DNA-templated borononucleic acid self assembly: a study of minimal complexity†
Renaud Barbeyron,
Anthony R. Martin,
Jean-Jacques Vasseur and
Michael Smietana*
Institut des Biomolécules Max Mousseron, UMR 5247 CNRS, Université de Montpellier, ENSCM Place Eugène Bataillon, 34095 Montpellier, France. E-mail: msmietana@um2.fr
First published on 1st December 2015
Abstract
Understanding and promoting self-assembling processes is a key issue that can give rise to interesting applications in nanotechnology and nanoengineering. A precise control at the molecular level is essential to increase the complexity of evolved DNA supramolecular architectures. In this article, we unveil the minimal level of complexity needed for promoting DNA-templated self-assembly of bifunctional oligonucleotides able to form internucleosidic boronate linkages. Our results highlight the key features required for an efficient control of the assembly/disassembly process, as well as the importance of primers to initiate the oligomerization.
Introduction
Mimicry of biological processes under abiotic conditions is a challenging step toward the reconstruction of life complexity with wide applications in bio- and nanotechnologies.1 The design of artificial biopolymers able to self-assemble in the absence of enzymatic activation provides such imitation. Moreover, apart from evolutionary strategies allowing the development of functional biopolymers and macromolecules, non-enzymatic templated reactions are of the utmost importance in RNA world scenarios.2 DNA- and RNA-templated methods allowing the transfer of genetic information through the enzyme-free ligation of natural or modified oligonucleotides has thus been largely studied and reviewed.3 While foremost studies aimed at forming phosphodiester bonds through chemical activation,1e,4 many modified internucleosidic linkages made of irreversible or reversible connections have been reported so far.2c,5 In fact, it has been proposed that reversible backbones preceded the contemporary phosphodiester linkage thus providing monomers the necessary adaptive behaviours to become functional polymers.2c,6 In our attempts to mimic the high-fidelity template-directed synthesis of informational nucleic acid sequences under enzyme-free conditions, we recently developed a dynamic enzyme- and activator-free DNA- and RNA-templated system based on the well-known boronic acid–boronate equilibrium (Fig. 1a).7 In these processes, oligonucleotides bearing a 5′-end boronic acid reacts with 3′-ended ribonucleotidic partners only in the presence of a complementary template (Fig. 1b). The newly-formed boronate internucleosidic linkage can then be switched off and on again through a variety of external stimuli (T, pH, anions) and the concept was recently applied to the DNA- and RNA-templated programmable self-assembly of bifunctional hexamers (Fig. 1c).8 The controlled and reversible templated oligomerization process is particularly appealing for both the design of nucleic acid-based assemblies of functional adaptive materials and to uncover new chemical structures allowing genetic information transfer and storage. As a matter of fact, the significance of the non-enzymatic template-directed replication of nucleic acid monomers spans from evolutionary processes to advanced bottom-up synthetic biology.1e,3c | ||
| Fig. 1 Schematic representation of (a) boronate formation, (b) DNA-templated ligation, (c) DNA-templated polymerization. | ||
The regio- and sequences specificities we previously observed with 6mers prompted us to investigate the implication of smaller sequences. The motivation behind this work was to identify the structural parameters and the minimal complexity needed for such self-assembly to happen. Indeed, these factors will not only allow us to increase our knowledge in the handling and programming of these units but also assist us in the design of non-enzymatic functional systems. Moreover, as boron species have been highlighted because of their potential prebiotic relevance, our results will also allow the exploration of prospective ancestral reversible internucleosidic linkages.9 We report here the results of our endeavour that have helped us determine for the first time the size limitation of this self-assembly process, its selectivity capabilities as well as the influence of external stimuli.
Results and discussion
Our initial experiments were focused on the determination of size limitation. Thus, DNA templates containing three successive repeats complementary to 5′-ended bifunctional 5mer (5′-TbnATGU, B5), 4mer (5′-TbnGTrA, B4) or 3mer (5′-TbnCrA, B3) were evaluated through thermal-denaturation studies at different pH values (Fig. 2). Control experiments using the corresponding unmodified shortmers C5, C4 and C3 did not feature any observable transition independently of the pH, thus indicating that the length of these short oligomers prohibited the formation of nicked duplexes (Table 1). | ||
| Fig. 2 Formation of boronate internucleosidic linkages through the self-assemblies of (a) pentamer B5, (b) tetramer B4 and (c) trimer B3. Bold letters represent RNA residues. | ||
In comparison, the use of stoichiometric amounts of probe B5 yielded a noticeable transition at Tm = 9.8 °C at pH 7.5 that increased to Tm = 14.9 °C at pH 9.5. This pH dependency confirmed the stabilization conveyed by the boronate linkages through boron rehybridization. Tetramer B4 did not induce any transition at pH 7.5, while a slight stabilization was observed at pH 9.5 (Tm = 7.2 °C). In contrast, trimer B3 showed no transition regardless of the pH value. These results confirmed the templated-assembly of the borono-oligonucleotides and their ability to respond to external stimuli. In addition, the results also highlighted the importance of the hybridization to the template in order for the oligomerization to proceed.
| ODN | Sequence (5′-3′) | ODN | Sequence (5′-3′)a |
|---|---|---|---|
| a Tbn refers to boronothymidine and bold letters represent RNA residues. | |||
| T5 | CC-(ACATA)3-CC | B5 | TbnATGU |
| T4 | CC-(TACA)3-CC | B4 | TbnGTA |
| T3 | CC-(TGA)3-CC | B3 | TbnCA |
| T5′ | CC-ACATA-ATACA-ACATA-CC | B5′ | TbnGTAU |
| T4′ | CC-TACA-TCAA-TACA-CC | B4′ | TbnTGA |
| T5p | CC-(ACATA)3-(TGA)3-CC | C5 | TATGU |
| T4p | CC-(TACA)3-(TGA)3-CC | C4 | TGTA |
| T3p | CC-(TGA)3-(TGA)3-CC | C3 | TCA |
| P | GG-TCATCATCA | C5′ | TGTAU |
| C4′ | TTGA | ||
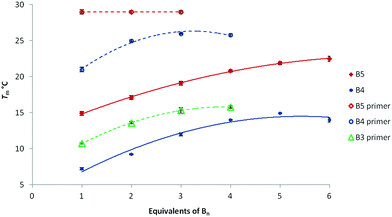
As for any equilibrium in water, boronate formation could also be favoured by adding an excess of one of the reactants; the formation of the boronate linkages could hence be facilitated by increasing the molarity of the bifunctional strands. To confirm this hypothesis, various excesses of B5, B4 and B3 with respect to the complementary template were evaluated at pH 9.5 through thermal denaturation experiments (Table 2). Once again, control experiments revealed that no transition could be observed even in the presence of 6 equivalents of the unmodified sequences. However, Tm values increased almost linearly when increasing the number of equivalents of B5 and B4, reaching a plateau at Tm = 21.9 °C and Tm = 14.9 °C, respectively, in the presence of 5 equiv. (Table 2, entries 1 and 2). Under these conditions, trimers B3 were not able to self-assemble thus demonstrating that with trimers the equilibrium is largely shifted toward the non-hybridized boronic acid sequence (Table 2, entry 3). Interestingly, in the presence of 5 equiv. of either B5 or B4, similar levels of stabilization could be reached at pH 7.5 by replacing hydroxide anions by cyanide ions. Indeed the use of a 3 mM solution of NaCN yielded transitions at Tm = 20.2 °C for B5 and Tm = 13.0 °C for B4 (see ESI†).
| Entry | Strand Bn | Tma [°C] according to the excess of Bn | |||||
|---|---|---|---|---|---|---|---|
| 1 eq. | 2 eq. | 3 eq. | 4 eq. | 5 eq. | 6 eq. | ||
| a Melting temperatures are obtained from the maxima of the first derivatives of the sigmoidal melting curve (A260 vs. temperature) recorded in a buffer containing 1 M NaCl and 10 mM of sodium cacodylate, template concentration 3 μM. Curve fits data were averaged from fits of three denaturation curves. Uncertainties were estimated from standard deviations of experimental melting temperatures.b Tm lower than 5 °C. | |||||||
| 1 | B5 | 14.9 ± 0.3 | 17.1 ± 0.3 | 19.1 ± 0.3 | 20.8 ± 0.2 | 21.9 ± 0.2 | 22.5 ± 0.4 |
| 2 | B4 | 7.2 ± 0.2 | 9.2 ± 0.1 | 12.0 ± 0.2 | 14.0 ± 0.1 | 14.9 ± 0.1 | 14.0 ± 0.3 |
| 3 | B3 | —b | —b | —b | —b | —b | —b |
Following these initial results, we then evaluated the reversibility of the process by cyclically changing the pH of the solution containing 5 equiv. of either B5 or B4 in the presence of the corresponding template by adding at 0 °C small aliquots of a 3 M aqueous solution of NaOH or HCl. As shown in Fig. 3, in both cases no transitions could be observed at pH 5.5 thus confirming the rupture of all boronate linkages and most probably all possible duplexes. Those can however be restored as initially observed by increasing the pH to 9.5. Applying several cycles of variations allowed the controlled formation/breaking of the oligomer without any loss of stability thus demonstrating the ability of these systems to behave as adaptive materials.
We next turned our attention towards selectivity issues by using templates exhibiting two mismatches on its central section (Fig. 4). Templates were designed as having a 5′-ATACA central section for T5′ and 5′-TCAA for T4′. In both cases, the presence of 5 equiv. of B5 or B4 at pH 9.5 did not induce any observable transition, thus demonstrating the total absence of mismatched duplexes and the high sequence selectivity of the self-assembly process.
Moreover addition of 5 equiv. of the complementary bifunctional building blocks B5′ (5′-TbnGTArU) or B4′ (5′-TbnTGrA) restored a well-defined transition (Tm = 20.0 and 13.5 °C respectively) confirming the possibility to selectively polymerize dynamically different bifunctional shortmers (Fig. 4).
Finally, by analogy with natural polymerization processes that use primers serving as starting points for DNA synthesis, we envisioned to promote the assembly of small bifunctional borono-oligonucleotides using a 11mer primer P (5′-GGTCATCATCrA) bearing a ribonucleotide at its 3′-end (Fig. 5). The melting temperature of the template/primer duplex was determined to be high enough (Tm = 51 °C) to avoid any overlapping with the transition resulting from short oligomers hybridization. In the presence of the primer only unmodified pentamers C5 showed a pH-independent low transition at Tm = 6.8 °C. With modified pentamers B5 and tetramers B4, a pH-dependant plateau was reached with 1 and 3 equiv. respectively. Indeed, duplexes were observed and stabilized when rising the pH from 7.5 to 9.5 with pentamers (Tm = 24.9 and 29.0 °C, Table 3, entry 1) and tetramers (Tm = 17.8 and 26.0 °C Table 3, entry 2).
 | ||
| Fig. 5 Formation of boronate internucleosidic linkages through a primer assisted polymerization of bifunctional strands. Bold letters represent RNA residues. | ||
| Entry | Template (3′-5′) | Stranda (5′-3) | Tmb [°C] | ||
|---|---|---|---|---|---|
| pH 7.5 | pH 9.5 | CNc | |||
| a Tbn refers to boronothymidine and bold letters represent RNA residues.b Melting temperatures are obtained from the maxima of the first derivatives of the sigmoidal melting curve (A260 vs. temperature) recorded in a buffer containing 1 M NaCl and 10 mM of sodium cacodylate, template concentration 3 μM; boronic strands concentration: B5 9 μM, B4 and B3 27 μM. Curve fits data were averaged from fits of three denaturation curves. Uncertainties were estimated from standard deviations of experimental melting temperatures.c Data were obtained in the presence of 3 mM NaCN.d Tm lower than 5 °C. | |||||
| 1 | CC(ATACA)3(AGT)3CC | TATGU | 6.8 ± 0.2 | 6.8 ± 0.2 | —d |
| TbnATGU | 24.9 ± 0.1 | 29.0 ± 0.3 | 27.0 ± 0.2 | ||
| 2 | CC(ACAT)3(AGT)3CC | TGTA | —d | —d | —d |
| TbnGTA | 20.5 ± 0.2 | 26.0 ± 0.1 | 24.0 ± 0.2 | ||
| 3 | CC (AGT)3(AGT)3CC | TCA | —d | —d | —d |
| TbnCA | —d | 15.3 ± 0.4 | 14.0 ± 0.3 | ||
Interestingly, while no transition could be observed with trimers at pH 7.5, increasing the pH to 9.5 allowed the formation of a noticeable transition up to Tm = 15.3 °C with 4 equiv. In all cases, similar degrees of stabilization were reached at pH 7.5 with cyanide anions (Table 3). These results highlight the pH and primer dependency of bifunctional trimers in the oligomerization process and suggest that trimers represent the current limit for the templated formation of boronate junctions (Fig. 6). Nevertheless, the results are still remarkable as it allows the oligomerization through reversible connections of probes as short as trimers.
Conclusions
We have shown that the self-assembly of short oligomers through the formation of boronate linkages is essentially controlled by templating effects. On–off oligomerization in response to pH triggers associated with sequence selectivity allows to precisely manipulate the position of multi-component systems varying in size and sequence. While primer assistance appears essential for oligomerization of trimers, the possibility of using systems with a diverse set of shortmers opens a plethora of exciting applications ranging from biosensing to novel materials with dynamic functionalities. While reducing the size of the bifunctional building blocks to dimers or ideally monomers is a long-term goal in the laboratory, our DNA-templated boronate strategy appears to reach its limit with trimers. Work is underway for the elaboration of new modifications allowing the formation of improved borono-based internucleosidic linkages.Acknowledgements
We thank the Agence Nationale de la Recherche (ANR “Prodygy”–11-JS07-005-01), the CNRS and the Région Languedoc-Roussillon (Programme "“Chercheur d'Avenir”).Notes and references
- (a) M. J. Doktycz and M. L. Simpson, Mol. Syst. Biol., 2007, 3, 125 CrossRef PubMed; (b) H. Liang, X.-B. Zhang, Y. Lv, L. Gong, R. Wang, X. Zhu, R. Yang and W. Tan, Acc. Chem. Res., 2014, 47, 1891 CrossRef CAS PubMed; (c) I. Hirao, M. Kimoto and R. Yamashige, Acc. Chem. Res., 2012, 45, 2055 CrossRef CAS PubMed; (d) S. Zhang, C. Switzer and J. C. Chaput, Chem. Biodiversity, 2010, 7, 245 CrossRef CAS PubMed; (e) M. Dorr, P. M. G. Loffler and P. A. Monnard, Curr. Org. Synth., 2012, 9, 735 CrossRef CAS; (f) V. Patzke and G. von Kiedrowski, Arkivoc, 2007, 293 CAS.
- (a) J. C. Leitzel and D. G. Lynn, Chem. Rec., 2001, 1, 53 CrossRef CAS; (b) J. Szostak, J. Syst. Chem., 2012, 3, 2 CrossRef CAS; (c) A. E. Engelhart and N. V. Hud, Cold Spring Harbor Perspect. Biol., 2010, 2, a002196 CAS.
- (a) L. E. Orgel, Acc. Chem. Res., 1995, 28, 109 CrossRef CAS PubMed; (b) K. K. Sadhu, M. Roethlingshoefer and N. Winssinger, Isr. J. Chem., 2013, 53, 75 CrossRef CAS; (c) K. Gorska and N. Winssinger, Angew. Chem., Int. Ed., 2013, 52, 6820 CrossRef CAS PubMed; (d) X. Y. Li and D. R. Liu, Angew. Chem., Int. Ed., 2004, 43, 4848 CrossRef CAS.
- (a) J. Sulston, R. Lohrmann, L. E. Orgel and H. T. Miles, Proc. Natl. Acad. Sci. U. S. A., 1968, 59, 726 CrossRef CAS; (b) L. E. Orgel, Crit. Rev. Biochem. Mol. Biol., 2004, 39, 99 CrossRef CAS PubMed; (c) L. Orgel, Science, 2000, 290, 1306 CrossRef CAS PubMed.
- (a) G. von Kiedrowski, Angew. Chem., Int. Ed., 1986, 25, 932 CrossRef; (b) A. Kaiser and C. Richert, J. Org. Chem., 2013, 78, 793 CrossRef CAS PubMed; (c) M. Hey, C. Hartel and M. W. Gobel, Helv. Chim. Acta, 2003, 86, 844 CrossRef CAS; (d) J. P. Schrum, A. Ricardo, M. Krishnamurthy, J. C. Blain and J. W. Szostak, J. Am. Chem. Soc., 2009, 131, 14560 CrossRef CAS PubMed; (e) C. Deck, M. Jauker and C. Richert, Nat. Chem., 2011, 3, 603 CrossRef CAS; (f) Z. Y. Li, Z. Y. J. Zhang, R. Knipe and D. G. Lynn, J. Am. Chem. Soc., 2002, 124, 746 CrossRef; (g) D. M. Rosenbaum and D. R. Liu, J. Am. Chem. Soc., 2003, 125, 13924 CrossRef CAS PubMed; (h) R. E. Kleiner, Y. Brudno, M. E. Birnbaum and D. R. Liu, J. Am. Chem. Soc., 2008, 130, 4646 CrossRef CAS PubMed; (i) B. D. Heuberger and C. Switzer, Org. Lett., 2006, 8, 5809 CrossRef CAS PubMed; (j) J. C. Chaput and C. Switzer, J. Mol. Evol., 2000, 51, 464 CAS; (k) S. L. Zhang, J. C. Blain, D. Zielinska, S. M. Gryaznov and J. W. Szostak, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17732 CrossRef CAS PubMed; (l) S. L. Zhang, N. Zhang, J. C. Blain and J. W. Szostak, J. Am. Chem. Soc., 2013, 135, 924 CrossRef CAS PubMed; (m) P. Hagenbuch, E. Kervio, A. Hochgesand, U. Plutowski and C. Richert, Angew. Chem., Int. Ed., 2005, 44, 6588 CrossRef CAS PubMed; (n) M. Rothlingshofer, E. Kervio, T. Lommel, U. Plutowski, A. Hochgesand and C. Richert, Angew. Chem., Int. Ed., 2008, 47, 6065 CrossRef PubMed; (o) K. Leu, E. Kervio, B. Obermayer, R. M. Turk-MacLeod, C. Yuan, J. M. Luevano, E. Chen, U. Gerland, C. Richert and I. A. Chen, J. Am. Chem. Soc., 2013, 135, 354 CrossRef CAS PubMed; (p) E. Kuruvilla, G. B. Schuster and N. V. Hud, ChemBioChem, 2013, 14, 45 CrossRef CAS; (q) E. Kervio, B. Claasen, U. E. Steiner and C. Richert, Nucleic Acids Res., 2014, 42, 7409 CrossRef CAS PubMed; (r) Y. Ura, J. M. Beierle, L. J. Leman, L. E. Orgel and M. R. Ghadiri, Science, 2009, 325, 73 CrossRef CAS PubMed; (s) H. D. Bean, F. A. L. Anet, I. R. Gould and N. V. Hud, Origins Life Evol. Biospheres, 2006, 36, 39 CrossRef CAS PubMed; (t) V. Patzke, J. S. McCaskill and G. von Kiedrowski, Angew. Chem., Int. Ed., 2014, 53, 4222 CrossRef PubMed; (u) A. E. Engelhart, B. J. Cafferty, C. D. Okafor, M. C. Chen, L. D. Williams, D. G. Lynn and N. V. Hud, ChemBioChem, 2012, 13, 1121 CrossRef CAS PubMed; (v) X. Y. Li, A. F. Hernandez, M. A. Grover, N. V. Hud and D. G. Lynn, Heterocycles, 2011, 82, 1477 CAS; (w) S. I. Walker, M. A. Grover and N. V. Hud, PLoS One, 2012, 7, e34166 CAS; (x) D. J. Hansen, I. Manuguerra, M. B. Kjelstrup and K. V. Gothelf, Angew. Chem., Int. Ed., 2014, 53, 14415 CrossRef CAS PubMed; (y) D. Li, X. Wang, F. Shi, R. Sha, N. C. Seeman and J. W. Canary, Org. Biomol. Chem., 2014, 12, 8823 RSC.
- (a) N. V. Hud, S. S. Jain, X. H. Li and D. G. Lynn, Chem. Biodiversity, 2007, 4, 768 CrossRef CAS PubMed; (b) I. Mamajanov, P. J. MacDonald, J. Ying, D. M. Duncanson, G. R. Dowdy, C. A. Walker, A. E. Engelhart, F. M. Fernandez, M. A. Grover, N. V. Hud and F. J. Schork, Macromolecules, 2014, 47, 1334 CrossRef CAS.
- (a) R. Badugu, J. R. Lakowicz and C. D. Geddes, Dyes Pigm., 2005, 64, 49 CrossRef CAS; (b) D. Luvino, D. Gasparutto, S. Reynaud, M. Smietana and J.-J. Vasseur, Tetrahedron Lett., 2008, 49, 6075 CrossRef CAS; (c) D. Luvino, M. Smietana and J. J. Vasseur, Tetrahedron Lett., 2006, 47, 9253 CrossRef CAS; (d) A. R. Martin, J. J. Vasseur and M. Smietana, Chem. Soc. Rev., 2013, 42, 5684 RSC; (e) S. D. Bull, M. G. Davidson, J. M. H. van den Elsen, J. S. Fossey, A. T. A. Jenkins, Y. B. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Z. Zhao and T. D. James, Acc. Chem. Res., 2012, 46, 312 CrossRef PubMed; (f) G. Springsteen and B. H. Wang, Tetrahedron, 2002, 58, 5291 CrossRef CAS.
- (a) D. Luvino, C. Baraguey, M. Smietana and J. J. Vasseur, Chem. Commun., 2008, 44, 2352 RSC; (b) A. R. Martin, I. Barvik, D. Luvino, M. Smietana and J. J. Vasseur, Angew. Chem., Int. Ed., 2011, 50, 4193 CrossRef CAS PubMed; (c) A. R. Martin, K. Mohanan, D. Luvino, N. Floquet, C. Baraguey, M. Smietana and J. J. Vasseur, Org. Biomol. Chem., 2009, 7, 4369 RSC; (d) M. Smietana, A. R. Martin and J. J. Vasseur, Pure Appl. Chem., 2012, 84, 1659 CrossRef CAS; (e) R. Barbeyron, J. Wengel, J. J. Vasseur and M. Smietana, Monatsh. Chem., 2013, 144, 495 CrossRef CAS; (f) R. Barbeyron, J.-J. Vasseur and M. Smietana, Chem. Sci., 2015, 6, 542 RSC.
- (a) R. Scorei and V. M. Cimpoiasu, Origins Life Evol. Biospheres, 2006, 36, 1 CrossRef CAS PubMed; (b) B. E. Prieur, C. R. Acad. Sci., 2001, 4, 667 CAS; (c) A. Ricardo, M. A. Carrigan, A. N. Olcott and S. A. Benner, Science, 2004, 303, 196 CrossRef CAS PubMed; (d) R. Scorei, Origins Life Evol. Biospheres, 2012, 42, 3 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details, melting curves and control experiments. See DOI: 10.1039/c5ra20767c |
| This journal is © The Royal Society of Chemistry 2015 |