Optimization of ultrasonic-assisted alkaline extraction of polysaccharides from Phellodendron amurense Rupr. pollen using response surface methodology and its structure features
Xuefei Wangab,
Hua Zhangac,
Zhenyu Wang*a and
Haina Baia
aDepartment of Food Science and Engineering, Harbin Institute of Technology, Harbin, China. E-mail: wangzy219001@163.com; Fax: +86 451 86282909; Tel: +86 451 86282909
bDepartment of Food and Environmental Engineering, East University of Heilongjiang, Harbin, China
cSchool of Material Science and Engineering Post-doctoral Research Station, Harbin Institute of Technology, Harbin, China
First published on 3rd December 2015
Abstract
To investigate extraction technology and determine the optimal extraction conditions of polysaccharides from Phellodendron amurense Rupr. pollen (PARPP), an alkaline extraction method was carried out to extract PARPP with ultrasonic treatment. The effects of three independent factors [NaOH concentration (X1: 0.4–0.6%), ultrasonic temperature (X2: 30–50 °C), ultrasonic time (X3: 30–50 min)] on the extraction yield of PARPP were optimized using a response surface methodology. The principal conclusions establish the optimal extraction conditions under the ultrasonic power 250 W for Phellodendron amurense Rupr. pollen were as follows: X1: 0.52%, X2: 43.02 °C, X3: 38.64 minutes. Verification experiment was carried out, and no significant difference was found between observed and estimated values for each response. Under such optimal conditions, the experimental yield was 4.133 ± 0.02%, which was compatible with the value predicted by model 4.157%, suggesting that the estimated models were reliable and valid for extract of polysaccharides. The UV, FT-IR and GC/MS analysis of PARPP showed that it was a type, or variety, of glycoprotein, and was composed of ribose, rhamnose, arabinose, xylose, mannose, glucose, galactose with the ratio of 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9
5.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.8
30.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4
6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2
3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.7
15.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.5.
18.5.
Introduction
Phellodendron amurense Rupr. (PAR) is a species of tree in the Rutaceae family, commonly called the Amur cork tree. The tree has leathery pinnate leaves and yellow clumped flowers. The name refers to the thick and corky bark of some (but not all) species of the genus. It is a major source of Huáng bò and one of the 50 fundamental herbs used in traditional Chinese medicine.Native to eastern Asia, northern China, Manchuria, Korea, Ussuri, Amur, and Japan, the Amur cork tree is considered invasive in many parts of North America. In China, the tree widely grows in Heilongjiang, Liaoning, and Jilin Province and in other places of northern China as well as in Mongolia. In China Phellodendron amurense Rupr. is used as a human therapeutic treatment internally. Either taken orally as a detoxification medication or applied for amendment individual's internal organ to have beneficial detoxification functions.
In recent years, polysaccharide has become a kind of potential therapeutic resources for its natural property, non-toxic and good curative effect. It has excellent bioactivity at the aspects of anti-fatigue, anti-tumor, antibacterial, radiation protection and strengthening organism immune system, etc.1 In modern pharmacological studies, the active components extracted from various varieties of plant pollen such as amino acids, vitamins, polysaccharides, and polyphenols are proven medications that decrease the danger of diabetes and mellitus affixation disease.2,3 Furthermore, Slađana et al. found the active component of pollen from various plants improved immunity by increasing the IgG content of the spleen index,4 as well as the thymus index and the activity of serum SOD, GSH-Px prevented hyperlipidemia and damage caused by radiotherapy injury.5–7
From our previous research, PAR pollen water extract was attested as to its credible ability to scavenge free radical and antioxidant activities that were correlated with the PAR pollen's high polysaccharide compound content.8 Therefore, in this research a variant of acid polysaccharide was separated from the PAR pollen with the objective of establishing the optimum extraction procedure of the PAR pollen polysaccharide (PARPP) with the purpose of maximizing the extraction yield.
