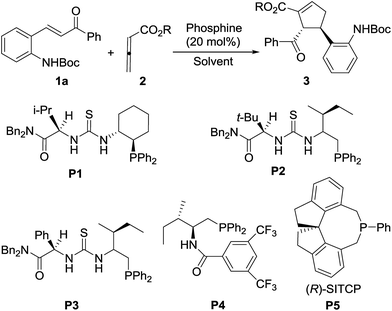
Phosphine-catalyzed asymmetric [3 + 2] annulation of chalcones with allenoates for enantioselective synthesis of functionalized cyclopentenes†
Zhenzhen Gao,
Chang Wang,
Chunhao Yuan,
Leijie Zhou,
Zhanhu Sun,
Yumei Xiao* and
Hongchao Guo*
Department of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: xiaoyumei23@126.com; hchguo@cau.edu.cn
First published on 1st December 2015
Abstract
Chiral phosphine-catalyzed asymmetric [3 + 2] annulation reaction of various Boc-amino-substituted chalcones with allenoates has been developed, leading to efficient formation of 1,4,5-trisubstituted cyclopentenes with high yields, excellent diastereoselectivities and enantioselectivities (up to 99% ee and up to 98% yield).
Nucleophilic phosphine-catalyzed cycloaddition reactions have been established as a reliable and powerful tool for the synthesis of carbo- and heterocycles from simple starting materials.1 In particular, phosphine-catalyzed [3 + 2] annulations of allenoates with electron-deficient olefins for cyclopentenes are one of most studied reactions, and considerable progress has been made since the pioneering contribution from Lu.2 In the presence of phosphines, a variety of activated olefins, including α,β-unsaturated enones,3 α,β-unsaturated esters,4 α,β-unsaturated cyanoethylenes5 and α,β-unsaturated acrylamides,6 could undergo [3 + 2] annulation with allenoates to afford medicinally important compounds.7 By employing a handful of chiral phosphine catalysts, asymmetric [3 + 2] annulation reactions have also been achieved.1 Among various electron-deficient alkenes, α,β-unsaturated enones are one of the most important substrates and has attracted much attention. In 2006, using a chiral phosphepine catalyst based on a binaphthyl skeleton, Fu developed the first asymmetric [3 + 2] annulation of ethyl allenoate and various α,β-unsaturated enones, affording highly functionalized cyclopentenes possessing two contiguous stereocenters in 54–76% yields and 75–90% ee.3a Subsequently, using a stoichiometric amount of a α-amino acid-based phosphine, Miller achieved enantioselective [3 + 2] annulation of allenoates with enones to give highly substituted cyclopentenes as single regio- and diastereomers in excellent yields with 87–93% ee.3b Although a catalytic amount of phosphine (20 mol%) still afforded 93% ee, but only 38% yield. Compared with the above two catalytic systems, using planar chiral 2-phospha[3]ferroceneophanes as the catalyst, Marinetti obtained better enantioselectivities (89–95% ee), but the yields ranged between 60 and 80% in most cases.3d Although the above successful examples have been reported, the yields were below 80% or ee's were below 90% in the [3 + 2] annulation reactions of many chalcones.3a,3b,3d Obviously, developing phosphine-catalyzed highly efficient and enantioselective [3 + 2] annulation of allenoates with chalcones still is a challenged issue.
Recently, in the area of asymmetric nucleophilic phosphine organocatalysis, the multifunctional chiral phosphines bearing nucleophilic phosphine and a key activation moiety on a molecular chiral backbone provided an effective strategy for achieving high catalytic activity and high enantioselectivity.1i,1m In several multifunctional chiral phosphines,3b,8 Boc-amino has been used as a requisite moiety to help achieve high yield and enantioselectivity. Inspired by this strategy, we envisioned that installing a Boc-amino moiety onto enones, thus leading to excellent yields and enantioselectivities for [3 + 2] annulation reaction, might be feasible (Scheme 1). Herein, using Boc-amino-substituted chalcones as substrates, we report chiral phosphine-catalyzed highly enantioselective [3 + 2] annulation of allenoates for synthesis of biologically important highly functionalized cyclopentenes9 (Scheme 1).
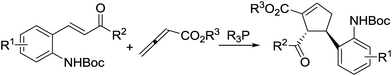 | ||
| Scheme 1 Phosphine-catalyzed enantioselective [3 + 2] annulation of Boc-amino-substituted chalcones with allenoates. | ||
Initially, we chose the reaction of 2-Boc-aminochalcone (1a) and ethyl allenoate (2a) as model reaction and examined several multifunctional chiral phosphines (P1–P4) in THF at room temperature (Table 1, entries 1–4). In the presence of 20 mol% of chiral phosphine, in these cases, the annulation reactions proceeded smoothly in THF at room temperature to afford the [3 + 2] cycloadduct 3aa as a single regioisomer and diastereomer in good yields, but with poor enantioselectivities (Table 1, entries 1–4). Subsequently, we turned our attention to a commercially available chiral phosphine (R)-SITCP (P5).10 To our delight, in the presence of 20 mol% of P5, the reaction worked efficiently to provide the product 3aa in 90% yield with 87% ee (entry 5). Next concise solvent screening revealed that dichloromethane (DCM) is the optimal solvent, resulting in the product 3aa in 92% yield with 94% ee (entries 6–7). We next investigated the influence of the variation of the ester moiety in allenoate structure. Changing the ester moiety of allenoate 2a to methyl led to a decrease of both yield and ee (entry 8). In contrast, using cyclohexyl allenoate 2c as the substrate, both yield and ee were increased to 93% and 96%, respectively (entry 9). Lowering the reaction temperature to 0 °C slightly increased yield and ee to 95% and 98%, respectively (entry 10). But further lowering the temperature to −20 °C, did not bring improvement (entry 11). The absolute configuration was identified by X-ray crystallographic analysis of the product 3ab.11 On the basis of the above screening of reaction conditions. The optimal reaction conditions were identified as follows: in the presence of 20 mol% chiral phosphine P5 using the allenoate 2c as the substrate in CH2Cl2 at 0 °C.
| Entry | Px | R | Solvent | t (h) | 3 | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|---|
a Reactions of 1a (0.1 mmol), 2 (0.15 mmol) and phosphine (0.02 mmol) were carried out in the solvent.b Isolated yields.c The ee values were determined by HPLC analysis using a chiral stationary phase. The regioisomer ratios are >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, determined by 1H NMR analysis of the crude products.d The reaction was carried out at 0 °C.e The reaction was carried out at −20 °C. 1, determined by 1H NMR analysis of the crude products.d The reaction was carried out at 0 °C.e The reaction was carried out at −20 °C. |
|||||||
| 1 | P1 | Et (2a) | THF | 3 | 3aa | 76 | 12 |
| 2 | P2 | Et (2a) | THF | 3 | 3aa | 76 | 20 |
| 3 | P3 | Et (2a) | THF | 3 | 3aa | 75 | 5 |
| 4 | P4 | Et (2a) | THF | 3 | 3aa | 85 | 33 |
| 5 | P5 | Et (2a) | THF | 3 | 3aa | 90 | 87 |
| 6 | P5 | Et (2a) | DCM | 3 | 3aa | 92 | 94 |
| 7 | P5 | Et (2a) | DCE | 3 | 3aa | 91 | 88 |
| 8 | P5 | Me (2b) | DCM | 3 | 3ab | 75 | 92 |
| 9 | P5 | Cy (2c) | DCM | 3 | 3ac | 93 | 96 |
| 10d | P5 | Cy (2c) | DCM | 7 | 3ac | 95 | 98 |
| 11e | P5 | Cy (2c) | DCM | 12 | 3ac | 95 | 98 |
With the optimal reaction conditions in hand, we investigated the scope of Boc-amino-substituted chalcones. As summarized in Table 2, various Boc-amino-substituted chalcone derivatives bearing different substituent patterns on the benzene rings were all very well tolerated in this reaction, giving the 1,4,5-trisubstituted cyclopentenes as a single diastereomer in good to excellent yields with excellent regioselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (entries 1–23). Regardless of the electron-donating or -withdrawing substituents at different position on the benzene rings in 2-Boc-aminochalcones 1, all the reactions were extremely efficient, affording the functionalized cyclopentenes in 69–98% yield with 94–98% ee (entries 1–22). In particular, alkyl enone 1w is also a compatible substrate, undergoing the reaction to give the corresponding product in 96% yield with 97% ee (entry 23).
1) and enantioselectivities (entries 1–23). Regardless of the electron-donating or -withdrawing substituents at different position on the benzene rings in 2-Boc-aminochalcones 1, all the reactions were extremely efficient, affording the functionalized cyclopentenes in 69–98% yield with 94–98% ee (entries 1–22). In particular, alkyl enone 1w is also a compatible substrate, undergoing the reaction to give the corresponding product in 96% yield with 97% ee (entry 23).
| Entry | R1/R2 in 1 | 3 | Yieldb (%) | eec (%) |
|---|---|---|---|---|
a Unless otherwise noted, reactions of 1a (0.1 mmol), 2 (0.15 mmol) and chiral phosphine P5 (0.02 mmol) were carried out in CH2Cl2 at 0 °C for 12 h.b Isolated yields.c The ee values were determined by HPLC analysis using a chiral stationary phase. The regioisomer ratios are >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, determined by 1H NMR analysis of the crude products.d The reaction completed within 7 h. 1, determined by 1H NMR analysis of the crude products.d The reaction completed within 7 h. |
||||
| 1d | H/Ph (1a) | 3ac | 95 | 98 |
| 2 | H/4-OMeC6H4 (1b) | 3bc | 97 | 95 |
| 3 | H/4-PhC6H4 (1c) | 3cc | 98 | 97 |
| 4 | H/3-BrC6H4 (1d) | 3dc | 93 | 96 |
| 5 | H/4-BrC6H4 (1e) | 3ec | 96 | 95 |
| 6 | H/4-CF3C6H4 (1f) | 3fc | 92 | 97 |
| 7 | 3-Me/Ph (1g) | 3gc | 96 | 97 |
| 8 | 3-Me/4-OMeC6H4 (1h) | 3hc | 94 | 97 |
| 9 | 3-Me/4-BrC6H4 (1i) | 3ic | 94 | 94 |
| 10 | 5-Me/4-PhC6H4 (1j) | 3jc | 85 | 96 |
| 11 | 5-Me/4-BrC6H4 (1k) | 3kc | 92 | 98 |
| 12 | 5-Me/4-CF3C6H4 (1l) | 3lc | 80 | 96 |
| 13 | 5 F/Ph (1m) | 3mc | 93 | 96 |
| 14 | 5 F/4-OMeC6H4 (1n) | 3nc | 98 | 97 |
| 15 | 5 F/4-PhC6H4 (1o) | 3oc | 69 | 98 |
| 16 | 4-Cl/Ph (1p) | 3pc | 81 | 97 |
| 17 | 5-Cl/Ph (1q) | 3qc | 92 | 97 |
| 18 | 5-Cl/4-BrC6H4 (1r) | 3rc | 87 | 98 |
| 19 | 5-Br/Ph (1s) | 3sc | 90 | 96 |
| 20 | 5-Br/4-OMeC6H4 (1t) | 3tc | 89 | 97 |
| 21 | 5-Br/4-BrC6H4 (1u) | 3uc | 80 | 96 |
| 22 | 6-Br/Ph (1v) | 3vc | 97 | 97 |
| 23 | H/Me (1w) | 3wc | 96 | 97 |
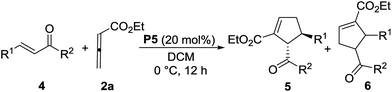
Using chiral phosphine P5 as the catalyst, we also tested the scope of other chalcones without Boc-amino group. With this kind of chalcones as the substrates, we found that allenoate 2a delivered better results than allenoate 2c did under the standard reaction conditions. As shown in Table 3, several chalcones with electron-donating or electron-withdrawing group could undergo asymmetric [3 + 2] annulation with allenoate 2a to give the desired cyclopentene derivatives as a single diastereomer in excellent regioselectivities and enantioselectivities. However, in comparison with those chalcones with Boc-amino group, some substrates displayed weak activities, leading to the products in moderate yields (entries 2, 4–6). In particular, using the amino substituted chalcone as the substrate instead of Boc-amino substituted chalcone, the reaction did not work (entry 7). It indicated that the bulky Boc-amino group is extremely important for achieving this asymmetric reaction and exerted dual influences on activity and stereoselectivity. The cyclopentene products are biologically important compounds9 and could also be further transformed into other useful functionalized cyclopentene derivatives. For example, treatment of the cyclopentene product (7) with MCPBA (m-chloroperoxybenzoic acid) in 1,2-dichloroethane (DCE) at room temperature for 12 h gave the epoxide (8) in 89% yield with excellent diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 96% ee (its absolute configuration has not been assigned) (Scheme 2). Unfortunately, 2D-NOESY spectra were not conclusive regarding the relative stereochemistry of the epoxide.
1) and 96% ee (its absolute configuration has not been assigned) (Scheme 2). Unfortunately, 2D-NOESY spectra were not conclusive regarding the relative stereochemistry of the epoxide.
| Entry | R1/R2 | 5 | Yieldb (%) | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6c 6c |
eec (%) |
|---|---|---|---|---|---|
| a Reactions of 4 (0.1 mmol), 2a (0.15 mmol) and chiral phosphine P5 (0.02 mmol) were carried out in CH2Cl2 at 0 °C for 12 h.b Isolated yields.c The ee values were determined by HPLC analysis using a chiral stationary phase. The regioisomer ratios were determined by 1H NMR analysis of the crude products. | |||||
| 1 | Ph/Ph (4a) | 5aa | 90 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
91 |
| 2 | 2-MeC6H4/Ph (4b) | 5ba | 68 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
95 |
| 3 | 4-MeC6H4/Ph (4c) | 5ca | 85 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
93 |
| 4 | 4-MeOC6H4/Ph (4d) | 5da | 40 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
90 |
| 5 | Ph/4-MeC6H4 (4e) | 5ea | 30 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
89 |
| 6 | 4-ClC6H4/4-MeC6H4 (4f) | 5fa | 45 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
92 |
| 7 | 2-NH2C6H4/Ph (4g) | — | 0 | — | — |
Experimental
General procedure for chiral phosphine-catalyzed asymmetric [3 + 2] annulation of chalcones with allenoates: under a nitrogen atmosphere, to a stirred solution of 2-tert-butoxycarbonyl aminochalcone 1 (0.05 mmol, 1.0 equiv.) in CH2Cl2 (1 mL) was sequentially added 2,3-butadienoate 2 (0.075 mmol, 1.5 equiv.) and the catalyst P5 (0.01 mmol, 20 mol%) via syringes. Then the resulting solution was vigorously stirred at 0 °C, and monitored by TLC. After the reaction was complete, the mixture was directly purified by column chromatography on silica gel (11% EtOAc/petroleum ether as the eluent) to furnish the corresponding product 3.Conclusions
In summary, we have developed chiral phosphine-catalyzed asymmetric [3 + 2] annulation of chalcones with allenoates to give multi-substituted cyclopentenes in good to excellent yields with excellent regioselectivities, diastereoselectivities and enantioselectivities. Compared with previous works on chiral phosphine-catalyzed [3 + 2] annulation reactions of chalcones with allenoates, the yields and enantioselectivities have remarkably been improved when 2-Boc-amino-substituted chalcones were used as the substrates.Acknowledgements
We are grateful for the financial support from NSFC (21172253 and 21372256) and the National S&T Pillar Program of China (2015BAK45B01).Notes and references
- For selected reviews on phosphine-promoted annulations, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (c) V. Nair, R. S. Menon, A. R. Sreekanth, N. Abhilash and A. T. Biju, Acc. Chem. Res., 2006, 39, 520 CrossRef CAS PubMed; (d) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (e) C. E. Aroyan, A. Dermenci and S. J. Miller, Tetrahedron, 2009, 65, 4069 CrossRef CAS; (f) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (g) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (h) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (i) S.-X. Wang, X. Y. Han, F. R. Zhong, Y. Q. Wang and Y. X. Lu, Synlett, 2011, 2766 CAS; (j) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (k) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (l) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (m) Y. M. Xiao, Z. H. Sun, H. C. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed; (n) P. Z. Xie and Y. Huang, Org. Biomol. Chem., 2015, 13, 8578 RSC.
- C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS.
- For [3 + 2] annulation reactions of α,β-unsaturated enones with allenes, see: (a) J. E. Wilson and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1426 CrossRef CAS PubMed; (b) B. J. Cowen and S. J. Miller, J. Am. Chem. Soc., 2007, 129, 10988 CrossRef CAS PubMed; (c) D. J. Wallace, R. L. Sidda and R. A. Reamer, J. Org. Chem., 2007, 72, 1051 CrossRef CAS PubMed; (d) A. Voituriez, A. Panossian, N. Fleury-Bregeot, P. Retailleau and A. Marinetti, J. Am. Chem. Soc., 2008, 130, 14030 CrossRef CAS; (e) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, Adv. Synth. Catal., 2009, 351, 1968 CrossRef CAS; (f) N. Pinto, M. Neel, A. Panossian, P. Retailleau, G. Frison, A. Voituriez and A. Marinetti, Chem.–Eur. J., 2010, 16, 1033 CrossRef CAS PubMed; (g) N. Pinto, P. Retailleau, A. Voituriez and A. Marinetti, Chem. Commun., 2011, 47, 1015 RSC; (h) D. Duvvuru, N. Pinto, C. Gomez, J.-F. Betzer, P. Retailleau, A. Voituriez and A. Marinetti, Adv. Synth. Catal., 2012, 354, 408 CrossRef CAS . For a [3 + 2] annulation reactions of α,β-unsaturated enones with 3-butynoates, see:; (i) M. Sampath and T.-P. Loh, Chem. Sci., 2010, 1, 739 RSC.
- For [3 + 2] annulation reactions of α,β-unsaturated esters with allenes, see: (a) M. Steurer, K. L. Jensen, D. Worgull and K. A. Jørgensen, Chem.–Eur. J., 2012, 18, 76 CrossRef CAS PubMed; (b) D. Wang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 2764 RSC; (c) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS; (d) A. Marinetti, M. Neel, J. Gouin and A. Voituriez, Synthesis, 2011, 2003 CrossRef; (e) X. N. Zhang and M. Shi, ACS Catal., 2013, 3, 507 CrossRef CAS; (f) S. Y. Lee, Y. Fujiwara, A. Nishiguchi, M. Kalek and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 4587 CrossRef CAS PubMed.
- For [3 + 2] annulation reactions of α,β-unsaturated cyanoethylenes with allenes, see: (a) M. Schuler, A. Voituriez and A. Marinetti, Tetrahedron: Asymmetry, 2010, 21, 1569 CrossRef CAS; (b) H. Xiao, Z. Chai, C. W. Zheng, Y. Q. Yang, W. Liu, J. K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed; (c) M. Gicquel, Y. Zhang, P. Aillard, P. Retailleau, A. Voituriez and A. Marinetti, Angew. Chem., Int. Ed., 2015, 54, 5470 CrossRef CAS PubMed.
- For [3 + 2] annulation reactions of α,β-unsaturated acrylamides with allenes, see: (a) Y. Fujiwara and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 12293 CrossRef CAS PubMed; (b) A. Voituriez, N. Pinto, M. Neel, P. Retailleau and A. Marinetti, Chem.–Eur. J., 2010, 16, 12541 CrossRef CAS PubMed; (c) X. Han, S.-X. Wang, F. Zhong and Y. Lu, Synthesis, 2011, 1859 CAS; (d) Q. Zhao, X. Han, Y. Wei, M. Shi and Y. Lu, Chem. Commun., 2012, 48, 970 RSC.
- For other examples of phosphine-promoted [3 + 2] annulations involving alkenes, see: (a) Z. Xu and X. Lu, Tetrahedron Lett., 1997, 38, 3461 CrossRef CAS; (b) G. Zhu, Z. Chen, Q. Jiang, D. Xiao, P. Cao and X. Zhang, J. Am. Chem. Soc., 1997, 119, 3836 CrossRef CAS; (c) S. G. Pyne, K. Schafer, B. W. Skelton and A. H. White, Chem. Commun., 1997, 67, 2267 RSC; (d) J.-C. Wang, S.-S. Ng and M. J. Krische, J. Am. Chem. Soc., 2003, 125, 3682 CrossRef CAS PubMed; (e) X.-F. Zhu, C. E. Henry and O. Kwon, Tetrahedron, 2005, 61, 6276 CrossRef CAS; (f) T. Q. Pham, S. G. Pyne, B. W. Skelton and A. H. White, J. Org. Chem., 2005, 70, 6369 CrossRef CAS PubMed; (g) Y. Xia, Y. Liang, Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS PubMed; (h) E. Mercier, B. Fonovic, C. Henry, O. Kwon and T. Dudding, Tetrahedron Lett., 2007, 48, 3617 CrossRef CAS; (i) C. E. Henry and O. Kwon, Org. Lett., 2007, 9, 3069 CrossRef CAS; (j) Y.-Q. Fang and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 5660 CrossRef CAS PubMed; (k) J. E. Wilson, J. Sun and G. C. Fu, Angew. Chem., Int. Ed., 2010, 49, 161 CrossRef CAS PubMed; (l) X.-C. Zhang, S.-H. Cao, Y. Wei and M. Shi, Org. Lett., 2011, 13, 1142 CrossRef CAS PubMed; (m) L.-J. Yang, S. Wang, J. Nie, S. Li and J.-A. Ma, Org. Lett., 2013, 15, 5214 CrossRef CAS PubMed; (n) J. Tian and Z. He, Chem. Commun., 2013, 49, 2058 RSC; (o) J. Marco-Martinez, V. Marcos, S. Reboredo, S. Filippone and N. Martin, Angew. Chem., Int. Ed., 2013, 52, 5115 CrossRef CAS PubMed.
- (a) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 767 CrossRef CAS PubMed; (b) F. Zhong, G.-Y. Chen, X. Han, W. Yao and Y. Lu, Org. Lett., 2012, 14, 3764 CrossRef CAS PubMed; (c) F. Zhong, X. Han, Y. Wang and Y. Lu, Chem. Sci., 2012, 3, 1231 RSC.
- (a) Y. Akhtar, M. B. Isman, P. M. Paduraru, S. Nagabandi, R. Nair and E. Plettner, J. Agric. Food Chem., 2007, 55, 10323 CrossRef CAS PubMed; (b) T. Sunazuka, T. Hirose and S. Omura, Acc. Chem. Res., 2008, 41, 302 CrossRef CAS PubMed.
- (a) S.-F. Zhu, Y. Yang, L.-X. Wang, B. Liu and Q.-L. Zhou, Org. Lett., 2005, 7, 2333 CrossRef CAS PubMed; (b) B. Liu, S.-F. Zhu, L.-X. Wang and Q.-L. Zhou, Tetrahedron: Asymmetry, 2006, 17, 634 CrossRef CAS; (c) W. Zhang, S.-F. Zhu, X.-C. Qiao and Q.-L. Zhou, Chem.–Asian J., 2008, 3, 2105 CrossRef CAS PubMed . For the reviews on the development of chiral spiro ligands and catalysts, see:; (d) J.-H. Xie and Q.-L. Zhou, Acc. Chem. Res., 2008, 41, 581 CrossRef CAS; (e) S.-F. Zhu and Q.-L. Zhou, in Privileged Chiral Ligands and Catalysts, Q.-L. Zhou, ed. Wiley-VCH, Weinheim, 2011, ch. 4, p. 137 Search PubMed.
- Crystallographic data for 3ab has been deposited with the Cambridge Crystallographic Data Centre as deposition number CCDC 1416080.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data and crystallographic data. CCDC 1416080. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra20603k |
| This journal is © The Royal Society of Chemistry 2015 |