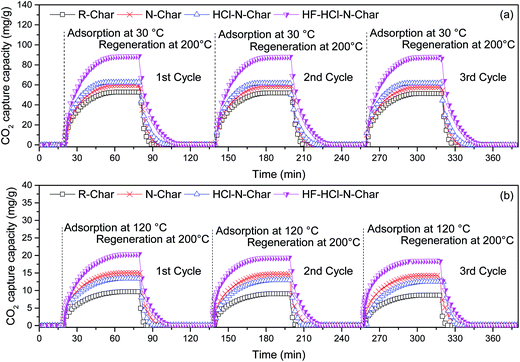
The coefficients of the intra-particle diffusion model and Bangham model for the CO2 adsorption are shown in Table 7. At 30 °C and 120 °C, k0 and qe of N-Char are greater than those of R-Char. This indicates that the ammonification treatment increases the CO2 adsorption rate and capacity of rice husk char. k0 of HCl–N-Char is smaller than that of N-Char, but there is a slight increase in qe at 30 °C. This may be because the diffusion of CO2 into the pores of the rice husk char is not the only rate-controlling step, film and pore diffusion both play important roles in CO2 adsorption.35 HF–HCl–N-Char obtains the maximum value of k0 and qe among all rice husk chars at the adsorption temperatures of 30 °C and 120 °C. When the adsorption temperature is 30 °C, the adsorption process is mainly controlled by physical adsorption and the optimal porous structure of HF–HCl–N-Char greatly improves the speed of pore diffusion.36 When the adsorption temperature increases to 120 °C, the adsorption process is dominated by chemical adsorption, and the improved nitrogen content and surface activity of HF–HCl–N-Char benefit chemical adsorption of CO2.26
| Sample | T (°C) | Intra-particle diffusion model | Bangham model | |||||
|---|---|---|---|---|---|---|---|---|
| k0 (mg (g min1/2)−1) | C (mg g−1) | R2 | qe (mg g−1) | K (min−n) | n | R2 | ||
| R-Char | 30 °C | 16.26 | −59.08 | 0.91 | 52.27 | 3.26 × 10−5 | 3.01 | 0.97 |
| N-Char | 20.86 | −82.25 | 0.92 | 57.35 | 2.28 × 10−6 | 3.81 | 0.96 | |
| HCl–N-Char | 19.55 | −68.95 | 0.87 | 61.95 | 8.07 × 10−6 | 3.47 | 0.96 | |
| HF–HCl–N-Char | 30.63 | −120.56 | 0.93 | 85.90 | 3.53 × 10−6 | 3.67 | 0.97 | |
| R-Char | 120 °C | 2.60 | −8.82 | 0.96 | 9.54 | 2.03 × 10−4 | 2.46 | 0.98 |
| N-Char | 3.94 | −12.56 | 0.93 | 14.81 | 2.50 × 10−4 | 2.44 | 0.98 | |
| HCl–N-Char | 3.45 | −10.87 | 0.94 | 13.23 | 3.11 × 10−4 | 2.37 | 0.98 | |
| HF–HCl–N-Char | 6.15 | −22.75 | 0.96 | 19.27 | 3.24 × 10−5 | 3.02 | 0.97 | |
Table 8 presented a comparison of the CO2 adsorption capacity of the biochars developed in this work with activated carbons and biochars derived from other precursors in the literature. It can be seen that compared to these carbonaceous adsorbents derived from other precursors, for example, almond shells, coffee grounds and rice straw, the biochar derived from raw rice husk with ammonia modification (N-Char) shows poorer CO2 adsorption capacity. However, the biochar derived from HF–HCl deashed rice husk (HF–HCl–N-Char) demonstrates significant improvement in CO2 adsorption capacity (from 57 mg g−1 to 86 mg g−1), making it more competitive to other biochars. It indicates that pre-deashing treatment of rice husk may be helpful to promote the utilization of rice husk in producing carbonaceous adsorbents.