There are many methods available for the extraction of polysaccharide from natural products some of which are solvent extraction, ultrasonic assisted extraction, microwave assisted extraction and supercritical CO2 extraction. The alkaline extract of Tricholoma crassum, reported by Pradip et al. (2012) was found to accelerate the dissolving of acidic polysaccharides by breaking the cell wall to extract the polysaccharide,9 and as reported by Wang et al. (2014), this technique had an enhanced antioxidant effect on Phellinus linteus polysaccharides.10
Ultrasonic assisted extraction is undoubtedly an emerging technology in the food industry; its use will contribute to the diffusion and dissolution of the active substances in a cell through ultrasonic cavitations, mechanical action, and thermal effect.11 In sum, alkaline extraction, when assisted by the ultrasonic technique, is able to improve the efficiency of polysaccharide extraction from a product when compared with the traditional hot water extract. The response surface methodology as an effective statistical method has recently been successfully applied to optimize complex processes by depicting the interaction relationship between response variables.12
A review of the relevant scientific literature on this subject found little or no scientific data and that it would appear that no research scientist had investigated the subject under discussion, though a number of research scientists have made inroads in other somewhat similar endeavours the implications from their research have proved to be valuable data for our research. Therefore, on this understanding, the study's objective was to isolate the polysaccharide from PAR pollen using the ultrasonic assisted method together with alkali solvent to evaluate the polysaccharide yields; UV, FT-IR, and GC-MS characterize the chemical profile of the bioactive component. The results from our study provide valuable data and a theoretical foundation for the component identification and functional research to expand comprehensive utilization of Phellodendron amurense Rupr. pollen.
Materials and methods
Materials
Methods
Following grinding by a basic IKA A11 grinder (Guangzhou Co., China); a powdered sample of PAR pollen was passed through a sieve with mesh size of 0.18 mm. Then, calculated amounts of this powder and petroleum ether, at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/v), were mixed uniformly for 3 hours at ambient room temperature, the suspension was then centrifuged using a LD5-2A at 4000 rpm for 10 minutes (Beijing Medical Centrifuge Co., China). This pretreatment process was repeated three times. Precipitation, in the centrifuge tube, dried at room temperature and was defatted. At that juncture, the PAR pollen was kept in sealed polyethylene bags at −20 °C until used. A batch of defatted PAR pollen (5 g) was then dissolved in an NaOH solution with a designed concentration ranging from 0.3% to 0.7% at a constant ratio of material to liquid (defatted pollen powder
3 (w/v), were mixed uniformly for 3 hours at ambient room temperature, the suspension was then centrifuged using a LD5-2A at 4000 rpm for 10 minutes (Beijing Medical Centrifuge Co., China). This pretreatment process was repeated three times. Precipitation, in the centrifuge tube, dried at room temperature and was defatted. At that juncture, the PAR pollen was kept in sealed polyethylene bags at −20 °C until used. A batch of defatted PAR pollen (5 g) was then dissolved in an NaOH solution with a designed concentration ranging from 0.3% to 0.7% at a constant ratio of material to liquid (defatted pollen powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH solutions = 1
NaOH solutions = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (w/v)). Extraction was carried out at the designed ultrasonic power and varied from 0 W to 250 W (KQ-250DDE, Kunshan, China), the ultrasonic temperature varied from 20 °C to 70 °C, and the ultrasonic time varied from 10 minutes to one hour. In order to prevent the increase of temperature in the sample as the water absorbs heat from the ultrasonic process, an additional water circulation device by self-developed was used to maintain a constant temperature, by which the temperature fluctuations of sample can be controlled within 2 °C and the effect of fluctuations on sample can be ignored.
30 (w/v)). Extraction was carried out at the designed ultrasonic power and varied from 0 W to 250 W (KQ-250DDE, Kunshan, China), the ultrasonic temperature varied from 20 °C to 70 °C, and the ultrasonic time varied from 10 minutes to one hour. In order to prevent the increase of temperature in the sample as the water absorbs heat from the ultrasonic process, an additional water circulation device by self-developed was used to maintain a constant temperature, by which the temperature fluctuations of sample can be controlled within 2 °C and the effect of fluctuations on sample can be ignored.
Following the ultrasonic treatment, and to accelerate the multiple polysaccharides dissolution, the sample was extracted, with hot water, for three hours at 85 °C. After that, the pH value of the mixture was adjusted to neutral by a phosphate buffer using an acidity meter (PB-10, Sartorius, Germany). As a consequence the centrifugal method removed the resultant protein precipitation by the changed sample conditions from alkaline to neutral.
Following the same centrifuged technique, as previously described, a liquid supernatant was concentrated to a third of the original volume, then precipitated by adding ethanol (24 h, 4 °C) to the final concentration of 80% (v/v). To obtain a crude polysaccharides solution, the precipitate was redissolved in a volumetric flask to a final volume of 100 mL. The polysaccharides content was measured by the phenol-sulfuric acid method using D-glucose as a standard. The percentage of PARPP extraction yield (%) was calculated as follows:
| Run | NaOH (%) X1 | Ultrasonic temperature (°C) X2 | Ultrasonic time (min) X3 | Extraction yield of PARPP (%) |
|---|---|---|---|---|
| a Source: developed for this research from Box–Behnken Design (BBD) of Design-Expert 7.1.3. | ||||
| 1 | 1 (0.6) | 0 (40 °C) | 1 (50 min) | 3.65 |
| 2 | 0 (0.5) | −1 (30 °C) | 1 | 3.83 |
| 3 | 0 | 0 | 0 (40 min) | 4.18 |
| 4 | 0 | 1 (50 °C) | 1 | 3.98 |
| 5 | 0 | −1 | −1 (30 min) | 3.86 |
| 6 | 0 | 1 | −1 | 3.97 |
| 7 | 0 | 0 | 0 | 4.18 |
| 8 | −1 | 1 | 0 | 3.29 |
| 9 | −1 | −1 | 0 | 3.32 |
| 10 | 0 | 0 | 0 | 4.2 |
| 11 | −1 | 0 | 1 | 3.42 |
| 12 | 1 | 1 | 0 | 3.84 |
| 13 | 1 | −1 | 0 | 3.51 |
| 14 | 0 | 0 | 0 | 3.99 |
| 15 | −1 | 0 | −1 | 3.39 |
| 16 | 1 | 0 | −1 | 3.78 |
| 17 | 0 | 0 | 0 | 4.09 |
Data from the BBD were analyzed by multiple regressions to fit the following quadratic polynomial model.
Results and discussion
Extraction yield of PARPP
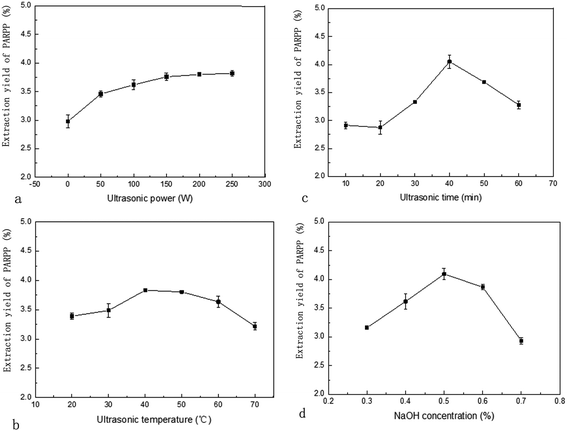
The extraction yield of the PARPP enhanced by ultrasonic power was shown in Fig. 1(a). Note that when the ultrasonic power exceeded 150 W the trend of the upward increased curve was slow. The maximum extraction yield of the PARPP was noted when the ultrasonic power was 250 W. Higher power will increase the polysaccharides diffusion coefficient and enhance the solubility of the polysaccharides in the extracting solvent. Similar results were found in the extraction of polysaccharide from Zagros oak leaf.15 For extraction and cost efficiency, the fixed ultrasonic power value of 250 W was used in this study.
The effect of different ultrasonic temperatures on the extraction of PARPP is shown in Fig. 1(b). The application of an appropriate high ultrasonic temperature enhanced extraction efficiency. There was a maximum value of polysaccharide extraction at the ultrasonic temperature of 40 °C.11,16 Above or below this value the extraction efficiency would slightly decease. A gradual increase in the ultrasonic temperature to 40 °C accelerated the ultrasonic propagation in the PARPP medium and enhanced the capacity of cavitation and, shortened the extraction time. However, when the ultrasonic temperature exceeded above a certain range of 40 °C, the vapour pressure increased leading to a reduced cavitation and a less efficient extraction.
As shown in Fig. 1(c), the extraction yield of PARPP significantly increased from 2.91% to 4.05% as the ultrasonic time increased from 10 minutes to 40 minutes and then negatively correlated by extending the ultrasonic time beyond 40 minutes. This outcome agreed with reports by other authors when extracting polysaccharides.17 This occurrence may well be caused by a mechanical shearing force being exerted by ultrasonic pressure to, in addition, decrease the extraction yield of polysaccharide and to then subsequently disrupt the PARPP chain structure.
The curve of NaOH concentration demonstrates that the PARPP yield increased with an increased concentration of NaOH until 0.5% and then began to decrease. Such variation trend may possibly be caused by the ability of a small amount of alkaline to break down the cell wall to accelerate the solubility of polysaccharide, the lipid saponification of raw material and to decrease interference from impurities. High concentration of NaOH may result in the decomposition of polysaccharide as well as increasing the rate of protein being dissolved that, in turn, will lead to the decline of the PARPP yield.18 The appearance of the polysaccharide was also affected in that the higher the concentration of NaOH the deeper the product colour.
Optimization of extraction parameters of PARPP
| Parameter | Sum of squares | df | Mean square | F-Value | P-Value | Significance |
|---|---|---|---|---|---|---|
| a *Significant at 0.01 < P < 0.05, **significant at P < 0.01.b Source: developed for this research from ANOVA of Design-Expert 7.1.3. | ||||||
| Model | 1.5117 | 9 | 0.1679 | 34.7032 | <0.0001 | ** |
| X1 | 0.2312 | 1 | 0.2312 | 47.7686 | 0.0002 | ** |
| X2 | 0.0392 | 1 | 0.0392 | 8.0991 | 0.0248 | * |
| X3 | 0.0018 | 1 | 0.0018 | 0.3719 | 0.5612 | — |
| X1X2 | 0.0324 | 1 | 0.0324 | 6.6942 | 0.0361 | * |
| X1X3 | 0.0064 | 1 | 0.0064 | 1.3223 | 0.2879 | — |
| X2X3 | 0.0004 | 1 | 0.0004 | 0.0826 | 0.7821 | — |
| X12 | 1.0275 | 1 | 1.0275 | 212.2975 | <0.0001 | ** |
| X22 | 0.0873 | 1 | 0.0873 | 18.0392 | 0.0038 | ** |
| X32 | 0.0231 | 1 | 0.0231 | 4.7638 | 0.0654 | — |
| Residual | 0.0338 | 7 | 0.0048 | |||
| Lack of fit | 0.0028 | 3 | 0.0009 | 0.1201 | 0.9435 | |
| Pure error | 0.0311 | 4 | 0.0078 | |||
| Cor total | 1.5456 | 16 | ||||
| R-Squared | 0.9781 | Adj. R-squared | 0.9499 | CV% = 1.83% | ||
| Y = −11.6200 + 49.1000X1 + 0.0732X2 + 0.0737X3 + 0.0900X1X2 − 0.0400X1X3 + 0.0001X2X3 − 49.4000X12 − 0.0014X22 − 0.0074X32 |
The ANOVA for the surface quadratic polynomial model response are summarized in Table 2, which follows. The high F value result (34.7032), as against the statistical significance of regression equation and low the P values (<0.0001), found evidence of a statistical significant effect.
The value of determination coefficient R2 and the adjusted determination coefficient Adj. R2 were respectively: 0.9781 and 0.9499. This outcome signified the fitted model explained 97.81% of the total variability within the values studied and the model had a good fit with the experimental data and the theoretical values of the PARPP yield. Furthermore, the result of lack of fit, the indication of the failure for a model representing the experimental data, was insignificant (P > 0.05). This outcome demonstrated that the developed quadratic regression model was sufficiently accurate and flawless for predicting a polysaccharide yield. A very low value of coefficient of variation (C.V. = 1.83%) clearly indicated the repeatability and reliability of the experimental values.15 As shown in the Table 2, the linear coefficients (X1, X2), quadratic term coefficients (X12, X22) and cross product coefficients (X1X2) were significant with their small P values (P < 0.05); the other term coefficient was not significant (P > 0.05). The F value showed that the successive order of factors influencing the extraction yield was NaOH concentration > ultrasonic temperature > ultrasonic time, and the order of interaction effect was X1X2 > X1X3 > X2X3.
 | ||
| Fig. 3 Contours plots showing the effect of NaOH concentration (X1), ultrasonic temperature (X2), and ultrasonic time (X3) on the yield of polysaccharides. (a) Effect of ultrasonic temperature and NaOH concentration on extraction yield of PARPP. (b) Effect of ultrasonic time and NaOH concentration on extraction yield of PARPP. (c) Effect of ultrasonic time and ultrasonic temperature on extraction yield of PARPP. Source: Fig. 2 and 3 data evaluated using Box–Behnken design principles. | ||
Fig. 2(a) and 3(a) illustrates the extraction yield of PARPP as a function of NaOH concentration and ultrasonic temperature at a fixed ultrasonic time (40 min). This result indicated that the influence of NaOH concentration on the extraction yield of PARPP was greater than that of ultrasonic temperature because the curved surface of NaOH concentration was steeper than the curved surface of the latter. The extraction yield of PARPP increased rapidly with the increase NaOH concentration from 0.4% to 0.53% and increased slowly with the increase of ultrasonic temperature from 30 °C to 42 °C. However, with the increasing of NaOH concentration from 0.53% to 0.6% and the increasing of ultrasonic temperature from 42 °C to 50 °C, the extraction yield of PARPP showed an apparent decrease.
The 3-D response surface plot and the 2-D contour plot, at varying ultrasonic time, and the NaOH concentration with a fixed ultrasonic temperature (0 level) are illustrated in Fig. 2(b) and 3(b). The steepness status of 3-D plot indicated that the most significant factor, an extraction yield of PARPP, was the NaOH concentration followed by the ultrasonic time. The extraction yield of the PARPP, i.e. the response value, was influenced significantly by the interaction between the NaOH concentration and the ultrasonic time by the elliptical contour shape of the 2-D plot above. When compared to the extending of ultrasonic time from 30 minutes to 37.5 minutes the extraction yield of PARPP increased more significant when NaOH concentration increased from 0.40%to 0.54%. The extraction yield of PARPP had a declining trend when the NaOH concentration exceeded 0.54% and ultrasonic time exceeded 37.5 minutes.
As shown in Fig. 2(c) and 3(c) where the extraction yield of PARPP was given as a function of ultrasonic temperature and ultrasonic time at a fixed NaOH concentration (0 level); the extraction yield of PARPP increased with increasing of ultrasonic temperature from 30 °C to 43 °C. However, beyond 43 °C the extraction yield of PARPP decreased with increasing ultrasonic temperature when the ultrasonic time was set. Similarly, when the ultrasonic temperature was set, the extraction yield of PARPP was also found to increase slightly by an extension of the ultrasonic time from 30 minutes to 39 minutes, at which time there was a slow decrease as the ultrasonic time continued to increase. Of note, that the maximum extraction yield of PARPP was achieved when the ultrasonic temperature and the ultrasonic time were respectively 43 °C and 39 minutes.
The 2-D contour plot shown in Fig. 3(c) demonstrates that the mutual interaction between ultrasonic temperature and ultrasonic time was not significant.
| NaOH concentration (%) | Ultrasonic temperature (°C) | Ultrasonic time (min) | Extraction yield of PARPP (%) | |
|---|---|---|---|---|
| a Source: developed for this research from the experimental value and predicted value under optimal condition. | ||||
| Optimum conditions (predicted) | 0.52 | 43.02 | 38.64 | 4.157 |
| Modified conditions (experimental) | 0.52 | 43 | 39 | 4.133 ± 0.02 |
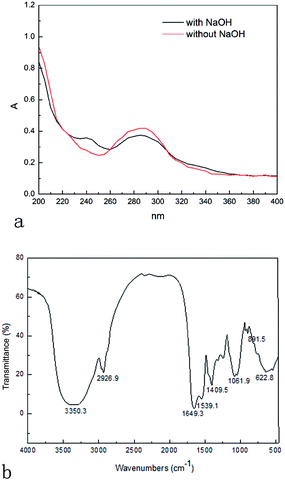
A glycoprotein is a glycoconjugate in which a protein carries carbohydrate chains covalently attached to its polypeptide backbone, usually via N-glycopeptide linkage and O-glycopeptide linkage. In order to determine the chemical bond between PARPP and protein, β-elimination reaction was adopted, according to which the N-linkage always keeps stable under low concentration alkaline condition, while O-linkage is easy to be broken and subsequently yield obvious absorption at 240 nm since the serine and threonine of glycosidic bonds translated into α-aminoacrylic acid and α-aminocrotonic acid, respectively under the same condition.20 As shown in Fig. 4(a) (the curve with NaOH) UV spectra after alkali treatment of PARPP were obtained and a stronger absorbance at 240 nm was observed, which suggested the existence of O-glycopeptide linkage.
In addition, Table 4 showed the amino acids composition of PARPP, seventeen amino acids were identified in the glycoprotein, which was mainly composed of Glu, Asp, Leu, Ser and Phe with the contents of 2.36%, 2.05%, 1.47%, 1.20% and 1.19%. The content of eight kinds of essential amino acid needed for human body was up to 38.06%. Especially contains serine and threonine, a kind of amino acid with hydroxyl group, they can connect to polysaccharide by O-glycopeptide linkage, which consistent with the above result identified in the β-elimination reaction.
| Amino acids | Content (%) | Amino acids | Content (%) |
|---|---|---|---|
| a Source: developed from Hitachi L8800 automatic amino acid analyzer. | |||
| Asp | 2.05 | Ile | 0.76 |
| Thr | 0.95 | Leu | 1.47 |
| Ser | 1.20 | Tyr | 0.68 |
| Glu | 2.36 | Phe | 1.19 |
| Gly | 1.11 | Lys | 0.63 |
| Ala | 1.12 | His | 0.37 |
| Cys | 0.21 | Arg | 0.31 |
| Val | 0.90 | Pro | 1.02 |
| Met | 0.51 | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of –CHO and of –COOH. This result indicated the characteristic peak of polysaccharide compounds. The featured signal vibration band for R–CO–NH–R was 1539 cm−1, which implied the existence of protein and was necessary to confirm that the PARPP mostly composed of glycoprotein.22,23 The FT-IR binding protein result was consistent with the above UV scan conclusion where the absorption peak reached 280 nm. The absorption bands at 1200–950 cm−1, for polysaccharide, occurred from the stretching vibration of glycosidic bond C–O–C, which was different from the position and intensity of the vibration between different polysaccharides. The absorption peak at 1061.9 cm−1 and 891.5 cm−1 indicated the vibration presence of a pyranose ring with a β-glycosidic linkage.24,25 β-Glycosidic bond existed in the most of plant polysaccharides with the bioactive prominent activity, especially β-(1→3) and β-(1→6) glycosidic bond on dextran main chains contributes to the anti-tumor effect of improving the immunity for organism since its special way of key links and the existence of intra-molecular hydrogen bond to form the molecular structure of spiral pattern, which unique configuration is easy to be accepted by the immune system.26 Analysis of FT-IR have proved the existence of β-glycosidic bond in the PARPP, so speculated that there maybe have partially correlation between this kind of chemical bond and previous research conclusion about antioxidant function of PARPP. Table 5 below, illustrates the PARPP – FT-IR characteristic peak.
O group of –CHO and of –COOH. This result indicated the characteristic peak of polysaccharide compounds. The featured signal vibration band for R–CO–NH–R was 1539 cm−1, which implied the existence of protein and was necessary to confirm that the PARPP mostly composed of glycoprotein.22,23 The FT-IR binding protein result was consistent with the above UV scan conclusion where the absorption peak reached 280 nm. The absorption bands at 1200–950 cm−1, for polysaccharide, occurred from the stretching vibration of glycosidic bond C–O–C, which was different from the position and intensity of the vibration between different polysaccharides. The absorption peak at 1061.9 cm−1 and 891.5 cm−1 indicated the vibration presence of a pyranose ring with a β-glycosidic linkage.24,25 β-Glycosidic bond existed in the most of plant polysaccharides with the bioactive prominent activity, especially β-(1→3) and β-(1→6) glycosidic bond on dextran main chains contributes to the anti-tumor effect of improving the immunity for organism since its special way of key links and the existence of intra-molecular hydrogen bond to form the molecular structure of spiral pattern, which unique configuration is easy to be accepted by the immune system.26 Analysis of FT-IR have proved the existence of β-glycosidic bond in the PARPP, so speculated that there maybe have partially correlation between this kind of chemical bond and previous research conclusion about antioxidant function of PARPP. Table 5 below, illustrates the PARPP – FT-IR characteristic peak.
| Characteristic peak (cm−1) | Functional groups |
|---|---|
| a Source: developed from TENSOR27 FT-IR spectrometer. | |
| 1200–950 | C–O–H or C–O–C |
| 3350.3 | O–H |
| 2926.9 | C–H |
| 1649.3 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 1061.9 | β-Glycosidic linkage |
| 1539.1 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N–H C–N–H |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9
5.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.8
30.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4
6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2
3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.7
15.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.5. Among them, arabinose, as the major monosaccharide moiety, accounted for 36.62% from which the polysaccharide extracted from the PAR pollen was speculated to be an arabinoxylan with a branch structure. Knowledge of the chemical composition of the glycoconjugates is important for understanding their applications and bioactivities. Pleurotus eryngii polysaccharide, a polysaccharide containing mannose, ribose, rhamnose, glucose has been found to act effectively as antioxidants and radical scavengers.27 Polysaccharides extracted from Actinidia arguta with the monosaccharide composition including mannose, rhamnose, glucose, galactose and arabinose, was also exhibited antioxidant activity and immunity activity.28 Previous research by Cao et al. (2011) found antitumor and immunomodulatory activity in the arabinoxylans of wheat bran.29 Therefore, we hypothesize that the early discovery concerning the antioxidant function of PARPP is directly related with the main components of arabinoxylans.8
18.5. Among them, arabinose, as the major monosaccharide moiety, accounted for 36.62% from which the polysaccharide extracted from the PAR pollen was speculated to be an arabinoxylan with a branch structure. Knowledge of the chemical composition of the glycoconjugates is important for understanding their applications and bioactivities. Pleurotus eryngii polysaccharide, a polysaccharide containing mannose, ribose, rhamnose, glucose has been found to act effectively as antioxidants and radical scavengers.27 Polysaccharides extracted from Actinidia arguta with the monosaccharide composition including mannose, rhamnose, glucose, galactose and arabinose, was also exhibited antioxidant activity and immunity activity.28 Previous research by Cao et al. (2011) found antitumor and immunomodulatory activity in the arabinoxylans of wheat bran.29 Therefore, we hypothesize that the early discovery concerning the antioxidant function of PARPP is directly related with the main components of arabinoxylans.8
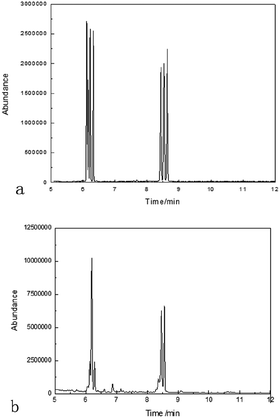
The retention time and molar ratio are illustrated in Table 6. The result from the monosaccharide composition of PARPP was consistent with the analysis of FT-IR for the existence of pyranose residues.
| Compound | Ret time (min) | Molar ratio |
|---|---|---|
| a Source: developed for this research from GC-MS analysis. | ||
| Ribose | 6.05 | 3.688137 |
| Rhamnose | 6.13 | 5.940915 |
| Arabinose | 6.2 | 30.79731 |
| Xylose | 6.29 | 6.433757 |
| Mannose | 8.36 | 3.218806 |
| Glucose | 8.46 | 15.56556 |
| Galactose | 8.56 | 18.45304 |
Conclusions
An ultrasonic assisted alkali extraction was found to be an effective method for improving Phellodendron amurense Rupr. pollen polysaccharide (PARPP) yield. The response surface method was used to optimize the extraction conditions of PARPP including NaOH concentration, ultrasonic time, and ultrasonic temperature. A second-order polynomial model gave a satisfactory description of the experimental data. In the case of 250 W ultrasonic power, optimal extraction conditions were performed as follows: NaOH concentration of 0.52%, ultrasonic temperature of 43.02 °C and ultrasonic time of 38.64 minutes. Under these specifications, the mean experimental value of PARPP extraction yield (4.13%) was achieved. This outcome corresponded satisfactorily with the predicted value by an analysis of variance. The polysaccharide yield increased 38.6% compared with non-ultrasonic assisted extraction (extraction yield 2.98%). A UV scan and analysis of amino acid composition indicated the existence of protein in the PARPP for emerging the absorption peak at 280 nm and different content of amino acid; an FT-IR spectroscopy demonstrated a similar result. The β-elimination reaction actually indicated the existence of O-glycopeptide linkage of glycoprotein.The analysis of the PARPP composition identified seven different monosaccharide composed of the polysaccharide extracted by the ultrasonic assisted technique with a NaOH solvent extracted from the PARPP. They were ribose, rhamnose, arabinose, xylose, mannose, glucose, galactose with a ratio of 3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.9
5.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.8
30.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.4
6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.2
3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.7
15.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.5.
18.5.
It is important to emphasize that the ultrasonic assisted assay mentioned in this research is an effective way to extract polysaccharide from Phellodendron amurense Rupr. pollen. Our research optimized and improved current technology to achieve the best polysaccharide yield with nominal monetary expense. At present, some polysaccharides such as lentinan, Ganoderma lucidum polysaccharide with a variety of important bioactivities and functions has become one of hotspots in the drug development and research. Results from this study also provide a better understanding of several factors associated with polysaccharide bioactivity including composition of monosaccharide, category of glycosidic bond and glycoprotein, and will allow for the meaningful identification of selected phytochemicals with biological property potential for further investigation and development into valued added foods and nutraceuticals, such as capsules or tablets, etc.
Conflicts of interest
The authors of the paper referenced above, have no financial and personal relationships with other people or organizations that could inappropriately influence (bias) this work.Acknowledgements
The authors thank Dr Baiyan Cai from Department of Food and Environmental Engineering, East University of Heilongjiang, Harbin, China, for his helpful suggestions and assistance. This work was supported by National Natural Science Foundation of China (No. 31401483) and Post-Doctoral Fund of Heilongjiang Province (LBH-Z14098). Zhenyu Wang designed and supervised the research project and provided financial support for the conduct of the research. Xuefei Wang finished all of the experiments and wrote the paper. Hua Zhang and Haina Bai assisted in research and conducted the informatics' analysis.References
- Y. Chen, M. Y. Xie and S. P. Nie, Food Chem., 2008, 107, 231–241 CrossRef CAS.
- F. L. Hu, H. Z. Xuan, W. Zhu, M. L. Chen and H. Z. Ying, Apiculture of China, 2003, 54, 9–11 Search PubMed.
- F. K. Zhao and B. H. Xu, Apiculture of China, 2013, 64, 38–42 Search PubMed.
- S. Žilić, J. Vančetović, M. Janković and V. Maksimović, J. Funct. Foods, 2014, 10, 65–74 CrossRef.
- S. K. Wang, C. X. Zhang, C. R. Lin, S. Y. Zuo and J. F. Qian, Feed Ind., 2013, 34, 11–15 Search PubMed.
- Y. L. Liu, J. W. Wang, J. M. Tong and S. Y. Li, Lab. Anim. Sci., 2013, 30, 41–43 Search PubMed.
- H. Zhang, W. H. Lu, C. G. Zhang and Y. C. Li, Sci. Technol. Food Ind., 2013, 34, 350–353 CAS.
- X. F. Wang, H. Zhang, Z. Y. Wang, Y. H. Du and H. N. Bai, Sci. Technol. Food Ind., 2014, 35, 139–143 CAS.
- P. Pradip, K. B. Sunil, K. S. Ipsita, K. N. Ashis, S. Surajit and D. Debsankar, et al., Carbohydr. Res., 2012, 362, 1–7 CrossRef PubMed.
- Z. B. Wang, J. J. Pei and H. L. Ma, et al., Carbohydr. Polym., 2014, 109, 49–55 CrossRef CAS PubMed.
- Y. Zheng, Y. Li and W. D. Wang, Carbohydr. Polym., 2014, 111, 315–323 CrossRef CAS PubMed.
- H. G. Chen, X. Zhou and J. Z. Zhang, Carbohydr. Polym., 2014, 111, 567–575 CrossRef CAS PubMed.
- Z. Zhao, H. T. Ji, D. B. Liu, Y. R. Ji and X. L. Hao, Journal of Jilin Normal University, 2015, 2, 112–116 Search PubMed.
- H. N. Zhang, H. L. Ma and W. Liu, et al., Carbohydr. Polym., 2014, 113, 380–387 CrossRef CAS PubMed.
- S. Tahmouzi, Carbohydr. Polym., 2014, 106, 238–246 CrossRef CAS PubMed.
- Y. T. Tian, Z. B. Xu, B. D. Zheng and Y. Martin Lo, Ultrason. Sonochem., 2013, 20, 202–208 CrossRef CAS PubMed.
- Y. L. Yan, C. H. Yu and J. Chen, et al., Carbohydr. Polym., 2011, 83, 217–224 CrossRef CAS.
- N. Khodaei and S. Karboune, Food Chem., 2013, 139, 617–623 CrossRef CAS PubMed.
- X. R. Liu, L. M. Zhang and J. N. Hu, Food Sci., 2013, 34, 96–102 CAS.
- T. T. Li, H. Zhang and C. E. Wu, Sci. Technol. Food Ind., 2014, 35, 100–103 CAS.
- M. Ka
![[c with combining breve]](https://www.rsc.org/images/entities/char_0063_0306.gif) uráková, P. Capek and V. Sasinková, Carbohydr. Polym., 2000, 43, 195–203 CrossRef.
uráková, P. Capek and V. Sasinková, Carbohydr. Polym., 2000, 43, 195–203 CrossRef. - Y. J. Li, M. C. Wu and J. Q. Deng, Sci. Technol. Food Ind., 2009, 30, 93–94 CAS.
- Y. S. Huang, Instrument Analysis, Chemical Industry Press, BeiJing, 2002 Search PubMed.
- L. Y. Guo, J. M. Ou and S. Z. Sun, Journal of Yunnan Normal University, 2005, 25, 48–50 Search PubMed.
- J. Chen and Y. Xiang, Mod. Food Sci. Technol., 2013, 29, 1544–1550 CAS.
- Z. Y. Sh, Z. W. Huang, J. H. Xiao and J. W. Cao, Food Sci., 2015, 13, 215–222 Search PubMed.
- Y. Y. Luo, D. Y. Ren, L. F. Chen and X. B. Yang, Sci. Technol. Food Ind., 2015, 36, 158–166 CAS.
- M. L. Zhao, L. L. Wang, L. Y. Liu and K. Zhong, Mod. Food Sci. Technol., 2015, 31, 46–50 Search PubMed.
- L. Cao, X. Z. Liu and T. X. Qian, et al., Int. J. Biol. Macromol., 2011, 48, 160–164 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |