Superhydrophobic attapulgite-based films for the selective separation of oils and organic solvents from water†
Weidong Liang,
Yuanrui Wang,
Hanxue Sun,
Pinsong Chen,
Zhaoqi Zhu and
An Li*
College of Petrochemical Technology, Lanzhou University of Technology, Langongping Road 287, Lanzhou 730050, P. R. China. E-mail: lian2010@lut.cn
First published on 26th November 2015
Abstract
Superwetting inorganic membranes or films have recently attracted considerable interest for the separation of oils or organics from water. In this work, attapulgite (ATP)-based films consisting mostly of ATP nano-crystals and cellulose fibers from wastepaper were fabricated by a suspension casting method. The as-synthesized ATP-based films exhibited good structural flexibility and tensile ability with σκ values of up to 6.14 MPa. After modification with PDMS, the surface wettability of the ATP-based films was tailored from being amphipathic to being superhydrophobic toward water and superoleophilic toward oils. Taking advantage of the simple fabrication, high mechanical stability and low cost, such superhydrophobic and superoleophilic ATP-based films could be used for the efficient separation of oily or organic contaminants from water and show great potential in such applications as purification, separation, oil spill cleanups and so on.
Introduction
Water pollution derived from crude oil spills, the petrochemical and metallurgical industries, pharmaceutical factories and toxic organic solvents has led to severe pollution on a global scale, and causes numerous environmental and ecological problems.1,2 The development of efficient technologies or new materials that can remove oils or organic solvents from water is of significant importance and is urgently demanded.3–7 Up to now, although a variety of separation technologies including mechanical separation, air flotation, filtration, coagulation, chemical de-emulsification and bioremediation have been developed and well studied,8–12 these methods usually suffer from their respective drawbacks including poor reusability, low oil separation capacity, high operation costs, successive oil/water separation processes and complicated techniques.13 Compared with those traditional separation technologies, membranes or films have the great advantages of a fast separation efficiency, low energy consumption and easy operation. The membrane can be classified as organic or inorganic. Compared with organic membranes, which usually suffer from complicated synthetic procedures, high costs, instability in some harsh practical conditions and the ease with which they are contaminated,14–17 the inorganic membranes have the advantages of thermal and mechanical stability, good regeneration, low cost and so on. In most cases, however, most inorganic membranes show poor selectivity and low efficiency for the separation of organics (especially nonpolar or weakly polar organics) from water due to their hydrophilic nature. In this regard, enhancing the surface wettability or increasing the affinity to organics should be a simple method for enhancing the overall separation performance of inorganic membranes. Up to now, a variety of inorganic membranes with superhydrophobicity and superoleophilicity, including a superwetting MnO2 membrane, a carbon nanofiber film, and a carbon nanotube membrane, have been reported and successfully used for the separation of organics from water.18–20 However, the need for complicated fabrication techniques or the high production costs are the major obstacles that hinder their large-scale practical application.Attapulgite (ATP) is one of the abundant, inexpensive clay minerals which is composed of hydrated magnesium aluminum silicate with a layered chain-like structure. In recent years, attapulgite has attracted considerable attention in different research fields owing to its natural nanochannel structure, large specific surface area, and exchange properties for large organic molecules. In our previous work,21 we developed a new kind of three-dimensional all-inorganic porous ATP adsorbent based on natural clay nanocrystals for the selective separation of organics or oils from water. However, the fabrication of ATP-based membranes for water/oils (or organics) separation has been rarely reported up to now.22,23 Thus, in this work, we fabricated ATP-based films consisting of rod-like ATP nano-crystals and cellulose fibers from wastepaper by a simple suspension casting method. After modification with materials of low-surface-energy, the ATP-based films exhibited superhydrophobic and superoleophilic properties. With advantages such as superwetting wettability, flexibility, and high thermal and mechanical stability, such ATP-based films show great potential in the effective remediation of oil spillages and chemical leakages.
Experimental section
Materials
Natural ATP [Mg5Si8O20(OH)2(OH)4·4H2O], aka palygorskite, supplied by Jiangsu Jiuchuan Nanomaterials Technology Co., Ltd., was treated to afford the purified ATP nano-crystals. Natural ATP was dispersed in distilled water with an appropriate amount of sodium hexametaphosphate and kept for 24 h at room temperature followed by washing with abundant amounts of water. The ATP was soaked in hydrochloric acid (1.0 mol L−1) for 24 h, washed with abundant distilled water and then dried at 70 °C overnight. The powder was finally milled through a 200-mesh screen.The recycled cellulose fibers were prepared from wastepaper (e.g. using commercially available facial tissues and napkin paper etc. made from wood pulp fiber or bamboo pulp fiber). In a typical procedure, the wastepaper was dried at 105 °C to a constant weight, followed by mixing with distilled water with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The mixture was kept for 4 h at room temperature under violent stirring.
50. The mixture was kept for 4 h at room temperature under violent stirring.
Preparation of the ATP-based films
A series of ATP-based films were fabricated according to a simple suspension casting method as shown in Fig. 1a. The purified ATP nano-crystals (6.0 g) were dispersed in distilled water (100 mL) in a 250 mL beaker with vigorous mechanical stirring. After being adequately dispersed, the recycled cellulose fibers suspension (60 mL, 1.2 g dry weight) was added. The mixture was kept under stirring for 30 min and then, taking appropriate suspension, filtrated. As a result, a wet film was obtained (about 90 mm in diameter and with a thickness that could be controlled by adjusting the filtering suspension volume). Subsequently, the film was well pressed by a vertical hydraulic sheeter and dried at 80 °C for 3 h to obtain the ATP-based film. The ratio of ATP to the recycled cellulose fibers was varied from 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 6
1 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Modification of the ATP-based film
The ATP-based film was immersed into a dilute solution of polydimethylsiloxane (PDMS) in toluene (0.25 mg mL−1) and dried in an oven at 80 °C for 12 h. The modified ATP-based film was named a PAT-film.Characterization
The morphology of the samples was examined using a scanning electron microscopy (SEM, JSM-6701F) instrument after coating the material with a Au film. The water and oil contact angle (CA) measurements were performed on a contact angle meter OCA20 (Dataphysics, Germany). X-ray photoelectron spectroscopy (XPS) analysis was performed on a ESCALAB 250Xi spectrometer (Thermon Scientific). The tensile stress and strain of the ATP-based film was measured using the DY-35 universal material testing machine at a ramp rate of 10 mm min−1. The thermal stability of the ATP-based film was studied by means of thermal gravity analysis (TGA) on a thermogravimetry analyzer (TGA/DSC1, Mettler Toledo) from room temperature to 500 °C with a heating rate of 10 °C min−1 and N2 as the carrier gas.Results and discussion
The ATP powder used in this work has a specific surface area of 209 m2 g−1 and a total pore volume of 0.46 cm3 g−1 (Fig. S1†) and contained the elements O, Si, Mg, Al and Ca (Fig. S2†). The micro-morphology of the purified ATP nano-crystals is shown in Fig. 1b. It can be seen that the ATP was composed of a large amount of rod-like nano-crystals with an average diameter of 15–50 nm and a rod length of 1 μm. The recycled cellulose fibers, as shown in Fig. 1c, presented a banded structure and were tens of micrometers in width and less than 1 μm in thickness. We also found that the recycled cellulose fibers have very uneven surfaces and open pores, which could facilitate the ATP nano-crystals entering the spaces in the cellulose fibers during the mixing of the ATP/cellulose fibers. As a result, the surfaces of the cellulose fibers and the open pores between the cellulose fibers were completely filled by the ATP nano-crystals. In combination with the following pressing-molded operation, the ATP and the cellulose fibers were woven together. As shown in Fig. 1d, the ATP-based film exhibited a compact and rough surface which was formed by the aggregation of the ATP nano-crystals and the cellulose fibers at the nanoscale. From the cross-section view (Fig. 1e), the ATP-based film has a very irregular multiple sheet layered structure.The mechanical property of the separation membrane was of great importance for its practical application. Thus, the tensile stress and strain of the as-prepared ATP-based films were investigated. As shown in Fig. 2a, the three kinds of ATP-based film with a different ratio between the ATP and cellulose fibers had similar tensile stress and strain curves. The tensile stress and strain yielded an atypical linear relationship and the strain value increased with the increase of the tensile stress. A maximum tensile stress value was observed for the ATP-based films. The small deformation can be partially or fully recovered with the removal of the tensile stress. A fracture occurred for the ATP-based films after the σκ was achieved, which was associated with a brittle fracture. The ATP-based film with the ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had the maximum σκ value of 6.14 MPa, suggesting the best tensile ability. Thus, the ATP-based film with the ratio of 5
1 had the maximum σκ value of 6.14 MPa, suggesting the best tensile ability. Thus, the ATP-based film with the ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the ATP and the cellulose fibers was chosen as the model for the next study. The ATP-based film also displayed a good structural flexibility and could be bent to a large degree (Fig. 2a inset), having great advantages over those traditional inorganic membrane materials which were usually too fragile and stiff to process.
1 between the ATP and the cellulose fibers was chosen as the model for the next study. The ATP-based film also displayed a good structural flexibility and could be bent to a large degree (Fig. 2a inset), having great advantages over those traditional inorganic membrane materials which were usually too fragile and stiff to process.
The thermal stability of the ATP-based film was investigated by TGA. As shown in Fig. 2b, the ATP-based film showed a very small weight loss below 100 °C, implying a loss of moisture. The obvious weight loss started at 350 °C, attributed to the carbonization of cellulose fibers. The whole weight loss of the ATP-based film was found to be 37.6 wt%, suggesting a high thermal stability.
Surface-coating with materials of low surface free energy has proven an efficient way to achieve a significant improvement of the wettability of materials and their selectivity for oils or organics from water.24 In this work, for the improvement of the surface wettability, the ATP-based film was immersed into a dilute toluene solution containing a precursor of PDMS (a kind of typical low-surface energy material) followed by drying, giving a robust and stable PDMS coating on the surface of the ATP-based film. It is worth pointing out that the PDMS coating barely changed the morphology of the ATP-based film (Fig. S3†) but improved its surface wettability. To investigate the effect of the modification with PDMS on the surface chemical compositions of the ATP-based film, XPS analyses of the ATP-based film before and after the modification with PDMS were conducted (Fig. 3). For the ATP-based film, the peaks at 532.13 eV, 284.93 eV, 153.73 eV and 102.53 eV are attributed to O1s, C1s, Si2s and Si2p, respectively. These peaks also appeared in the PAT-film. However, compared with the ATP-based film, the intensity of the Si2p peak in the XPS spectrum of the PAT-film was increased, which could be attributed to the deposition of the PDMS film on the surface of the PAT-film.25,26
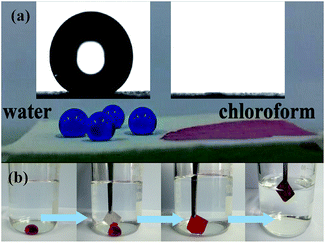
The PAT-film showed a static water CA value of 163° (Fig. 4a (inset) left) and a water sliding angle ca. 4.2°, suggesting an excellent surface superhydrophobic property. Moreover, the PAT-film exhibited a superoleophilic property with a kerosene CA of nearly zero (Fig. 4a (inset) right). Such excellent superhydrophobic and superoleophilic properties of the PAT-film make the water droplets keep mostly spherical and they roll on the surface of the PAT-film. While, the oils or organics (for example chloroform, dyed with Red oil O) were completely immersed into the PAT-film (Fig. 4a). By combination with the SEM and XPS analysis, the surface superhydrophobicity and superoleophilicity of the PAT-film should be attributed to the rough surface composed of the ATP nano-crystals and cellulose fibers at the nanoscale and the hydrophobic PDMS coating on the PAT-film, which are two key factors for the fabrication of superwetting surfaces.27,28 Due to its surface superhydrophobicity and superoleophilicity, the PAT-film can be used directly for the separation of organics from water. When using the PAT-film to touch the chloroform droplets (dyed with Red oil O) under water, the chloroform droplets could be adsorbed by the PAT-film because of its superoleophilic nature, and we did not find any water droplets or even water stains attached on the surface of the film during the whole process (Fig. 4b).
For cleaning organic pollutants from water, Fig. 5 shows the separation process of separating chloroform from water with the PAT-film. On contacting the mixture of chloroform (dyed with Red oil O) and water, the chloroform permeated through the PAT-film and was collected due to the superoleophilicity and micro-nano pores of the PAT-film. However, the water could not get through the PAT-film even after keeping it for 24 h after the separation of the chloroform, because of the superhydrophobic and low water-adhesion properties of the PAT-film. Notably, no water was observed in the collected chloroform or remaining chloroform in the collected water, and the separation efficiency of the PAT-film was calculated to be ca. 91% (ESI†), suggesting a high selectivity and an excellent separation performance of the PAT-film.
 | ||
| Fig. 5 The selective separation process of the PAT-film. Water was dyed with methylene blue and chloroform was dyed with Red oil O for clear observation. | ||
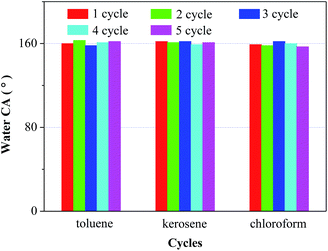
Moreover, the recyclability of a film or membrane plays an important role in evaluating its practical usage performance in oil/chemical cleanup applications. In this case, due to its good thermal stability, the PAT-film fouled by toluene, kerosene and chloroform can be restored through heat treatments at 105 °C, 110 °C and 65 °C, respectively. The water CA of the PAT-film remained above 150°, relating to superhydrophobicity, even after five cycles of the separation/heating test (Fig. 6), which suggested a stable recycling performance and showed promise for practical use. Moreover, the PAT-film exhibited superhydrophobicity towards water with a broad range of pH values. The CA value still maintained above 150° (Fig. S4†), suggesting excellent stability and reusability. These findings suggest that the PAT-film should be an ideal candidate for the separation of oils or organic compounds from water.
Conclusions
ATP-based films consisting of ATP nano-crystals and cellulose fibers from wastepaper were fabricated by a suspension casting method. The ATP-based films exhibited a good tensile ability with σκ values of up to 6.14 MPa and good structural flexibility. After modification with PDMS, the ATP-based films showed superhydrophobicity with a water CA value of 163° and superoleophilicity. With advantages such as simple fabrication, low cost, excellent surface wettability, and high thermal and mechanical stability, such ATP-based films could be used for the efficient separation of oily or organic contaminants from water and be used as ideal candidates in such applications as purification, separation, oil spill cleanups and so on.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (Grant No. 51263012, 51262019, 51462021 and 51403092), Gansu Provincial Science Fund for Distinguished Young Scholars (Grant No. 1308RJDA012), Support Program for Hongliu Young Teachers (Q201411), Hongliu Elitist Scholars of LUT (J201401) and Fundamental Research Funds for the Universities of Gansu Province.Notes and references
- T. Dalton and D. Jin, Mar. Pollut. Bull., 2010, 60, 1939 CrossRef CAS PubMed.
- M. Toyoda and M. Inagaki, Spill Sci. Technol. Bull., 2003, 8, 467 CrossRef CAS.
- H. Sun, F. Wang, P. Liu, C. Wang, B. Sun, Z. Jiang and S. Xiao, Adv. Mater., 2006, 18, 1968 CrossRef.
- H. Bi, X. Xie, K. Yin, L. Zhou, S. Wan, B. He, F. Xu, F. Banhart, L. Sun and R. S. Ruoff, Adv. Funct. Mater., 2012, 22, 4421 CrossRef CAS.
