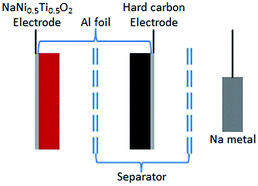
A type of sodium-ion full-cell with a layered NaNi0.5Ti0.5O2 cathode and a pre-sodiated hard carbon anode
Hongbo Wangac,
Yazhou Xiaoa,
Chuang Sunb,
Chao Lai*b and
Xinping Ai*c
aChina Aviation Lithium Battery Co. Ltd., Luoyang 471003, China
bSchool of Chemistry and Chemical Engineering, Jiangsu Key Laboratory of Green Synthetic Chemistry for Functional Materials, Jiangsu Normal University, Xuzhou 221116, China. E-mail: laichao@jsnu.edu.cn
cCollege of Chemistry and Molecular Science, Wuhan University, Wuhan 430072, China
First published on 27th November 2015
Abstract
A new structure of sodium-ion full-cell with a layered NaNi0.5Ti0.5O2 cathode and a pre-sodiated hard carbon anode is reported. The pre-sodiation of the hard carbon anode, achieved via a facile approach in a three-electrode battery, can significantly enhance the initial coulombic efficiency of the sodium-ion full-cell. As a consequence, a much higher capacity is obtained in the hard carbon/NaNi0.5Ti0.5O2 sodium-ion battery. Based on the cathode mass, the full-cell with the pre-sodiated hard carbon anode can exhibit a reversible capacity of 93 mA h g−1.
Lithium-ion batteries are widely applied in portable electronic products and are considered as the best candidates for electric vehicles and hybrid electric vehicles in recent decades.1–3 However, mass production of lithium-ion batteries is hindered by cost and the shortage of lithium resources. In sharp contrast to lithium, abundant sodium resources offer an economic potential for energy storage. Therefore, room-temperature sodium-ion batteries have attracted much attention as large-scale energy storage devices in recent years.4–6
To develop sodium-ion batteries, the novel design of electrodes is still urgent. For anodes in sodium-ion batteries, most work focused on hard carbon materials due to their large interlayer distance and disordered structure, which can facilitate sodium-ion insertion–extraction.7–15 Typical cathode materials are more varied compared with anode materials, such as polyanions and layered transition-metal oxides.13–20 More recently, layered NaNi0.5Ti0.5O2 was proposed as a cathode material which exhibits promising charge–discharge curves in a metal sodium half-cell.21 However, to the best of our knowledge, there is no literature reported on the performance of layered NaNi0.5Ti0.5O2 in sodium-ion full-cells, which is important for its further application.
For the first time, we report a sodium-ion full-cell coupled between a hard carbon anode and a NaNi0.5Ti0.5O2 cathode. Moreover, it is well known that it is difficult for cathode materials to achieve full charge due to the solid electrolyte interphase (SEI) layer forming on the anode surface, mainly leading to a low coulombic efficiency, and the initial unstable SEI always results in a poor cycle life.15,22 In order to address these issues, a lithium pre-doping strategy has been widely used in lithium-ion batteries and lithium-ion hybrid capacitors.23–25 Based on this, for the first time, we employed a sodium pre-doping strategy in an assembled sodium-ion full-cell. As presented in the schematic structure in Fig. 1, a metal sodium foil was used as an extra sodium source and separated by a separator to produce a three-electrode battery. Therefore, sodiated hard carbon could be achieved during the discharge process (sodium-ions insertion) for the metal sodium/hard carbon half-cell. The electrochemical performance of the sodium pre-doping hard carbon/NaNi0.5Ti0.5O2 sodium-ion full-cell then could be evaluated, and a significantly enhanced capacity was obtained.
The XRD pattern of the as-prepared NaNi0.5Ti0.5O2 sample is shown in Fig. 2. All of the diffraction peaks (black lines) can be indexed to the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group with the α-NaFeO2 structure apart from a trace impurity of NiO (marked with *), consistent with reported literature.21 Rietveld refinement was conducted in order to calculate the lattice parameters of the as-prepared layered NaNi0.5Ti0.5O2 material. It is obvious that the calculated XRD patterns from the refined profile (red dots) are in good agreement with the experimental data, for which the refined lattice parameters are a = b = 3.3003(6) Å and c = 16.1410(2) Å.
m space group with the α-NaFeO2 structure apart from a trace impurity of NiO (marked with *), consistent with reported literature.21 Rietveld refinement was conducted in order to calculate the lattice parameters of the as-prepared layered NaNi0.5Ti0.5O2 material. It is obvious that the calculated XRD patterns from the refined profile (red dots) are in good agreement with the experimental data, for which the refined lattice parameters are a = b = 3.3003(6) Å and c = 16.1410(2) Å.
SEM images shown in Fig. 3 demonstrate the morphologies of the as-prepared NaNi0.5Ti0.5O2 sample and commercial hard carbon material. The as-prepared NaNi0.5Ti0.5O2 sample is composed of agglomerate primary particles which form large micro-sized secondary particles (Fig. 3a and b). For the commercial hard carbon shown in Fig. 3c and d, it is an irregular bulk material.
 | ||
| Fig. 3 SEM images of the as-prepared NaNi0.5Ti0.5O2 sample (a and b) and commercial hard carbon material (c and d). | ||
The electrochemical performances of the NaNi0.5Ti0.5O2 cathode and hard carbon anode in sodium-ion half-cells at room temperature were evaluated, as shown in Fig. 4a and b. The NaNi0.5Ti0.5O2 electrode delivers a reversible capacity of 109 mA h g−1 when cycled between 4.1 V and 2.0 V vs. Na/Na+ at a current of 10 mA g−1. The coulombic efficiency of the first cycle is 85.5%, as shown in Fig. 4a. In addition, the initial three charge–discharge curves overlap well, indicating excellent reversible sodium-ion insertion–extraction behavior. The hard carbon electrode exhibits a reversible capacity of 230 mA h g−1 at a current of 20 mA g−1 as shown in Fig. 4b, and the initial coulombic efficiency is 83.8%. The initial irreversible capacity is about 45 mA h g−1, due to the formation of SEI on the hard carbon electrode surfaces, which consists of mainly inorganic species in sodium-ion batteries.26 Fig. 4c is the first charge–discharge curve of the hard carbon/NaNi0.5Ti0.5O2 sodium-ion full-cell. The initial coulombic efficiency is 64.7% with a specific capacity of 82 mA h g−1 based on the cathode active material when testing between 4.0 V and 1.5 V at a current of 10 mA g−1. Considering the obvious irreversibility in the first cycle, we adopted a three-electrode pouch cell as mentioned in Fig. 1 to pre-dope sodium-ions into the anode electrode. The sodium pre-doping was conducted by an electrochemical discharge process of the hard carbon anode using the metal sodium foil as the counter electrode. The current density for pre-sodiation is 10 mA g−1, and the discharge capacity is approximately 45 mA h g−1. A typical voltage profile of the pre-sodiation process for the hard carbon anode is displayed in the inset of Fig. 4c. The first cycle voltage curve for the pre-sodiated hard carbon/NaNi0.5Ti0.5O2 full-cell is also demonstrated in Fig. 4c. In contrast, the initial coulombic efficiency is enhanced to 73.0% with a specific capacity of 93 mA h g−1 based on the cathode mass. The pre-sodiated full-cell shows improved capacity. After the first charge–discharge cycle at a current of 10 mA g−1, the cycle performance is also much improved, as the corresponding capacity retentions are 72.0% and 69.5% after 100 cycles for with and without pre-sodiated full-cells, respectively. The improved cycle performance may originate from a more stable surface passivation layer including SEI forming on the carbon electrode surface in the pre-sodiated hard carbon/NaNi0.5Ti0.5O2 full-cell during the first cycle, which can be ascribed to the fact that the pre-doping process is an effective scavenger for moisture in the system.23 Further work on the optimization of the NaNi0.5Ti0.5O2 sample and development of a high performance sodium-ion battery will be carried out in the near future.
In summary, a novel type of sodium-ion full-cell constructed with a NaNi0.5Ti0.5O2 cathode and a pre-sodiated hard carbon anode is investigated. The pre-doping of sodium ions can be realized via a three-electrode structured battery, in which metal sodium foil is placed behind the carbon anode and the sodiation process is completed by the electrochemical sodium intercalation of the anode using the sodium foil as the counter electrode. After coupling with NaNi0.5Ti0.5O2 cathode, the full-cell delivers a higher capacity and cycle stability in comparison with conventional hard carbon/NaNi0.5Ti0.5O2 sodium-ion batteries. At a current of 10 mA g−1, the battery consisting of the NaNi0.5Ti0.5O2 cathode and pre-sodiated hard carbon anode can exhibit a reversible capacity of 93 mA h g−1 based on the cathode mass, while it is only 82 mA h g−1 without the pre-sodiation process.
Experimental section
Materials
TiO2 powder was obtained from Shanghai Pengbo Titanium Dioxide Co. Ltd., China. Hard carbon material was purchased from Kureha Co., Japan. All other chemicals were used directly without further purification.Preparation of NaNi0.5Ti0.5O2
A conventional high-temperature synthetic approach was employed to prepare the NaNi0.5Ti0.5O2 sample. First, 1.20 g of TiO2 powder was dispersed in 50 mL distilled water under ultrasonication at room temperature for 1 h. 4.37 g of Ni(NO3)2·6H2O and 1.67 g of Na2CO3 (5% excess condition) were added to the suspension under magnetic stirring. After it turned to a uniform sticky suspension, the mixture was transferred to an oven at 80 °C to evaporate the water. Then, the mixture was ground, heated in air to 900 °C in a muffle furnace for 12 h, and transferred to an argon-filled chamber before cooling to 200 °C. Finally, dark-green powder was obtained, sieved by a 200-mesh screen and used in all of the electrochemical and structural studies.Characterization
Powder X-ray diffraction (XRD) was carried out on a Rigaku Miniflex 600 X-ray diffractometer using Cu-Kα radiation. Data was collected over the range of 10° ≤ 2θ ≤ 80° at a scanning rate of 2° min−1. Rietveld refinement of the diffraction pattern was performed using the RIETAN-2000 program. The morphologies of samples were investigated using a ZEISS EVO-18 scanning electron microscope (SEM).Electrochemical measurements
All the electrodes were prepared using a doctor-blade casting process. The NaNi0.5Ti0.5O2 electrode consists of 80 wt% active material, 12 wt% Super P, and 8 wt% polyvinylidene fluoride (PVDF). The hard carbon electrode contains 94 wt% active material, 1 wt% Super P, and 5 wt% PVDF. The current collectors for the cathode and anode electrodes are both metal aluminum foil. The electrodes were dried thoroughly and punched into suitable sizes. In the metal sodium half-cells, circular disks with a diameter of 12 mm were carried out for CR2025 cell testing. In the sodium-ion full-cells, squares with a size of 30 mm × 30 mm were used to fabricate pouch cells and the anode to cathode capacity loading ratio was 1.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A 1.0 M solution of NaClO4 in propylene carbonate was used as the electrolyte and a glass fiber was used as the separator. Electrochemical evaluation was performed on a Land CT2001A cell testing instrument.
1. A 1.0 M solution of NaClO4 in propylene carbonate was used as the electrolyte and a glass fiber was used as the separator. Electrochemical evaluation was performed on a Land CT2001A cell testing instrument.
Acknowledgements
This work was supported by funding from the 863 Program of China (2013AA050901) and National Natural Science Foundation of China (51202094 and 51572116).Notes and references
- E. Karden, S. Ploumen, B. Fricke, T. Miller and K. Snyder, J. Power Sources, 2007, 168, 2 CrossRef CAS.
- M. Armand and J.-M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- P. Poizot and F. Dolhem, Energy Environ. Sci., 2011, 4, 2003 CAS.
- M. Sawicki and L. L. Shaw, RSC Adv., 2015, 5, 53129 RSC.
- H. Pan, Y.-S. Hu and L. Chen, Energy Environ. Sci., 2013, 6, 2338 CAS.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947 CrossRef CAS.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271 CrossRef CAS.
- R. Alcantara, J. M. Jimenez-Mateos, P. Lavela and J. L. Tirado, Electrochem. Commun., 2001, 3, 639 CrossRef CAS.
- R. Alcantara, P. Lavela, G. F. Ortiz and J. L. Tirado, Electrochem. Solid-State Lett., 2005, 8, A222 CrossRef CAS.
- Y. Cao, L. Xiao, M. L. Sushko, W. Wang, B. Schwenzer, J. Xiao, Z. Nie, L. V. Saraf, Z. Yang and J. Liu, Nano Lett., 2012, 12, 3783 CrossRef CAS PubMed.
- Y. Bai, Z. Wang, C. Wu, R. Xu, F. Wu, Y. Liu, H. Li, Y. Li, J. Lu and K. Amine, ACS Appl. Mater. Interfaces, 2015, 7, 5598 CAS.
- M. Dahbi, N. Yabuuchi, K. Kubota, K. Tokiwa and S. Komaba, Phys. Chem. Chem. Phys., 2014, 16, 15007 RSC.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859 CrossRef CAS.
- C.-Y. Yu, J.-S. Park, H.-G. Jung, K.-Y. Chung, D. Aurbach, Y.-K. Sun and S.-T. Myung, Energy Environ. Sci., 2015, 8, 2019 CAS.
- D. Kim, E. Lee, M. Slater, W. Lu, S. Rood and C. S. Johnson, Electrochem. Commun., 2012, 18, 66 CrossRef CAS.
- P. Barpanda, G. Oyama, S.-I. Nishimura, S.-C. Chung and A. Yamada, Nat. Commun., 2014, 5, 4358 CAS.
- X. Zhu, Y. Fang, X. Ai, H. Yang and Y. Cao, J. Alloys Compd., 2015, 646, 170 CrossRef CAS.
- W. Song, X. Ji, Z. Wu, Y. Zhu, F. Li, Y. Yao and C. E. Banks, RSC Adv., 2014, 4, 11375 RSC.
- M. Nose, H. Nakayama, K. Nobuhara, H. Yamaguchi, S. Nakanishi and H. Iba, J. Power Sources, 2013, 234, 175 Search PubMed.
- X. Li, D. Wu, Y.-Y. Zhou, L. Liu, X.-Q. Yang and G. Ceder, Electrochem. Commun., 2014, 49, 51 CrossRef CAS.
- H. Yu, S. Guo, Y. Zhu, M. Ishida and H. Zhou, Chem. Commun., 2014, 50, 457 RSC.
- D. Liu, Y. Wang, Y. Xie, L. He, J. Chen, K. Wu, R. Xu and Y. Gao, J. Power Sources, 2013, 232, 29 CrossRef CAS.
- Y. Li and B. Fitch, Electrochem. Commun., 2011, 13, 664 CrossRef CAS.
- H. Wang, C. Lai, Y. Zhou and X. Ai, Mater. Lett., 2015, 160, 250 CrossRef CAS.
- X. Sun, X. Zhang, H. Zhang, N. Xu, K. Wang and Y. Ma, J. Power Sources, 2014, 270, 318 CrossRef CAS.
- S. Komaba, T. Ishikawa, N. Yabuuchi, W. Murata, A. Ito and Y. Ohsawa, ACS Appl. Mater. Interfaces, 2011, 3, 4165 CAS.
| This journal is © The Royal Society of Chemistry 2015 |