| Adsorbent | Precursor | SBET (m2 g−1) | Temperature (°C) | CO2 adsorption capacity (mg g−1) | Reference |
|---|---|---|---|---|---|
| Activated carbon | — | 1323 | 36 | 76 | 5 |
| Activated carbon | Palm shells | 889 | 30 | 74 | 26 |
| Activated carbon | Peat | 942 | 30 | 97 | 37 |
| Biochar | Almond shells | 822 | 25 | 97 | 38 |
| Biochar | Coffee grounds | 84 | 25 | 62 | 39 |
| Biochar | Rice straw | 122 | 20 | 77 | 40 |
| N-Char | Rice husk | 231 | 30 | 57 | This work |
| HCl–N-Char | Rice husk | 255 | 30 | 62 | This work |
| HF–HCl–N-Char | Rice husk | 420 | 30 | 86 | This work |
4. Conclusions
In this work, HCl pre-deashing treatment and HF–HCl pre-deashing treatment for developing efficient CO2 adsorbents were compared. The corresponding nitrogen-enriched chars demonstrate significant differences in physicochemical properties, which affect the CO2 adsorption performance greatly. The most notable conclusions are summarized as follows:(1) HCl pre-deashing treatment successfully removes Mg, Ca, and Fe from the rice husk, but fails to remove Si, which forms a Si-rich protective layer hindering the pore structure development during one-step ammonification. HF–HCl pre-deashing efficiently removes Mg, Ca, Fe, and Si, which leads to the collapse of the Si-rich layer and greatly improves the pore structure of the derived nitrogen-enriched char. The micropore surface area of HF–HCl–N-Char was 549.61 m2 g−1, much greater than those of N-Char (303.10 m2 g−1) and HCl–N-Char (313.72 m2 g−1).
(2) Though HCl pre-deashing increases the surface oxygen functional groups of the precursor, it fails to enhance the introduction of nitrogen functional groups. By contrast, HF–HCl pre-deashing reduces the surface oxygen functional groups of the precursor, but increases the effective active oxygen functional groups, which introduce nitrogen functional groups. Hence, it promotes the introduction of nitrogen functional groups.
(3) HCl pre-deashing fails to improve the adsorption capacity and adsorption rate of rice husk char, while HF–HCl pre-deashing successfully increases the adsorption capacity and rate at the adsorption temperatures of 30 °C and 120 °C.
Acknowledgements
The authors wish to express their sincere thanks to the financial support from the National Basic Research Program of China (2013CB228102), the National Natural Science Foundation of China (51276075 and 51476067), the Special Fund for Agro-scientific Research in the Public Interest (201303095) and the innovation foundation of Huazhong University of Science and Technology (2014TS118 and CX14-035). The study also benefits from the technical support from Analytical and Testing Center in Huazhong University of Science & Technology (http://atc.hust.edu.cn).References
- S. Hu, J. Xiang, L. Sun, M. Xu, J. Qiu and P. Fu, Fuel Process. Technol., 2008, 89, 1096–1105 CrossRef CAS.
- W. T. Tsai, M. K. Lee and Y. M. Chang, Bioresour. Technol., 2007, 98, 22–28 CrossRef CAS PubMed.
- B. D. Park, S. G. Wi, K. H. Lee, A. P. Singh, T. H. Yoon and Y. S. Kim, Biomass Bioenergy, 2003, 25, 319–327 CrossRef CAS.
- D. Long, M. Wang, J. Wang, W. M. Qiao and L. Ling, RSC Adv., 2014, 4, 61456–61464 RSC.
- J. Przepiórski, M. Skrodzewicz and A. W. Morawski, Appl. Surf. Sci., 2004, 225, 235–242 CrossRef.
- N. Sun, C. Sun, J. Liu, H. Liu, C. E. Snape, K. Li, W. Wei and Y. Sun, RSC Adv., 2015, 5, 33681–33690 RSC.
- C. Chen, J. Kim and W. S. Ahn, Fuel, 2012, 95, 360–364 CrossRef CAS.
- A. Heidari, H. Younesi, A. Rashidi and A. A. Ghoreyshi, Chem. Eng. J., 2014, 254, 503–513 CrossRef CAS.
- J. Yu, Y. Le and B. Cheng, RSC Adv., 2012, 2, 6784 RSC.
- R. S. Jassal, M. S. Johnson, M. Molodovskaya, T. A. Black, A. Jollymore and K. Sveinson, J. Environ. Manage., 2015, 152, 140–144 CrossRef CAS PubMed.
- M. G. Plaza, S. García, F. Rubiera, J. J. Pis and C. Pevida, Sep. Purif. Technol., 2011, 80, 96–104 CrossRef CAS.
- X. Zhang, S. Zhang, H. Yang, Y. Feng, Y. Chen, X. Wang and H. Chen, Chem. Eng. J., 2014, 257, 20–27 CrossRef CAS.
- T. H. Liou and S. J. Wu, J. Hazard. Mater., 2009, 171, 693–703 CrossRef CAS PubMed.
- D. Vamvuka, S. Troulinos and E. Kastanaki, Fuel, 2006, 85, 1763–1771 CrossRef CAS.
- Y. Shen, C. Areeprasert, B. Prabowo, F. Takahashi and K. Yoshikawa, RSC Adv., 2014, 4, 40651–40664 RSC.
- W. M. A. W. Daud and A. H. Houshamnd, J. Nat. Gas Chem., 2010, 19, 267–279 CrossRef CAS.
- L. Wang, J. Xue, B. Gao, P. Gao, C. Mou and J. Li, RSC Adv., 2014, 4, 64744–64746 RSC.
- G. Yin, Z. Liu, Q. Liu and W. Wu, Chem. Eng. J., 2013, 230, 133–140 CrossRef CAS.
- P. Das, A. Ganesh and P. Wangikar, Biomass Bioenergy, 2004, 27, 445–457 CrossRef CAS.
- H. K. Fouad and J. Radioanal, Nucl. Chem., 2010, 285, 193–197 CrossRef CAS.
- D. Ma, Z. Cao, H. Wang, X. Huang, L. Wang and X. Zhang, Energy Environ. Sci., 2012, 5, 8538–8542 CAS.
- N. Louvain, A. Fakhry, P. Bonnet, M. El Ghozzi, K. Guérin, M. T. Sougrati, J. C. Jumas and P. Willmann, CrystEngComm, 2013, 15, 3664–3671 RSC.
- X. Zhang, S. Zhang, H. Yang, J. Shao, Y. Chen, Y. Feng, X. Wang and H. Chen, Energy, 2015, 91, 903–910 CrossRef CAS.
- C. L. Mangun, K. R. Benak, J. Economy and K. L. Foster, Carbon, 2001, 39, 1809–1820 CrossRef CAS.
- O. Ioannidou and A. Zabaniotou, Renewable Sustainable Energy Rev., 2007, 11, 1966–2005 CrossRef CAS.
- M. S. Shafeeyan, W. M. A. W. Daud, A. Houshmand and A. Arami Niya, Appl. Surf. Sci., 2011, 257, 3936–3942 CrossRef CAS.
- H. Yang, Y. Yuan and S. C. E. Tsang, Chem. Eng. J., 2012, 185–186, 374–379 CrossRef CAS.
- F. Ahmad, W. M. A. W. Daud, M. A. Ahmad and R. Radzi, Chem. Eng. Res. Des., 2013, 91, 1028–1038 CrossRef CAS.
- T. H. Liou, Mater. Sci. Eng., A, 2004, 364, 313–323 CrossRef.
- M. S. Shafeeyan, W. M. A. W. Daud, A. Houshmand and A. Shamiri, J. Anal. Appl. Pyrolysis, 2010, 89, 143–151 CrossRef CAS.
- A. Heidari, H. Younesi, A. Rashidi and A. Ghoreyshi, J. Taiwan Inst. Chem. Eng., 2014, 45, 579–588 CrossRef CAS.
- R. J. J. Jansen and H. van Bekkum, Carbon, 1994, 32, 1507–1516 CrossRef CAS.
- V. S. Mane, I. D. Mall and V. C. Srivastava, Dyes Pigm., 2007, 73, 269–278 CrossRef CAS.
- M. S. Bilgili, J. Hazard. Mater., 2006, 137, 157–164 CrossRef CAS PubMed.
- E. Tütem, R. Apak and Ç. F. Ünal, Water Res., 1998, 32, 2315–2324 CrossRef.
- M. G. Plaza, C. Pevida, A. Arenillas, F. Rubiera and J. J. Pis, Fuel, 2007, 86, 2204–2212 CrossRef CAS.
- M. G. Plaza, S. García, F. Rubiera, J. J. Pis and C. Pevida, Chem. Eng. J., 2010, 163, 41–47 CrossRef CAS.
- M. G. Plaza, C. Pevida, B. Arias, J. Fermoso, F. Rubiera and J. J. Pis, Energ. Procedia, 2009, 1, 1107–1113 CrossRef CAS.
- M. Plaza, A. González, C. Pevida, J. Pis and F. Rubiera, Appl. Energy, 2012, 99, 272–279 CrossRef CAS.
- Y. Huang, P. Chiueh, C. Shih, S. Lo, L. Sun, Y. Zhong and C. Qiu, Energy, 2015, 84, 75–82 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |