Ultralow friction regime from the in situ production of a richer fullerene-like nanostructured carbon in sliding contact
Yongfu Wangabc,
Junmeng Guoc,
Junyan Zhang*a and
Yong Qinbd
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, P. R. China. E-mail: zhangjunyan@licp.cas.cn; Tel: +86-931-4968295
bInstitute of Nanoscience and Nanotechnology, Lanzhou University, Lanzhou 730000, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
dThe Research Institute of Biomedical Nanotechnology, Lanzhou University, Lanzhou 730000, China
First published on 1st December 2015
Abstract
Hitherto, among carbon-based thin films, fullerene-like hydrogenated carbon (FL-C:H) films exhibit unique ultralow friction and wear in open or humid air, but the mechanisms responsible for the friction regime are still not clear. Here, we provide definitive experimental evidences obtained from the wear tracks and debris of FL-C:H films, and show that FL-C:H films’ surface undergoes gradual transformation into a richer and more stable fullerene-like nanostructure, due to the increasing content of pentagonal and heptagonal carbon rings in addition to the six-membered graphene rings, as a result of thermal and strain effects from the repeated friction. The sliding-induced in situ promotion of the FL structures at frictional contact leads to ultralow friction and wear in open air. The results establish an excellent low friction and wear regime for the structural film, and develop the lubrication mechanisms of carbon-based films.
1. Introduction
A sustainable society is a long-term target being chased by human beings, which is to some extent dependent on energy efficiency. In machines, a large amount of energy is generally consumed to overcome the friction of moving parts. Novel lubricant materials are desired to reduce the friction of machines, and among them, carbon-based thin films such as diamond-like carbon (DLC) thin films are considered as potential next generation solid lubricants, and it is found that the construction of nanostructures bestow carbon thin films with excellent low friction behaviors. For instance, although hydrogenated diamond-like amorphous carbon (a-C:H) films exhibit excellent friction and wear resistance and extended lifetime in vacuum or inert gas, their frictional properties remain poor in the presence of humidity or oxygen, thereby limiting their further applications since machines are generally used in open air.1–4 But designing special structures such as fullerene-like (FL) ones5–9 and composite structures10,11 can improve their performances. Recently, an important advancement in these areas is the discovery of FL nanostructures in hydrogenated carbon thin films. The shape of the structures is represented by the curved fragments with five- and seven-membered carbon rings in addition to six-membered rings, and it is similar to those found in carbon nanotubes and bucky onions. The FL-C:H film,8,9,12 with a high hardness (19–26 GPa) and extremely high elastic recovery (75–95%), exhibits ultralow friction (10−3) and wear in humid environments, but the mechanism responsible for the ultralow friction behaviors of these films is not well understood.Hitherto, even if DLC films contain special nanostructures, such as fullerene-like nanostructures,8,13 their tribological studies have fixed patterns, and their low friction regimes have been attributed to the friction-induced rehybridization of an interfaced amorphous structure as well as to the passivation of surface dangling bonds. Passivation is proposed because friction and wear for DLC films are lower in environments containing H2, H2O and glycerol, than in a vacuum and inert gas.14–16 A similar mechanism has also been reported for the lubrication of ultrananocrystalline diamond by water molecules.17 However, these molecules are not able to totally passivate dangling bonds, especially in situations where dissociation and removal of surface termination occurs during sliding.18,19 In other words, sliding inevitably induces the sp3 → sp2 rehybridization of carbon. An sp2-rich tribofilm forming on the sliding surface of DLC films has been observed in experiments18,20–26 and simulations.27,28 It is believed that the richer sp2-bonded carbon atoms compared to the starting carbon-based materials are grouped into amorphous (rehybridization) or layered graphite-like (graphitization) structures. But the fixed patterns lead to a lack of detail on how FL nanostructures may evolve under friction and how they may affect the ultralow friction and wear behavior of FL-C:H films. For example, are they graphitized or involved in the formation of other lubricious structures?
Previously, FL nanostructures within FL-C:H films have been shown to contribute to ultralow friction and wear in open air.29 However, an in situ formed tribofilm is always present at the sliding interface, which is known to be a key factor to control the frictional properties of the films. This underlines the need to characterize not only the film bulk composition, but also the interfacial structure of the tribofilm, for use in practical tribological applications. Here, we provide definitive experimental evidence, and show that the richer sp2-bonded carbon atoms compared to the starting FL-C:H film evolve towards richer FL structures. We think that the ultralow friction comes from both the inherent FL structure within the films and the newly formed FL structures within the tribofilm, apart from environmental conditions. We expect that the result can enrich the understanding of FL-C:H films’ lubrication mechanism and develop the lubrication mechanism of carbon-based films.
2. Experimental
2.1 Film preparation
FL-C:H films were produced on Si (001) substrates using a direct current plasma chemical vapor deposition system. Prior to the deposition, the deposition chamber was pumped down to 10−4 Pa followed by introducing a mixed gas of methane and hydrogen in the feedstock. The mixed gas was CH4 = 10 SCCM, and H2 = 0, 2.5, 5, 7.5 and 10 SCCM. The distance between the two electrodes was fixed at about 5 cm, with a DC negative voltage of 800 V. The thickness is about 500 nm.2.2 Characterization of wear tracks and debris
Friction tests were performed on a reciprocating ball on-disc tribotester in open air and at room temperature (25 °C). The Al2O3 balls were loaded against the films by the normal force of 20 N generating a contact pressure of about 2000 MPa, the sliding speed was fixed at 60 cm s−1, and the amplitude was 5 mm. The diameter of all balls was 5 mm, and their surface roughness was 0.052 μm. The sliding time was 30 min. Such load and speed conditions in humid air with a relative humidity (RH) of about 20% drive the system into a realistic working state. The HRTEM images and Raman spectra of the wear tracks and debris were revealed by HRTEM (FEI Tecani F30) and a Raman spectrometer (Jobin-Yvon HR-800) with an excitation wavelength of 532 and 632 nm respectively. Due to the debris floating on the surface of deionized water, the TEM samples were prepared by dipping the holey Cu grids into the water and scooping out the debris from the surface. The chemical states of the films were determined using PHI-5702 multifunctional X-ray photoelectron spectroscopy (XPS, operating with Al Kα radiation and a detecting chamber pressure of below 10−6 Pa). The XRD patterns were collected by powder X-ray diffraction (XRD, D/max2550HB+/PC, 40 kV, 450 mA, with Cu KR (λ = 1.54 Å)).3. Results and discussion
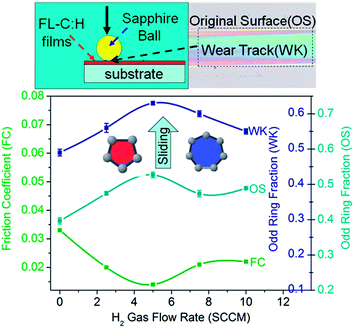
In this work, FL-C:H films on Si (001) substrates were deposited by a plasma-enhanced chemical vapor deposition technique using CH4 and H2 as feedstock.29 An analysis method of their Raman spectra is introduced that permits the amorphous and FL nanostructures of these films to be distinguished.12,30,31 This method consists of four vibrational bands centred at about 1200, 1380, 1470 and 1560 cm−1, three with A-type symmetry (from five- (5A1), six- (6A1g), and seven-membered rings (7A1)) and the last one with E-type symmetry (from six-membered rings (6E2g)),32–34 respectively. These rings are present in fullerenes and FL carbon, and the 1200 cm−1 mode usually has a companion mode at around 1480 cm−1.30 The total relative intensity fraction of 7A1 and 5A1 is a measure of FL nanostructures,29,31,35 as curvature and cross-linking are caused by the introduction of odd membered rings in an otherwise hexagonal carbon structure.6,36 The films at 5 SCCM have ultralow friction and wear (0.011 and 1.48 × 10−8 mm3 Nm−1).29 The effect of H2 flow rate on the structure and frictional behavior is discussed in a manner described elsewhere.293.1 Characteristics of the wear tracks of the as-deposited FL-C:H films
As shown in Fig. 1b, a new and low peak near 710 cm−1 is observed in the wear tracks, but seems to be non-existent in the originally deposited surface (OS5). This peak is previously observed in the Raman spectra acquired from the curvature in graphitic planes and carbon nano-onions.12,37–39 Due to the presence of the near 1200 cm−1 peak for OS5 and the central wear tracks of the films, their 532 nm Raman spectra in the region from 1000 to 2000 cm−1 are decomposed into four Gaussian peaks at about 1230, 1365, 1470 and 1560 cm−1, respectively corresponding to 7A1, 6A1g, 5A1 and 6E2g, as introduced in the method above. The total relative intensity fraction of 7A1 and 5A1 is a measure of FL nanostructures, and thus the odd carbon ring fraction in the samples is summarized in Fig. 2. After sliding friction, the odd ring fraction (62%) in the wear tracks is higher than that (52%) of OS5, indicating that the structural changes may be the promotion of their own FL nanostructures during sliding. Moreover, each of the FL-C:H films prepared at 0, 2.5, 5, 7.5 and 10 SCCM exhibit lower friction, and the related wear track has a higher odd ring fraction than its originally deposited surface. Therefore, the Raman results from the wear tracks may infer that an ultralow friction coefficient measured for the films may simply be a consequence of the creation of FL nanostructures during sliding.3.2 Characteristics of the wear debris of the as-deposited FL-C:H films
At the beginning of sliding tests, wear materials containing FL nanostructures mainly deposited in front of the sliding ball and later covered the main part of the contact area,40,41 resulting in the formation of a tribolayer. After ultimate sliding, the tribolayer still adhered to the ball surface. When the ball surface with the tribolayer slid on a new slip way of the films, the tribolayer was detached from the counterface as a result of plough of protruding regions of the new asperity contacts, or extruded out of the contact interface during sliding, and thus wear debris was formed (Fig. 3a). Among sliding, the tribolayer was subject to friction heat and shear strain effects. If possible, the films’ tribo-chemical and structural changes may be triggered, and the extent of reaction or rehybridization can vary with the subjected time. In other words, wear debris around wear track is an important representation of the films’ interfacial structure changes. All friction experimental results were repeatedly performed more than three times, and the mean friction coefficient was 0.011.In order to gain more insight into the interfacial structure changes of the FL-C:H films, XPS analysis has been performed. The C 1s spectra of the films deposited at 5 SCCM, shown in Fig. 3a and b, can be deconvoluted into three bands at around 284.3, 285.3, and 286.1 eV. The bands are respectively assigned to sp2 bonds in graphite, sp3 bonds in diamond and C–O bonds formed on the film surface due to the free σ-bonds passivated by adsorbates such as water and oxygen molecules in open air.42,43 The relative content of each bond is listed in Table 1. As a result of cyclic sliding, the sp2 fraction obviously rises from 65.0% in the original surface to 80.4% in the wear debris, and the sp3 fraction decreases from 27.2% to 10.7%, but the C–O bonds’ fraction slightly increases from 7.8% to 8.9%. The low oxidation during the friction process in humid air is consistent with the studies in which FL-C:H films have less sensitivity to H2O and O2 molecules in air.8,29,41,44 FL-C:H films are known to have high hardness and elastic recovery originating from their unique FL nanostructures. Thus, under friction, the structures with rich sp2 bonds can stably exist rather than be broken at a longer time after the beginning of sliding, due to elastic deformation by bond angle deflection.44 This indicates that during sliding, the sp3-hybridized bonds are preferentially broken and primarily converted to sp2-hybridized bonds but not free σ-bonds within the film tribo-surface, shown as follows:
| C(sp3 bond) → primary C(sp2 bond) | (1) |
| FL-C:H film | ||
|---|---|---|
| Original surface (OS5) | OS5 + wear debris | |
| sp2 content (%) | 65.0 | 80.4 |
| sp3 content (%) | 27.2 | 10.7 |
| C–O content (%) | 7.8 | 8.9 |
| C 1s position (eV) | 284.47 | 284.36 |
| Friction | 0.011 | |
What is a primary C(sp2 bond)? Where is it formed? Is it formed at the tribolayers’ exposed surface, or within the tribolayers? Is it grouped into amorphous (rehybridization) or layered graphite-like (graphitization) structures mentioned in the introduction? X-ray diffraction (XRD) analysis has been performed at the original surface (OS5) and wear debris and tracks of the FL-C:H films, to obtain further information about their chemical composition and structure (Fig. 4). The film pattern shows three peaks at about 2θ = 69.1°, 33° and 22.4°. The two peaks at 2θ = 69.1° and 33° are from the silicon substrate, i.e., Si (004) and Si (002), respectively. The weak peak at 2θ = 22.4° is consistent with these studies12,29 which revealed the presence of fullerene-like or onion-like nanoparticles using HRTEM and Raman peaks near 400, 710 and 1200 cm−1. Provided that this peak corresponds to the (002) reflection, it shows a significantly downward shift compared to the normal graphite at 2θ = 26.5°. It decreases with the increase of the curvature of the graphene layers,45 as is expected for highly curved sheets with many non-six-membered rings. After sliding, the peak about 2θ = 22.4° becomes obvious, while a new broad peak around 2θ = 15° shows up. The peaks stem from the creation of pentagonal and heptagonal carbon rings with partial enrichment of the FL structures in the wear debris and tracks, because the characteristic peaks of 3D sp2-coordinated carbon materials, such as C60 or C70 including odd carbon rings, are mainly distributed in the range below 30°.46,47 In fact, diffraction methods do not provide unequivocal evidence that pentagons and heptagons are present. Nevertheless, in the range below 25°, fullerenes, nanotubes and carbon onions containing these odd rings have characteristic diffraction peaks but graphite has no such features. Therefore, it may be inferred that under friction, a C(sp2 bond) evolves toward crystalline order, analogous to a fullerene structure, though such order is poorly crystallized. The tribo-chemical reaction is shown as follows:
| C(sp3 bond) → primary C(sp2 bond) → pentagons and heptagons | (2) |
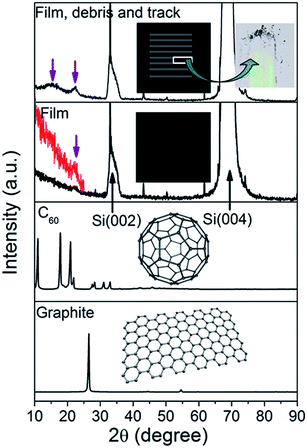 | ||
| Fig. 4 Experimental XRD patterns from the films (OS5) and wear debris showing that the debris has a structure analogous to that of C60, far from that of nc-graphite. | ||
Raman spectroscopy is an effective way to distinguish fullerene-like nanostructures within hydrogenated carbon films, due to its sensitivity to pentagonal and heptagonal rings.29,31,35 In this paper, we have tried to superimpose the Raman signal of the debris on the films’ original surface signal. Fig. 5a shows the wear debris accumulated at the surrounding area of the wear tracks. The debris volume increases from D51 to D54 in sequence. When the OS5 and wear debris at D51 are observed (in Fig. 5b), both the spectra exhibit similar profiles, due to the fact that there is too little D51 debris to influence the Raman signal of OS5. However, as the debris at D52 and above is detected, the Raman shapes are quite different from that of OS0, suggesting a structural transition. From Fig. 5d, the G peak dramatically shifts from 1527 at D51 to 1556 cm−1 at D54, and the ID/IG shifts towards a higher value in order from 1.61 at D51 to 2.65 at D54. These are indicative of the conversion of sp3- to sp2-bonded carbon during sliding, consistent with the XPS result above.
These are usually not final results, however, some interesting potential data have not been exposed. For example, apart from those observed peaks (710, 850 and 1200 cm−1) and a new peak at around 520 cm−1 from the silicon substrate,30 two additional peaks near 400 and 1050 cm−1 appear in all the Raman spectra from the wear debris of the FL-C:H films deposited at 5 SCCM (Fig. 5b), and are more obviously observed in Fig. 5c. A similar band also appears in the Raman spectra of nanoparticle carbon nitride films37 and carbon nano-onions.38 Recently, we investigated in detail the Raman spectra of fullerene-like carbon nitride (FL-CNx)30 and FL-C:H films12 and confirmed that the peak at about 400 cm−1 is attributed to FL or onion-like nanostructures. Interestingly, with extended analysis of the Raman spectra from the wear debris, the odd ring (7A1 and 5A1) fraction increases from 52% at OS5 to 70% at D54. Combined with the higher odd ring fraction in the central wear tracks than in the corresponding original surfaces of the FL-C:H films grown at 0, 2.5, 5, 7.5 and 10 SCCM (Fig. 2), it is inferred that the sp3-bonded carbon atoms in the films undergo tribo-chemical reactions whereby they may not simply form sp2-bonded carbons but probably form pentagonal and heptagonal carbon rings across the interface, leading to the promotion of the FL nanostructures and the decrease of the friction.
Fig. 6 shows 632 nm Raman spectra taken from the wear debris particles present on the 5 SCCM FL-C:H films. The spectra show changes in shape and intensity similar to the 532 nm spectra. Some additional peaks at about 490, 710, 860, 1050, 1200 and 1360 cm−1 can be distinguished as broad shoulders or as local maxima, especially for D4 and D5. The spectra in the range of 1000–2000 cm−1 were evaluated by a Gaussian fit with four Gaussian peaks at about 1230, 1365, 1470, and 1560 cm−1. These peaks from pentagons (5A1) and heptagons (7A1) are more obvious and intense from Fig. 6a–d, indicating the formation of richer FL structures with a higher content of pentagons and heptagons compared to the starting film.
In addition, the tribo-chemical reaction of C(sp3-bond) → pentagons and heptagons (eqn (2)) can be further confirmed by the 532 nm Raman analysis of the films and wear debris at 0 SCCM (Fig. 7a–d). The films have the lowest FL content (Fig. 2). From Fig. 7d, the G peak dramatically shifts from 1525 at D01 to 1547 cm−1 at D04, and ID/IG shifts towards a higher value in the order from 1.57 at D01 to 2.38 at D04. These are indicative for the conversion of sp3- to sp2-bonded carbon during sliding. From extended analysis of the Raman spectra from the wear debris, the odd ring (7A1 and 5A1) fraction increases from 40% at OS0 to 67% at D04 (Fig. 7d), and additional peaks near 400 and 1050 cm−1 compared to the starting film are observed.
HRTEM has been performed to confirm the interfacial structure changes of the FL-C:H films at 5 SCCM. Fig. 8a shows the film wear debris around the track, and the superposition and agglomeration of the wear debris due to the rubbing process can be observed by comparing the sets in Fig. 8b and c. Fig. 8b shows that the FL nanostructures are different from both graphite and turbostratic carbon. The basal planes in the structure were covalently curved and cross-linked by tetrahedral sp3 bonds which have a much shorter bond length than van der Waals bonds.5,48 The planes are therefore unlike the normally flat sp2-hybridized planes of graphite. From Fig. 8c, the restacking of disordered FL-C clusters in thicker regions confuses the image and prevents clear observation of the curved sheets. Nevertheless, an obviously increasing number of curved sheets are observed in the direction of the arrow (Fig. 8c), and in thinner areas (Fig. 8d). From Fig. 8d, we can also see open spherical particles much like carbon onion nanoparticles. The broken curved lines of the particles may mean that it has remaining amorphous carbon and lacks the lamellar structure of well-crystallized onions.
Given the above, we reveal that friction inevitably induces interfacial structure changes of FL-C:H films, and with the continuous sliding of the film against Al2O3 under a contact pressure of about 2000 MPa and a sliding speed at 60 cm s−1, there are an increasing number of sp2-bonded carbon atoms compared to at the starting film grouped into FL nanostructures with richer pentagons and heptagons, as shown by eqn (3) and in Fig. 9. The newly formed FL structures within the tribofilm, with the film’s inherent FL structure, contribute to the ultralow friction and wear (0.011 and 1.48 × 10−8 mm3 Nm−1).29 We expect that the results can enrich the understanding of FL-C:H films’ lubrication mechanism and develop the lubrication mechanism of carbon-based films.
| C(sp3 bond) → primary C(sp2 bond) → pentagons and heptagons → FL structures | (3) |
3.3 The friction-induced structural evolution analysis of the as-deposited FL-C:H films
We report on the structural changes within the FL-C:H films as a result of cyclic annealing and strain relaxation during sliding. Simulations49 have proven highly successful in understanding the formation mechanism of FL structured carbon, whereby a variety of precursors including amorphous carbon films and even nanodiamonds form large, well-aligned and closed structures if a sufficient annealing temperature and annealing time are provided. Experimentally, modest thermal annealing (from 300 °C) of amorphous carbon films containing considerable fractions of sp2 carbon has resulted in the formation of carbon nanocomposites with the evolution of five- and seven-membered rings.34 Moreover, FL-C:H films are shown to be annealed at 200 and 300 °C in a vacuum, the structures of the films will be reorganized with the creation of odd rings, and the films will be endowed with excellent tribological properties.35 In this paper, the flash temperature at the sliding contact area is tentatively estimated as 200–1250 °C during the first cycle, according to the literature.50–52 Repeated sliding friction is expected to further raise the temperature, probably up to the point where nanostructured sp3 clusters transform to curved sp2 clusters, i.e. FL structures.Besides, frictional shear inevitably causes dissociation and removal of surface termination (for example, hydrogen removal),16,18,19 and high local stress during initial sliding.27 This drives the formation of dangling σ-bonds or radicals from the cleavage of C–C or C–H bonds on the top layer of the films,53 and the atomic migration or rearrangements at the friction interface region.27 In the presence of water, these radicals chemisorb water molecules to form hydroperoxide groups (–COOH) by a series of tribo-chemical interactions.53 Meanwhile, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds at the edge of the FL clusters react with the O and OH radicals (atmospheric), and thus transform into –C–OH groups. The friction load brings the –COOH and –C–OH groups closer, leading to the trigger of reactions as ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes54 and the formation of six-membered graphene rings at the edge of FL clusters. So, the graphite-like sheets at the edge of the clusters are extended. The highly strained environment within the tribolayer will force curvature in large extended graphite-like sheets, and thus pentagons and heptagons are grouped into the sheets, leading to structure relaxation of the interfacial C–C network, analogous to their stress model in the films;31 at this stage, these rings may favor the passivation of edge dangling bonds in the sheets,55,56 reducing adhesive interaction between two sliding surfaces. Additionally, along with repeated sliding, an increasing sp2 content is directly related to a lower density, which further gives the tribolayer lower compressive stress surroundings. As a result, cross-linking among the planes and distortion of the graphite-like sheets by odd rings is produced gradually, and finally a richer FL structural carbon network is formed. When this network resides in the midst of the interfacial region, it forms a lubricating layer, which leads to low friction and small wear in open air.
C bonds at the edge of the FL clusters react with the O and OH radicals (atmospheric), and thus transform into –C–OH groups. The friction load brings the –COOH and –C–OH groups closer, leading to the trigger of reactions as ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes54 and the formation of six-membered graphene rings at the edge of FL clusters. So, the graphite-like sheets at the edge of the clusters are extended. The highly strained environment within the tribolayer will force curvature in large extended graphite-like sheets, and thus pentagons and heptagons are grouped into the sheets, leading to structure relaxation of the interfacial C–C network, analogous to their stress model in the films;31 at this stage, these rings may favor the passivation of edge dangling bonds in the sheets,55,56 reducing adhesive interaction between two sliding surfaces. Additionally, along with repeated sliding, an increasing sp2 content is directly related to a lower density, which further gives the tribolayer lower compressive stress surroundings. As a result, cross-linking among the planes and distortion of the graphite-like sheets by odd rings is produced gradually, and finally a richer FL structural carbon network is formed. When this network resides in the midst of the interfacial region, it forms a lubricating layer, which leads to low friction and small wear in open air.
4. Conclusions
In summary, we provide definitive experimental evidence obtained from the wear tracks and debris of FL-C:H films, and show that a richer FL nanostructure tribolayer compared to the starting film residing in the midst of an interfacial region leads to ultralow friction and wear in open air. The cyclic annealing and strain relaxation induced by sliding is attributed to an increase in sp2 bonding and the pentagonal and heptagonal carbon ring fraction, leading to the promotion of a 3D fullerene-like nanostructure within the film surface. The results can enrich the understanding of the lubrication mechanism of FL-C:H films.Acknowledgements
Y. W. and J. Z. designed the study and wrote the paper. Y. W. prepared the FL-C:H films and analyzed the resulting data. Y. W. and J. G. collected the data. All authors commented on the manuscript and discussed the results. This work was supported by the Major State Basic Research Development Program of China (973 Program) (No 2013CB632304).References
- A. Erdemir and C. Donnet, Tribology of diamond-like carbon films: recent progress and future prospects, J. Phys. D: Appl. Phys., 2006, 39(18), R311–R327 CrossRef CAS.
- J. Jiang, S. Zhang and R. D. Arnell, The effect of relative humidity on wear of a diamond-like carbon coating, Surf. Coat. Technol., 2003, 167(2–3), 221–225 CrossRef CAS.
- W. Zhang and A. Tanaka, Tribological properties of DLC films deposited under various conditions using a plasma-enhanced CVD, Tribol. Int., 2004, 37(11–12), 975–982 CrossRef CAS.
- J. Andersson, R. A. Erck and A. Erdemir, Friction of diamond-like carbon films in different atmospheres, Wear, 2003, 254(11), 1070–1075 CrossRef CAS.
- H. SjÖstrÖm, S. StafstrÖm, M. Boman and J.-E. Sundgren, Superhard and elastic carbon nitride thin films having fullerenelike microstructure, Phys. Rev. Lett., 1995, 75, 1336–1339 CrossRef PubMed.
- I. Alexandrou, H.-J. Scheibe, C. J. Kiely, A. J. Papworth, G. A. J. Amaratunga and B. Schultrich, Carbon films with an sp2 network structure, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 10903–10907 CrossRef CAS.
- J. G. Buijnsters, M. Camero, R. Gago, A. R. Landa-Canovas, C. Gómez-Aleixandre and I. Jiménez, Direct spectroscopic evidence of self-formed C60 inclusions in fullerene-like hydrogenated carbon films, Appl. Phys. Lett., 2008, 92, 141920 CrossRef.
- C. Wang, S. Yang, Q. Wang, Z. Wang and J. Zhang, Super-low friction and super-elastic hydrogenated carbon films originated from a unique fullerene-like nanostructure, Nanotechnology, 2008, 19, 225709 CrossRef PubMed.
- X. Liu, J. Yang, J. Hao, J. Zheng, Q. Gong and W. Liu, A near-frictionless and extremely elastic hydrogenated amorphous carbon film with self-assembled dual nanostructure, Adv. Mater., 2012, 24(34), 4614–4617 CrossRef CAS PubMed.
- F. Zhao, H. X. Li, L. Ji, Y. F. Mo, W. L. Quan, W. Du, H. D. Zhou and J. M. Chen, Superlow friction behavior of Si-doped hydrogenated amorphous carbon film in water environment, Surf. Coat. Technol., 2009, 203(8), 981–985 CrossRef CAS.
- J. Jiang, J. Hao, P. Wang and W. Liu, Superlow friction of titanium/silicon codoped hydrogenated amorphous carbon film in the ambient air, J. Appl. Phys., 2010, 108(3), 033510 CrossRef.
- Q. Wang, C. Wang, Z. Wang, J. Zhang and D. He, Fullerene nanostructure-induced excellent mechanical properties in hydrogenated amorphous carbon, Appl. Phys. Lett., 2007, 91, 141902 CrossRef.
- J. G. Buijnsters, M. Camero, L. Vázquez, F. Agulló-Rueda, R. Gago, I. Jiménez, C. Gómez-Aleixandre and J. M. Albella, Tribological study of hydrogenated amorphous carbon films with tailored microstructure and composition produced by bias-enhanced plasma chemical vapour deposition, Diamond Relat. Mater., 2010, 19, 1093–1102 CrossRef CAS.
- O. L. Eryilmaz and A. Erdemir, On the hydrogen lubrication mechanism(s) of DLC films: An imaging TOF-SIMS study, Surf. Coat. Technol., 2008, 203(5–7), 750–755 CrossRef CAS.
- C. Matta, L. Joly-Pottuz, M. de Barros Bouchet, J. Martin, M. Kano, Q. Zhang and W. Goddard, Superlubricity and tribochemistry of polyhydric alcohols, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78(8), 085436 CrossRef.
- J. Andersson, R. A. Erck and A. Erdemir, Frictional behavior of diamondlike carbon films in vacuum and under varying water vapor pressure, Surf. Coat. Technol., 2003, 163–164, 535–540 CrossRef CAS.
- A. Konicek, D. Grierson, P. Gilbert, W. Sawyer, A. Sumant and R. Carpick, Origin of Ultralow Friction and Wear in Ultrananocrystalline Diamond, Phys. Rev. Lett., 2008, 100(23), 235502 CrossRef CAS PubMed.
- Y. Liu, A. Erdemir and E. I. Meletis, An investigation of the relationship between graphitization and frictional behavior of DLC coatings, Surf. Coat. Technol., 1996, 86–87, 564–568 CrossRef CAS.
- J. A. Harrison and D. W. Brenner, Simulated tribochemistry: An atomic-scale view of the wear of diamond, J. Am. Chem. Soc., 1994, 116, 10399–10402 CrossRef CAS.
- J. C. Sanchez-Lopez, A. Erdemir, C. Donnet and T. C. Rojas, Friction-induced structural transformations of diamondlike carbon coatings under various atmospheres, Surf. Coat. Technol., 2003, 163–164, 444–450 CrossRef CAS.
- A. P. Merkle, A. Erdemir, O. L. Eryilmaz, J. A. Johnson and L. D. Marks, In situ TEM studies of tribo-induced bonding modifications in near-frictionless carbon films, Carbon, 2010, 48(3), 587–591 CrossRef CAS.
- Y. Wang, L. Wang, S. C. Wang, G. Zhang, J. K. Wood Robert and Q. Xue, Nanocomposite Microstructure and Environment Self-Adapted Tribological Properties of Highly Hard Graphite-Like Film, Tribol. Lett., 2010, 40(3), 301–310 CrossRef CAS.
- Y. Liao, R. Pourzal, M. A. Wimmer, J. J. Jacobs, A. Fischer and L. D. Marks, Graphitic tribological layers in metal-on-metal hip replacements, Science, 2011, 334(6063), 1687–1690 CrossRef CAS PubMed.
- T. H. Abdullah, M. Wakayama, T. Tokoroyama, H. Kousaka, N. Umehara, Y. Mabuchi and T. Higuchi, Wear behaviour of tetrahedral amorphous diamond-like carbon (ta-C DLC) in additive containing lubricants, Wear, 2013, 307(1–2), 1–9 CrossRef.
- P. Wang, M. Hirose, Y. Suzuki and K. Adachi, Carbon tribo-layer for super-low friction of amorphous carbon nitride coatings in inert gas environments, Surf. Coat. Technol., 2013, 221, 163–172 CrossRef CAS.
- T. Kunze, M. Posselt, S. Gemming, G. Seifert, A. R. Konicek, R. W. Carpick, L. Pastewka and M. Moseler, Wear, Plasticity, and Rehybridization in Tetrahedral Amorphous Carbon, Tribol. Lett., 2013, 53(1), 119–126 CrossRef.
- T. B. Ma, Y. Z. Hu and H. Wang, Molecular dynamics simulation of shear-induced graphitization of amorphous carbon films, Carbon, 2009, 47(8), 1953–1957 CrossRef CAS.
- T. B. Ma, L. F. Wang, Y. Z. Hu, X. Li and H. Wang, A shear localization mechanism for lubricity of amorphous carbon materials, Sci. Rep., 2014, 4, 3662 Search PubMed.
- Y. Wang, J. Guo, K. Gao, B. Zhang, A. Liang and J. Zhang, Understanding the ultra-low friction behavior of hydrogenated fullerene-like carbon films grown with different flow rates of hydrogen gas, Carbon, 2014, 77, 518–524 CrossRef CAS.
- C. Wang, S. Yang, H. Li and J. Zhang, Elastic properties of a-C: N:H films, J. Appl. Phys., 2007, 101(1), 013501 CrossRef.
- Q. Wang, C. Wang, Z. Wang, J. Zhang and D. He, The correlation between pentatomic and heptatomic carbon rings and stress of hydrogenated amorphous carbon films prepared by dc-pulse plasma chemical vapor deposition, Appl. Phys. Lett., 2008, 93(13), 131920 CrossRef.
- T. Doyle and J. Dennison, Vibrational dynamics and structure of graphitic amorphous carbon modeled using an embedded-ring approach, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51(1), 196–200 CrossRef CAS.
- M. P. Siegal, D. R. Tallant, L. J. Martinez-Miranda, J. C. Barbour, R. L. Simpson and D. L. Overmyer, Nanostructural characterization of amorphous diamondlike carbon films, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 10451–10462 CrossRef CAS.
- M. P. Siegal, D. R. Tallant, P. N. Provencio, D. L. Overmyer, R. L. Simpson and L. J. Martinez-Miranda, Ultrahard carbon nanocomposite films, Appl. Phys. Lett., 2000, 76, 3052–3054 CrossRef CAS.
- Q. Wang, D. He, C. Wang, Z. Wang and J. Zhang, The evolution of the structure and mechanical properties of fullerenelike hydrogenated amorphous carbon films upon annealing, J. Appl. Phys., 2008, 104(4), 043511 CrossRef.
- S. J. Townsend, T. J. Lenosky, D. A. Muller, C. S. Nichols and V. Elser, Negatively curved graphitic sheet model of amorphous carbon, Phys. Rev. Lett., 1992, 69(6), 921–924 CrossRef CAS PubMed.
- R. Debdulal, C. Manish, H. Niklas, T. W. Clyne and G. A. J. Amaratunga, Probing carbon nanoparticles in CNx thin films using Raman spectroscopy, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70(3), 035406 CrossRef.
- D. Roy, C. Manish, H. Wang, N. Sano, I. Alexandrou, T. W. Clyne and G. A. J. Amaratunga, Characterisation of carbon nano-onions using Raman spectroscopy, Chem. Phys. Lett., 2003, 373(1–2), 52–56 CrossRef CAS.
- V. D. Blank, S. G. Buga, G. A. Dubitsky, N. R. Serebryanaya, M. Popov Yu and B. Sundqvist, High-pressure polymerized phases of C60, Carbon, 1998, 36, 319–343 CrossRef CAS.
- H. Ronkainen, J. Koskinen, J. Likonen, S. Varjus and J. Vihersalo, Characterization of wear surfaces in dry sliding of steel and alumina on hydrogenated and hydrogen-free carbon films, Diamond Relat. Mater., 1994, 3, 1329–1336 CrossRef CAS.
- X. Wang, P. Wang, S. Yang and J. Zhang, Tribological behaviors of fullerene-like hydrogenated carbon (FL-C:H) film in different atmospheres sliding against Si3N4 ball, Wear, 2008, 265(11–12), 1708–1713 CrossRef CAS.
- A. Johnson Jacqueline, H. Diane, B. Woodford John, Z. Alexander, A. Gee Ian, L. Eryilmaz Osman and E. Ali, Top-surface characterization of a near frictionless carbon film, Diamond Relat. Mater., 2007, 16(2), 209–215 CrossRef.
- C. Wang, S. Yang, Q. Wang, Z. Wang and J. Zhang, Comparative study of hydrogenated diamondlike carbon film and hard hydrogenated graphitelike carbon film, J. Appl. Phys., 2008, 103(12), 123531 CrossRef.
- L. Ji, H. Li, F. Zhao, W. Quan, J. Chen and H. Zhou, Fullerene-like hydrogenated carbon films with super-low friction and wear, and low sensitivity to environment, J. Phys. D: Appl. Phys., 2010, 43(1), 015404 CrossRef.
- Z. Q. Li, C. J. Lu, Z. P. Xia, Y. Zhou and Z. Luo, X-ray diffraction patterns of graphite and turbostratic carbon, Carbon, 2007, 45(8), 1686–1695 CrossRef CAS.
- S. Yoshimoto, J. Amano and K. Miura, Synthesis of a fullerene/expanded graphite composite and its lubricating properties, J. Mater. Sci., 2010, 45(7), 1955–1962 CrossRef CAS.
- K. Zhang, Y. Zhang and S. Wang, Enhancing thermoelectric properties of organic composites through hierarchical nanostructures, Sci. Rep., 2013, 3, 3448 Search PubMed.
- G. A. J. Amaratunga, M. Chhowalla, C. J. Kiely, I. Alexandrou, R. Aharonov and R. M. Devenish, Hard elastic carbon thin films from linking of carbon nanoparticles, Nature, 1996, 383, 321–323 CrossRef CAS.
- D. W. M. Lau, D. G. McCulloch, N. A. Marks, N. R. Madsen and A. V. Rode, High-temperature formation of concentric fullerene-like structures within foam-like carbon: Experiment and molecular dynamics simulation, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 233408 CrossRef.
- T. LeHuu, H. Zaidi, D. Paulmier and P. Voumard, Transformation of sp3 to sp2 sites of diamond like carbon coatings during friction in vacuum and under water vapour environment, Thin Solid Films, 1996, 290–291, 126–130 CrossRef CAS.
- Z. F. Zhou, K. Y. Li, I. Bello, C. S. Lee and S. T. Lee, Study of tribological performance of ECR–CVD diamond-like carbon coatings on steel substrates, Wear, 2005, 258(10), 1589–1599 CrossRef CAS.
- Y. Liu, A. Erdemir and E. I. Meletis, A study of the wear mechanism of diamond-like carbon films, Surf. Coat. Technol., 1996, 82, 48–56 CrossRef CAS.
- H. Li, T. Xu, C. Wang, J. Chen, H. Zhou and H. Liu, Tribochemical effects on the friction and wear behaviors of diamond-like carbon film under high relative humidity condition, Tribol. Lett., 2005, 19(3), 231–238 CrossRef CAS.
- M. A. Kabbani, C. S. Tiwary, P. A. Autreto, G. Brunetto, A. Som, K. R. Krishnadas, S. Ozden, K. P. Hackenberg, Y. Gong, D. S. Galvao, R. Vajtai, A. T. Kabbani, T. Pradeep and P. M. Ajayan, Ambient solid-state mechano-chemical reactions between functionalized carbon nanotubes, Nat. Commun., 2015, 6, 7291 CrossRef CAS PubMed.
- D. Ugarte, Curling and closure of graphitic networks under electron-beam irradiation, Nature, 1992, 359, 707–709 CrossRef CAS PubMed.
- A. Chuvilin, U. Kaiser, E. Bichoutskaia, N. A. Besley and A. N. Khlobystov, Direct transformation of graphene to fullerene, Nat. Commun., 2010, 2, 450–453 CAS.
| This journal is © The Royal Society of Chemistry 2015 |