- A. Asatekin and A. M. Mayes, Environ. Sci. Technol., 2009, 43, 4487 CrossRef CAS PubMed.
- P. H. H. Duong and T. S. Chung, J. Membr. Sci., 2014, 452, 117 CrossRef CAS.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Commun., 2012, 3, 1025 CrossRef.
- R. Hosokawa, N. Sakaguchi and H. Okuyama, Int. Biodeterior. Biodegrad., 2010, 64, 519 CrossRef CAS.
- R. C. Prince, R. M. Garrett, R. E. Bare, M. J. Grossman, T. Townsend, J. M. Suflita, K. Lee, E. H. Owens, G. A. Sergy, J. F. Braddock, J. E. Lindstrom and R. R. Lessard, Spill Sci. Technol. Bull., 2003, 8, 145 CrossRef CAS.
- J. Rubio, M. L. Souza and R. W. Smith, Miner. Eng., 2002, 15, 139 CrossRef CAS.
- A. Bayat, S. F. Aghamiri, A. Moheb and G. R. Vakili-Nezhaad, Chem. Eng. Technol., 2005, 28, 1525 CrossRef CAS.
- X. Huang and T. T. Lim, Desalination, 2006, 190, 295 CrossRef CAS.
- D. Wu, Z. Yu, W. Wu, L. Fang and H. Zhu, RSC Adv., 2014, 4, 53514 RSC.
- M. O. Adebajo, R. L. Frost, J. T. Kloprogge, O. Carmody and S. Kokot, J. Porous Mater., 2003, 10, 159 CrossRef CAS.
- M. Toyoda and M. Inagaki, Carbon, 2000, 38, 199 CrossRef CAS.
- V. K. Gupta, P. J. M. Carrott, M. Carrott, M. L. Carrott and D. Suhas, Crit. Rev. Environ. Sci. Technol., 2009, 39, 783 CrossRef.
- J. Zhang and S. Seeger, Adv. Funct. Mater., 2011, 21, 4699 CrossRef CAS.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. Suib, J. Kong and F. Stellacci, Nat. Nanotechnol., 2008, 3, 332 CrossRef CAS PubMed.
- H. Liu, C. Cao, F. Wei, P. Huang, Y. Sun, L. Jiang and W. Song, J. Mater. Chem. A., 2014, 2, 3557 CAS.
- K. Song, A. Gao, X. Cheng and K. Xie, Carbohydr. Polym., 2015, 130, 381 CrossRef CAS.
- W. Liang, Y. Liu, H. Sun, Z. Zhu, X. Zhao, A. Li and W. Deng, RSC Adv., 2014, 4, 12590 RSC.
- J. Li, L. Yan, H. Li, F. Zha and Z. Lei, RSC Adv., 2015, 5, 53802 RSC.
- J. Li, L. Yan, H. Li, F. Zha and Z. Lei, J. Mater. Chem. A, 2015, 3, 14696 CAS.
- L. Feng, S. Li, Y. Li, H. Li, L. Zhang, J. Zhai, Y. Song, B. Liu, L. Jiang and D. Zhu, Adv. Mater., 2002, 14, 1857 CrossRef CAS.
- H. Sun, A. Li, X. Qin, Z. Zhu, W. Liang, J. An, P. La and W. Deng, ChemSusChem, 2013, 6, 2377 CrossRef CAS.
- Z. Fan, X. Qin, H. Sun, Z. Zhu, C. Pei, W. Liang, X. Bao, J. An, P. La, A. Li and W. Deng, ChemPlusChem, 2013, 78, 1282 CrossRef CAS.
- H. Sun, P. La, Z. Zhu, W. Liang, B. Yang, H. Zhao, J. Pei and A. Li, J. Mater. Sci., 2014, 49, 6855 CrossRef CAS.
- X. Bao, J. Cui, H. Sun, W. Liang, Z. Zhu, J. An, B. Yang, P. La and A. Li, Appl. Surf. Sci., 2014, 303, 473 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21791a |
| This journal is © The Royal Society of Chemistry 2015 |