Codelivery of doxorubicin and p53 by biodegradable micellar carriers based on chitosan derivatives
Guan-Hai Wang†
ab,
Hui-Kang Yang†c,
Yi Zhaod,
Da-Wei Zhang*e,
Li-Ming Zhang*f and
Jian-Tao Lin*ab
aDongguan Scientific Research Center, Guangdong Medical University, Dongguan 523808, China. E-mail: linjt326@163.com; wanggh0101@163.com
bGuangdong Key Laboratory for Research and Development of Natural Drugs, Guangdong Medical University, Zhanjiang 523024, China
cDepartment of Radiology, Guangzhou First People’s Hospital, Guangzhou Medical University, Guangzhou 510180, China. E-mail: 123086302@qq.com
dDepartment of Microbiology and Immunology, School of Basic Medicine, Guangdong Medical University, Dongguan 523808, China. E-mail: zhaoyicomnet@gmail.com
eDepartment of Pharmacology, School of Medicine, Guangdong Medical University, Dongguan 523808, China. E-mail: D.w.zhang@163.com; ceszhlm@mail.sysu.edu.cn
fDSAPM Lab, PCFM Lab, Institute of Polymer Science, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China
First published on 27th November 2015
Abstract
In this work, novel biodegradable cationic micelles were prepared based on poly-(N-ε-carbobenzyloxy-L-lysine) (PZLL) and chitosan (CS) by click reaction, and applied for co-delivery of doxorubicin (DOX) and p53 plasmid. The structure of the copolymer was characterized by 1H NMR and FTIR. The loading amount of DOX in the micelles was 12.8%. Fluorescence spectra confirmed that DOX interacted via π–π stacking with micelles when DOX was encapsulated into the micelles. In particular, its complexation with plasmid DNA was investigated using agarose gel electrophoresis, flow cytometry, zeta potential, and particle size analyses as well as transmission electron microscopy observations. The results showed that the copolymers have a strong pDNA condensation ability and provide protection of pDNA against deoxyribonuclease I degradation. CS-g-PZLL/DOX/p53 nanoparticles showed good gene transfection efficiency in vitro. Fluorescence images and flow cytometry tests revealed that p53 and DOX could be efficiently transported into Hela tumor cells simultaneously, and the optimum N/P ratio for p53 transfection was 20/1. For co-delivery analysis, the obtained CS-g-PZLL/DOX/p53 complexes showed a better inhibitory effect on Hela tumor cells than DOX or p53 used singly.
1. Introduction
To date, cancer treatment still faces serious challenges. Chemotherapy is one of the reliable choices for the treatment of many cancers. However, treatment with chemotherapy has been limited because of the emergence of multi-drug resistance (MDR), which is commonly associated with cancer cell over expression of drug transporter proteins.1 A novel approach to address cancer drug resistance is to take advantage of the co-delivery of anticancer drugs and nucleic acid using multi-functionalized nanocarriers. Co-delivery of anticancer drugs and nucleic acid to the tumor site could efficiently control the drug transporter proteins. This promising approach may sidestep MDR and lead to an improved therapeutic effect.For the co-delivery of drugs and genes while maintaining their biological functions, there has been an increasing interest in the development of multifunctional polymeric carriers using polymers,2–4 liposomes5,6 dendrimers,7,8 silica,9,10 quantum dot11 based nanoparticles and so on. As one of the most promising nanocarrier systems, self-assembled cationic polymeric micelles have been widely utilized as drug and gene co-delivery systems. Cationic micelles are very effective nano-carriers for the co-delivery of genes and drugs into various cancer cell lines. Shi et al. prepared a series of cationic micelles based on triblock copolymers (MPEG–PCL-g-PEI) to deliver doxorubicin and the gene Msurvivin T34A. Their results showed that DOX and the gene were successfully co-delivered to the MCF-7 and CT26 cells. Introduction of T34A in combination with doxorubicin could greatly reduce systemic toxicity as well as improve the anti-tumor efficiency.12 Lee et al. designed an amphiphilic copolymer poly{(N-methyldietheneamine sebacate)-co-[(cholesteryl oxocarbonylamido ethyl) methyl bis ammonium bromide] sebacate} [P(MDS-co-CES)] to deliver human TRAIL and paclitaxel simultaneously. They found that the co-delivery nanoparticulate system induced synergistic anti-cancer activities with relatively low toxicity in non-cancerous cells.13 However, these kinds of cationic polymers are still associated with problems of biodegradability, biocompatibility and cytotoxicity, which need to be overcome for in vivo application.
In this work, novel cationic micelles based on a polysaccharide and polypeptide were prepared by click chemistry, and applied for co-delivery of doxorubicin (DOX) and p53 plasmid. Poly-(N-ε-carbobenzyloxy-L-lysine) (PZLL) is a hydrophobic polypeptide derivate. Due to its good biocompatibility and biodegradability, PZLL has been widely used as the hydrophobic inner cores of micelles.14–16 DNA can be bound tightly to the surfaces of the micelles because of the amino groups in the chitosan chains and PZLL branch chains. Their proton buffering capability, DNA condensation ability, protection of pDNA against deoxyribonuclease I degradation, in vitro cytotoxicity and gene transfection efficiency into Hela cells were investigated via acid–base titration, agarose gel electrophoresis, MTT, flow cytometry assay and fluorescence microscopy. Their drug loading capacity and in vitro release behavior were studied using DOX as a model drug. The co-delivery of an anti-cancer DOX and functional gene (p53 plasmid) into Hela cells was also investigated and discussed in this study.
2. Experimental section
2.1. Materials
N-ε-Carbobenzyloxy-L-lysine, doxorubicin hydrochloride, azido propylamine phthalic anhydride, hydrazine monohydrate, N-bromosuccinimide (NBS), triphenylphosphine (TPP), copper sulfate, 1-methyl-2-pyrrolidinone (NMP) and triphosgene were purchased from Aladdin Chemical Reagent Co., Ltd. (China). Chitosan (Mw = 10 kDa) was purchased from Haidebei Marine Bioengineering Co. Ltd. (China). Propargylamine, sodium azide (NaN3, 99%) and sodium ascorbate (99%) were purchased from Alfa Aesar. The Dulbecco’s modified Eagle medium (DMEM), trypsin–ethylenediaminetetraacetic acid (trypsin–EDTA), and fetal bovine serum (FBS) were purchased from Gibco-BRL (Canada). Polyethylenimine (PEI, 25 kDa) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (U.S.A.). Deoxyribonuclease I (DNaseI) was purchased from Feibo Life Sciences (China). The plasmid p53 was obtained from Invitrogen. All other reagents were analytical grade and were used as received.2.2. Preparation of CS-g-PZLL copolymers
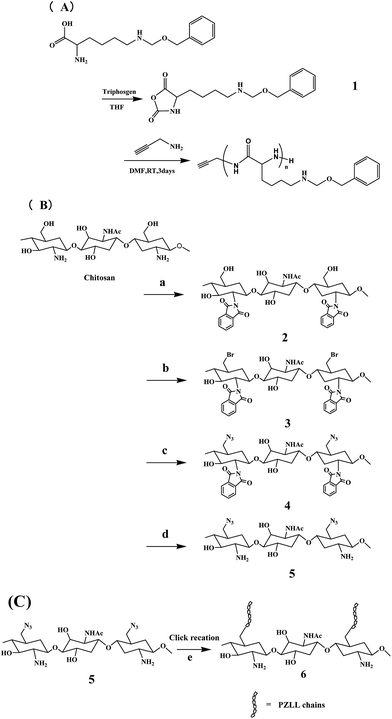
CS-g-PZLL was prepared by click reaction of α-alkyne-poly-(N-ε-carbobenzyloxy-L-lysine) (α-alkyne-PZLL) and azide focal point chitosan (6-N3–CS), as shown in Scheme 1. First, α-alkyne-PZLL (Scheme 1A) was synthesized following a procedure reported by Lin.17In brief, 5.0 g of N-ε-carbobenzyloxy-L-lysine (17.8 mmol) was reacted with 3 g of triphosgene (10.1 mmol) using tetrahydrofuran (THF) as solvent. The reaction time was 1 h and reaction temperature was 50 °C. After reaction, the solvent was removed in vacuo, and the obtained residue was first dissolved in ethyl acetate, then washed with cold 5% NaHCO3 solution. The ethyl acetate layer was collected and dried using anhydrous Na2SO4. The ethyl acetate was removed in vacuo and a ε-carbobenzyloxy-L-lysine N-carboxyanhydride (Lys (Z)-NCA) white solid with a yield of 77% was obtained. After that, 1.0 g of Lys (Z)-NCA was reacted with 0.01 g of propargylamine in anhydrous dimethylformamide (DMF), and the reaction time was 3 days. After reaction, methanol was added into the solution and a white powder deposit was obtained. The chemical structure was confirmed by 1H NMR and FTIR. 1H NMR (400 MHz, D2O): δ 7.36–7.41 (5H, m, –Ph), 5.08 (s, 2H, –OCH2Ph), 3.95 (s, 2H, –CCH2NH–), 2.89 (d, 2H, –NHCH2CH2), 1.7 (t, 2H, –CHCH2CH2–), 1.24 (m, 2H, –CHCH2CH2–). IR (KBr, cm−1): 3299, 3053, 2970, 1645, 1544, 1258, 1165, 1013, 539.
Second, 6-N3–CS (Scheme 1B) was synthesized by a similar method as that reported by Deng et al.18 In brief, chitosan and phthalic anhydride were dissolved in DMF and reacted for 8 h at 120 °C to obtain N-phthaloyl-chitosan (2). (2) was then reacted with N-bromosuccinimide to obtain 6-bromide-6-deoxy-N-phthaloyl-chitosan (3), and after that, (3) was reacted with sodium azide in N-methylpyrrolidone for 8 h at 80 °C. After reaction, the solution was filtered and precipitated with ethanol, and the precipitate was collected and washed using acetone three times, then dried under vacuum to get 6-azido-6-deoxy-N-phthaloyl-chitosan (4) with a yield of 73%. 1H NMR (400 MHz, D2O): δ 3.30–3.90 (m, D-glucosamine unit, H-3, H-4, H-5, H-6, H-60), 3.34–3.78 (2H, –CONHCH2–), 2.90 (protons next to amines). IR (KBr, cm−1): 3442, 2930, 2114, 1667, 1382, 1071, 1013, 657.
Finally, CS-g-PZLL was synthesized as shown in Scheme 1C. α-Alkyne-PZLL (3 mmol), 6-N3–CS (1 mmol) and the catalyst (CuSO4·5H2O/sodium ascorbate, 0.5 mmol/1 mmol) were dissolved in 20.0 mL of DMSO/water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixture solution and reacted at 50 °C for 24 h. The mixture was then precipitated with ethanol and purified by dialysis in water for 2 days. After dialysis and lyophilization, the CS-g-PZLL was collected as a brown powder with a yield of 87%. 1H NMR (400 MHz, D2O): δ 7.36–7.41 (5H, m, –Ph), 5.08 (s, 2H, –OCH2Ph), δ 3.30–3.90 (m, D-glucosamine unit, H-3, H-4, H-5, H-6, H-60), 3.34–3.78 (2H, –CONHCH2–), 2.90 (protons next to amines) 1.7 (t, 2H, –CHCH2CH2–), 1.24 (m, 2H, –CHCH2CH2–). IR (KBr, cm−1): 3299, 3053, 2930, 1645, 1382, 1258, 1165, 1013, 539.
1, v/v) mixture solution and reacted at 50 °C for 24 h. The mixture was then precipitated with ethanol and purified by dialysis in water for 2 days. After dialysis and lyophilization, the CS-g-PZLL was collected as a brown powder with a yield of 87%. 1H NMR (400 MHz, D2O): δ 7.36–7.41 (5H, m, –Ph), 5.08 (s, 2H, –OCH2Ph), δ 3.30–3.90 (m, D-glucosamine unit, H-3, H-4, H-5, H-6, H-60), 3.34–3.78 (2H, –CONHCH2–), 2.90 (protons next to amines) 1.7 (t, 2H, –CHCH2CH2–), 1.24 (m, 2H, –CHCH2CH2–). IR (KBr, cm−1): 3299, 3053, 2930, 1645, 1382, 1258, 1165, 1013, 539.
2.3. DOX loading and in vitro release
DOX was loaded using a dialysis method reported by Lin et al.9 Briefly, 10 mg of CS-g-PZLL and 5.0 mg of DOX hydrochloride were first dissolved using 5.0 mL of DMSO as solvent, and for neutralization of HCl, a drop of triethylamine was then added to the solution. The complete dissolved solution was then transferred to a dialysis bag (MWCO 3000) and subjected to dialysis against distilled water for 48 h. After that, the dialysis solution was filtered through a 0.45 μm filter and then lyophilized. To investigate the interactions between CS-g-PZLL and DOX, the obtained CS-g-PZLL/DOX complex was analysed using obtained fluorescence spectra. The excitation wavelength was 330 nm and the fluorescence emission spectra were recorded in the range from 400 to 700 nm. To determine the loading amount of DOX, the obtained DOX/CS-g-PZLL was dissolved in DMSO and analyzed using UV-vis spectrophotometry (TU-1900, China) at 480 nm. It was found that the loading amount of DOX was 12.8%. The loading amount of DOX was calculated according to the following equation:| LC = M1/M0 × 100% |
The release of DOX from CS-g-PZLL was assayed at 37 °C in PBS buffer of pH 5.8 (to simulate the pH of a tumor) and 7.4 (to simulate the pH of blood plasma). A predetermined amount of the DOX/CS-g-PZLL complexes in 5.0 mL of PBS (pH 5.0 and 7.4) was sealed in a dialysis bag (MWCO = 3 kDa), and then the dialysis bag was submerged in 20 mL of the corresponding buffer. At predetermined time intervals, 2.0 mL of aqueous solution was taken out for drug concentration measurement and replaced by an equal volume of fresh PBS. The released DOX in the incubation buffer was analyzed using UV-vis spectrophotometry (TU-1900, China) at 480 nm. All measurements were performed in triplicate.
2.4. Plasmid binding
DNase I was added to the CS-g-PZLL/p53 complexes (N/P 5, 10 and 20) to examine the protective ability of CS-g-PZLL against DNase degradation. DNase I and the complexes were incubated at 37 °C for 30 min, after that, EDTA (4.0 mL, 250 mM) and sodium dodecyl sulfate (SDS) solution (4.0 mL, 10%, w/v) were added and the mixture was incubated at room temperature for another 1 h. The samples were then electrophoresed on the 1.0% agarose gel to examine the integrity of the DNA.
2.5. In vitro cytotoxicity assay
An in vitro cytotoxicity assay of CS-g-PZLL and DOX loaded CS-g-PZLL nanoparticles was performed against Hela cells using the MTT assay. Five multiple holes were set for every sample. Briefly, Hela cells were cultured on 96-well plates at a density of 1 × 104 cells per well and incubated in a humidified atmosphere of 5% CO2 at 37 °C for 12 h. After that, the growth medium was replaced with 100 μL of complete DMEM containing an indicated amount of sample and the cells were further incubated for 24 h. Then 10 μL of MTT (0.5 mg mL−1) in PBS solution was added to each well, and the cells were incubated for another 4 h to form formazan crystals. Finally, the medium was removed and 100 μL of DMSO was added to each well. The optical density values of the samples were measured at 490 nm using a MRX-Microplate Reader (Thermo, USA). Cells treated with the same amount of PBS were used as a control. The relative cell viability was calculated as follow:| Cell viability (%) = [A490 (sample)/A490 (control)] × 100 |
3. Result and discussion
3.1. Synthesis and characterization of CS-g-PZLL
Fig. 1 shows the 1H NMR spectra of the CS-g-PZLL, 6-N3–CS, and PZLL. As seen in the spectrum of the CS-g-PZLL, the new peaks at 5.0, and 7.0–7.30 ppm showed the presence of the PZLL compared to the spectrum of the 6-N3–CS. Moreover, the peak at 8.1 ppm in the spectrum of the CS-g-PZLL indicated the presence of the triazole proton, which could be attributed to the formation of 6-N3–CS and PZLL by the click reaction.Fig. 2 shows the FTIR spectra of the CS-g-PZLL, N3–CS and PZLL. As seen in the FTIR spectrum of N3–CS, the characteristic vibration band for the azide group at 2110 cm−1 demonstrated that azide groups were successfully incorporated in N3–CS. A new peak at 1450 cm−1 appeared in the samples of CS-g-PZLL, which is attributed to the 1,2,3-triazole structure formed during click modification.17 The FTIR data indicated that the PZLL dendrimer was successfully grafted onto the chitosan chains via click chemistry.
3.2. DOX-loaded micelles and in vitro drug release
The in vitro release of drugs from the micelles was investigated at pH 7.4 and 5.0 to imitate the pH of blood and a tumor site. DOX was chosen as the model drug, and DOX was encapsulated into the hydrophobic core of the micelles. Fig. 3A shows the interaction of DOX and micelles by fluorescence spectra. It was found that the fluorescence of the complex was nearly completely quenched when DOX was encapsulated into the micelles, confirming the π–π stacking interaction between DOX and the phenyl groups of the PZLL chains.19,20 Moreover, the π–π stacking interaction helps to improve the high loading amount of DOX. It was found that the loading amount of DOX in the micelles was 12.8%. As shown in Fig. 3B, the release profiles showed the sustained release behaviors of DOX at pH 7.4 and pH 5.0, where 35.7% and 37.8% of the loaded DOX was released at pH 7.4 and pH 5.0 in the initial 12 h, and the accumulated release reached 61.4% and 75.4% after 96 h, respectively (Fig. 3B). It is interesting to find that the release rate and released amount of DOX at pH 5.0 were a little higher than those at pH 7.4, which revealed a promoted drug release behavior at the tumor site. DOX was released from micelles mainly by free diffusion. The better solubility of DOX at pH 5.0 than at pH 7.4 promoted the rapid release behavior. Moreover, the pH-responsive property of the copolymers leads to the higher release rate at lower pH. Since a tumor shows a lower extracellular pH, this observation demonstrated that the CS-g-PZLL micelle might show potential applications as a tumor-targeting drug delivery platform.The co-delivery of drugs and genes has become the primary strategy in cancer and other disease therapy in recent years, because this technique could promote synergistic actions, improve target selectivity and deter the development of drug resistance. For the co-delivery of antitumor drugs and genes while maintaining their chemo-physical properties and biological functions, a multifunctional carrier in which gene and drug could be loaded simultaneously is necessary. In previous studies, cationic polymeric micelles have been widely used as drug and gene co-delivery carriers. Zhu et al.22 prepared biodegradable cationic micelles based on the self-assembly of PDMAEMA–PCL–PDMAEMA triblock copolymers as siRNA and paclitaxel co-delivery carriers. The results demonstrated that combinatorial delivery of VEGF siRNA and paclitaxel showed an efficient knockdown of VEGF expression. Zheng et al.23 prepared polypeptide cationic micelles based on poly(ethylene glycol)-b-poly(L-lysine)-b-poly(L-leucine) (PEG–PLL–PLLeu) triblock copolymers as docetaxel (DTX) and siRNA-Bcl-2 co-delivery vectors. The results showed that the co-delivery of DTX and siRNA-Bcl-2 (siRNA that suppresses the expression of the anti-apoptotic Bcl-2 gene) significantly inhibited tumor growth as compared to the individual siRNA or DTX treatment.
To confirm the cell inhibition effect of the complexes containing both DOX and p53, we evaluated their cytotoxic effects using a MTT assay. The results showed that CS-g-PZLL was non-toxic at the concentration used in this assay, while the samples containing p53 showed an obvious cytotoxicity, where the cell viability was decreased to 70, 58 and 55% at the N/P ratios of 10, 20 and 40 (Fig. 6C). A further inhibitory effect was found in the co-delivery group, where the cell viability was decreased to 63, 41 and 44%, respectively. The better effect may be attributed to the released DOX being able to damage DNA; meanwhile, p53 could instigate mRNA transcription to down-regulate protein expression. The result suggested that the co-delivery induces synergistic actions and leads to an effective method for tumor therapy.
4. Conclusion
For the co-delivery of an anti-tumor drug and gene to tumor cells, a new cationic micelle consisting of poly-(N-ε-carbobenzyloxy-L-lysine) (PZLL) and chitosan has been synthesized, and used to co-deliver DOX and p53 for cancer therapy. CS-g-PZLL/DOX/p53 nanoparticles showed good gene transfection efficiency in vitro, and could deliver p53 and DOX simultaneously to Hela tumor cells. Through co-delivery analysis, the obtained CS-g-PZLL/DOX/p53 complexes were shown to have a better inhibitory effect on Hela tumor cells than p53 used singly. Such a cationic micelle delivery system may be used as a potential multifunctional vector for future cancer therapy applications.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (81402563), Traditional Chinese Medicine Bureau Foundation of Guangdong Province (20141158), Doctoral Research Program of Guangdong Medical College (XB1303 and XB1387) and Special Foundation for Young Innovation Scientists of Department of Education of Guangdong Province (4CX14119G and 4CX14118g).References
- X.-B. Xiong and A. Lavasanifar, ACS Nano, 2011, 5, 5202–5213 CrossRef CAS PubMed.
- Y. Wang, S. Gao, W.-H. Ye, H. S. Yoon and Y.-Y. Yang, Nat. Mater., 2006, 5, 791–796 CrossRef CAS PubMed.
- Q.-D. Hu, H. Fan, Y. Ping, W.-Q. Liang, G.-P. Tang and J. Li, Chem. Commun., 2011, 47, 5572–5574 RSC.
- M. Ma, Z. Yuan, X. Chen, F. Li and R. Zhuo, Acta Biomater., 2012, 8, 599–607 CrossRef CAS PubMed.
- M. S. Suh, G. Shim, H. Y. Lee, S.-E. Han, Y.-H. Yu, Y. Choi, K. Kim, I. C. Kwon, K. Y. Weon and Y. B. Kim, J. Controlled Release, 2009, 140, 268–276 CrossRef CAS PubMed.
- Z. Xu, Z. Zhang, Y. Chen, L. Chen, L. Lin and Y. Li, Biomaterials, 2010, 31, 916–922 CrossRef CAS.
- S. Liu, Y. Guo, R. Huang, J. Li, S. Huang, Y. Kuang, L. Han and C. Jiang, Biomaterials, 2012, 33, 4907–4916 CrossRef CAS.
- T. L. Kaneshiro and Z.-R. Lu, Biomaterials, 2009, 30, 5660–5666 CrossRef CAS PubMed.
- J.-T. Lin, C. Wang, Y. Zhao and G.-H. Wang, Mater. Res. Express, 2014, 1, 035403 CrossRef.
- M. Akin, R. Bongartz, J. G. Walter, D. O. Demirkol, F. Stahl, S. Timur and T. Scheper, J. Mater. Chem., 2012, 22, 11529–11536 RSC.
- J.-M. Li, Y.-Y. Wang, M.-X. Zhao, C.-P. Tan, Y.-Q. Li, X.-Y. Le, L.-N. Ji and Z.-W. Mao, Biomaterials, 2012, 33, 2780–2790 CrossRef CAS.
- S. Shi, K. Shi, L. Tan, Y. Qu, G. Shen, B. Chu, S. Zhang, X. Su, X. Li and Y. Wei, Biomaterials, 2014, 35, 4536–4547 CrossRef CAS.
- A. L. Lee, Y. Wang, S. Pervaiz, W. Fan and Y. Y. Yang, Macromol. Biosci., 2011, 11, 296–307 CrossRef CAS PubMed.
- H.-Y. Wen, H.-Q. Dong, W.-j. Xie, Y.-Y. Li, K. Wang, G. M. Pauletti and D.-L. Shi, Chem. Commun., 2011, 47, 3550–3552 RSC.
- J. Ding, J. Chen, D. Li, C. Xiao, J. Zhang, C. He, X. Zhuang and X. Chen, J. Mater. Chem. B, 2013, 1, 69–81 RSC.
- C. Deng, X. Chen, H. Yu, J. Sun, T. Lu and X. Jing, Polymer, 2007, 48, 139–149 CrossRef CAS.
- J.-T. Lin, Y. Zou, C. Wang, Y.-C. Zhong, Y. Zhao, H.-E. Zhu, G.-H. Wang, L.-M. Zhang and X.-B. Zheng, Mater. Sci. Eng., C, 2014, 44, 430–439 CrossRef CAS.
- J. Deng, Y. Zhou, B. Xu, K. Mai, Y. Deng and L.-M. Zhang, Biomacromolecules, 2011, 12, 642–649 CrossRef CAS.
- M. Dong, L. Zong-Hua, Z. Qian-Qian, Z. Xiao-Yan, Z. Yi, S. Yun-Feng, L. Jian-Tao and X. Wei, Macromol. Rapid Commun., 2013, 34, 548–552 CrossRef PubMed.
- D. Ma, J. Lin, Y. Chen, W. Xue and L. M. Zhang, Carbon, 2012, 50, 3001–3007 CrossRef CAS.
- M. Metzke, N. O’Connor, S. Maiti, E. Nelson and Z. Guan, Angew. Chem., Int. Ed., 2005, 44, 6529–6533 CrossRef CAS PubMed.
- C. Zhu, S. Jung, S. Luo, F. Meng, X. Zhu, T. G. Park and Z. Zhong, Biomaterials, 2010, 31, 2408–2416 CrossRef CAS PubMed.
- C. Zheng, M. Zheng, P. Gong, J. Deng, H. Yi, P. Zhang, Y. Zhang, P. Liu, Y. Ma and L. Cai, Biomaterials, 2013, 34, 3431–3438 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |