Fixed bed adsorption of diquat dibromide from aqueous solution using carbon nanotubes†
Anthony B. Dichiara,
Samuel F. Harlander and
Reginald E. Rogers*
Department of Chemical Engineering, Rochester Institute of Technology, Rochester, NY 14623, USA. E-mail: reginald.rogers@rit.edu; Tel: +1-585-475-4157
First published on 10th July 2015
Abstract
While carbon nanotubes (CNTs) are increasingly studied as attractive adsorbents for wastewater treatments, research on adsorption characteristics of these materials is mostly restricted to batch studies. However, it is well known that continuous flow techniques can be more efficient in removing contaminants. The present work exploits the attributes of different types of CNTs in a fixed bed column for the dynamic uptake of diquat dibromide from aqueous solutions.
The development of sustainable and efficient technologies to purify water has been motivated by insufficient drinking water and inadequate sanitation in various locations around the world.1 Whereas many purification treatments are either expensive or operationally intensive, adsorption-based methods are simple to implement for point-of-use water applications. These techniques are attractive because they are capable of separation even with exceedingly low bulk solution concentrations, a regime where most other mass transfer operations are challenged due to the small concentration gradients incurred.2 Adsorption consists of the transfer of undesirable chemicals from a fluid phase (either gas or liquid) to the surface of a solid adsorbent in a transient process. Adsorption processes are limited in their ability to remove contaminants by the capacity of the adsorbent itself; once the adsorbent reaches equilibrium with the surrounding fluid, it can no longer adsorb more molecules.3 Opportunities exist for enhanced adsorption using advanced nanostructures to increase the number of binding sites to enable interactions with more chemicals.4–6 Among newly emerged materials, carbon nanotubes (CNTs) are commonly recognized as promising candidates for future separation systems due to their high surface areas, large delocalized π electrons and hydrophobic surfaces.7 The attributes of these nanostructures have been widely exploited in batch processes for the removal of diverse contaminants from aqueous environments, with reported uptake capacities being significantly higher than activated carbon (AC), the most widely used adsorbent to date.8–13 However, as pointed out by a recent review,14 there have been very few studies that have explored the potential of CNTs in continuous flow water purification systems, and in most cases, CNTs are mixed with a packing media, like sand, which reduces the overall efficiency of the system.15,16
Fixed-bed techniques are desirable not only because they can provide immediate on-demand removal of contaminants directly at a water supply, but also because they have the potential to enhance the capacity of adsorbent materials to hold contaminants.17 Batch adsorption is a first step in examining the efficacy of an adsorbent to remove given chemicals, but is often not a convenient method for large scale water purification. In a batch process, the adsorbent is in equilibrium with the residual concentration in the bulk, while in a fixed bed system, fluid is fed continuously through a packed column of adsorbent, which ultimately becomes fully loaded with contaminants when the adsorbent achieves equilibrium with the feed concentration. Material balances on the solution dictate that the residual concentration in the bulk is significantly lower than that of the feed. Typical isotherms that govern equilibrium (e.g. Langmuir or Freundlich) being very steep at low concentrations, small changes in bulk concentration can lead to large changes in adsorbed contaminants. Accordingly, the adsorbent uptake can be substantially larger in fixed bed operations than in batch processes when operated in concentration ranges where the equilibrium isotherm has a large slope.18–22 Apart from the potential to increase the adsorption capacity, fixed-bed processes have a unique advantage in that up to a certain time, all fluid exiting the column will have zero contaminants—the nature of the transient adsorption in the column, where an equilibrium front moves through the column at a certain speed, affords this important result.17 By contrast, equilibrium imposes that there be a residual bulk concentration of contaminant in a batch process. The success of prior batch adsorption studies,8–13 then, motivates the examination of CNTs in fixed-bed processes.
Although fixed-bed adsorption may be used for a variety of liquid and gaseous separations, the present research examine the removal of contaminants from aqueous solutions. Granular activated carbon (GAC) has recently been examined in fixed bed processes for the removal of organic compounds and heavy metals.23,24 These studies demonstrated that significant uptake of such contaminants is possible providing opportunities to expand the focus to advance carbonaceous materials. The widespread use of pesticides in agricultural practices leads us to consider specifically the uptake of pesticide residues from water. Despite recent reports,25–27 the use of CNTs for pesticide removal appears to be less studied than for other organic contaminants such as dyes. Diquat dibromide (DqDb), one of the most widely used pesticides to regulate plant growth, poses serious health and environmental risks due to its ubiquitous and toxic nature.27 Its presence in water can be caused by aerial drift and field runoff during spraying applications where it can bond strongly to mineral and organic and does not degrade significantly over time. Concentrated solutions of DqDb may cause severe skin irritations, diarrhea, kidney failure, and liver damage.28,29 Therefore, it is important to control the concentration of DqDb from hydrological systems. To this end, the present study focuses on the efficacy of AC and various types of CNTs as fixed-bed adsorbents.
The setup for fixed bed adsorption studies is depicted in Fig. 1 and detailed in ESI.† Briefly, a gravity-fed fixed-bed column packed with different carbonaceous adsorbents was operated with DqDb feed concentrations of 15 and 25 μg mL−1. The solution was fed by a peristaltic pump (Langer instruments BT100-2J) used to maintain a set hydrostatic head above the bed to achieve a constant flow rate through the column. The performance of the system was evaluated at 20 °C by measuring the effluent concentration at the outlet of the column at different time intervals by UV/vis absorption spectroscopy (Perkin Elmer Lambda 950 UV/Vis/NIR) using a measured extinction coefficient from Beer's law analysis.
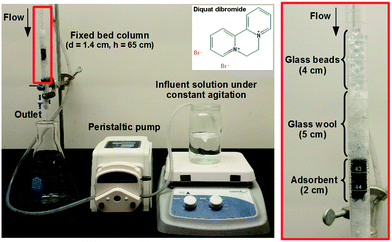 | ||
| Fig. 1 Photographs of the fixed bed column and the adsorbent bed used in this study. The 2D structure of DqDb is shown in the inset. | ||
Breakthrough curves were obtained, from which the efficacy of the adsorbent systems could be examined. Breakthrough curves were expressed in terms of normalized concentration, defined as the ratio of the outlet concentration, Ct, to the feed concentration, C0, as a function of time. For definiteness, the breakthrough time, tb, and the exhaustion time, te, were taken as the times to reach Ct/C0 = 0.1 and Ct/C0 = 0.9, respectively. Note that the breakthrough time is associated with the threshold level for solute concentration, and the exhaustion time is taken to be the time at which the bed is close to full loading. Different definitions can thus be used in practice depending on the specific application. Both breakthrough and exhaustion times were estimated through interpolation of experimental data. In general, the efficiency of the bed can be extracted from experimental data as follows. The accumulation of solute over any time interval may be obtained by direct integration of an overall mass balance on the column in any time interval. The quantity of pesticides retained in the column at the exhaustion point, me, for a given volumetric flow rate, Q, may thus be expressed by the following equation.
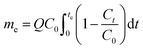 | (1) |
Similarly, the total amount of pesticides retained in the column up to the breakthrough time, mb, is determined as:
 | (2) |
It is assumed that the mass of solute in the liquid in the column is small compared with that adsorbed to the solid at breakthrough and exhaustion. Accordingly, the percentage of the bed that is saturated at the breakthrough time, θ, is given in terms of me and mb as:17
 | (3) |
The quantity θ provides the utilization efficiency of the adsorbent in a fixed bed process, and may be used in engineering design of fixed bed columns during scale up.21 Additionally, the breakthrough time, tb, is of significant interest. For a given volumetric flow rate, the volume of liquid that can be treated to a desired level of purity, Vtreated, given by eqn (4). This relation indicates that the longer the breakthrough time, the larger the amount of fluid that can be purified.
| Vtreated = Qtb | (4) |
Granular AC and hydroxyl (OH)-functionalized SWCNTs containing 3.9% OH groups were purchased from Calgon Carbon Corp. (Filtrasorb) and Cheap Tubes Inc., respectively. Their purity level is higher than 90 wt% and they were used as-received without further treatment. Pristine single-wall and multi-wall CNTs (SWCNTs and MWCNTs) from Cheap Tubes Inc. were purified by non-oxidative acid and thermal treatments to remove metal particles and amorphous carbon.† As revealed by electron microscopy,† the size of AC is about 150 μm, while the CNT length is below 20 μm and the individual tube mean diameter of MWCNTs and SWCNTs is below 15 and 2 nm, respectively. The external surface of AC has a morphology characterized by irregular cavities and pores (Fig. 2a), while distinct bundles and ropes of CNTs are nested together forming a CNT network with hierarchical porosity (Fig. 2b and c). The CNTs are able to closely contact with one another due to strong π–π interactions along their length axis. All carbonaceous materials display strong peaks near 1336 and 1590 cm−1 in their Raman spectra, assigned to the D band and G band, respectively. The G band corresponds to the stretching vibration of carbon sp2 bonds in a hexagonal lattice, whereas the D band is associated with the vibration of carbon atoms with dangling bonds in an amorphous network.30 The integrated intensity ratios of the D band to the G band (ID/IG) listed in Table 1 reflect the degree of graphitization of the carbon surfaces for each type of adsorbent. Both SWCNTs and MWCNTs appear to be composed of more completely crystallized sp2-bonding graphitic surfaces than SWCNT-OH and granular AC, with SWCNTs showing the highest structural integrity as indicated by the lowest value of ID/IG. The Brunauer–Emmett–Teller (BET) surface areas reported by the manufacturers of the different adsorbents are also summarized in Table 1. Among the carbonaceous materials, granular AC has the largest specific surface area (SSA = 850 m2 g−1) followed by pristine and oxidized SWCNTs (SSA = 407 m2 g−1) and by MWCNTs (SSA = 233 m2 g−1).
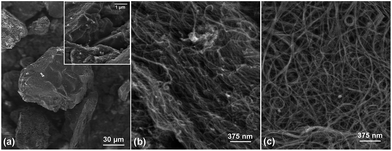 | ||
| Fig. 2 Representative SEM images of purified (a) granular AC, (b) MWCNTs and (c) SWCNTs prior to adsorption treatment. | ||
| Adsorbents | AC | MWCNT | SWCNT | SWCNT-OH |
|---|---|---|---|---|
| SSA (m2 g−1) | 850* | 233* | 407* | 407* |
| ID/IG | 1.1 | 0.7 | 0.2 | 0.9 |
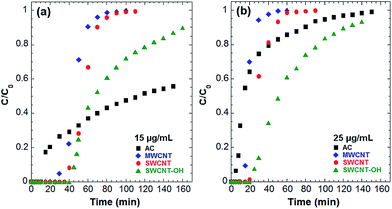
Continuous flow experiments of aqueous solutions of DqDb were conducted to determine the adsorption capacity of a fixed bed system packed with AC, MWCNT and SWCNT adsorbents. Fig. 3 provides the breakthrough curves for DqDb in columns packed with AC (■), MWCNTs (●), SWCNTs (♦) and SWCNTs-OH (▲) for a 2 cm bed depth and different feed concentrations. Key features of the breakthrough curves, extracted using eqn (1)–(3), are summarized in Table 2.
| Adsorbent | C0 (μg mL−1) | tb (min) | te (min) | mb (mg) | me (mg) | θ (%) |
|---|---|---|---|---|---|---|
| AC | 15 | 14 | — | 0.51 | — | — |
| AC | 25 | 6 | 80 | 0.48 | 1.38 | 35.5 |
| MWCNT | 15 | 32 | 60 | 1.21 | 1.44 | 83.3 |
| MWCNT | 25 | 15 | 29 | 1.01 | 1.15 | 87.8 |
| SWCNT | 15 | 41 | 70 | 1.56 | 1.79 | 87.2 |
| SWCNT | 25 | 23 | 42 | 1.46 | 1.71 | 85.4 |
| SWCNT-OH | 15 | 47 | 160 | 1.59 | 2.81 | 56.6 |
| SWCNT-OH | 25 | 28 | 126 | 1.51 | 2.49 | 60.7 |
Fig. 3 shows that while all breakthrough curves have a similar “S” shape, adsorption is affected by changes in initial concentration. It can be observed that the breakthrough curves become steeper at higher concentration of DqDb. This is consistent with other studies reporting the fixed bed adsorption of different chemicals on carbonaceous materials.15,16,31 Increasing the feed concentration corresponds to increase in the concentration gradient which reduces the time required to reach breakthrough and exhaustion (Table 2) regardless of the type of adsorbent used to pack the column. Consequently, decreasing the inlet concentration of DqDb improves the treatment of larger volume of effluent as reflected by longer breakthrough curve due to reduced mass transfer coefficient and slower saturation of the system.9,25 The number of DqDb molecules available per volume of the feed for adsorption on the carbonaceous materials increases as the concentration of DqDb is varied from 15 to 25 μg mL−1. This suggests that the binding sites become more quickly saturated at 25 μg mL−1 than at 15 μg mL−1, resulting in a slight decrease of the uptake of DqDb in the former case (Table 2).
Besides the feed concentration, the nature of adsorbent used to pack the column also greatly influences the adsorption of DqDb in fixed bed systems. As observed in Fig. 3, larger breakthrough times are incurred when CNTs are used as adsorbents instead of AC, regardless of the feed concentration. According to eqn (4), this indicates that for the same volumetric flow rate, Q, larger amounts of contaminated water can be treated under similar conditions when CNTs are used to pack the fixed bed column instead of AC. The volume of liquid that can be decontaminated to a desired level of purity increases in the order of granular AC > MWCNTs > SWCNTs > SWCNTs-OH. Furthermore, by comparing the different values of mb in Table 2, it can be seen that CNTs exhibit much higher uptakes than AC, which is consistent with previous batch adsorption studies (Fig. S1).†9–11 At similar bed depths, the retention of DqDb at breakthrough on MWCNTs, SWCNTs and SWCNTs-OH shows respective improvements over AC by a factor of 2.2, 3 and 3.2 regardless of the feed concentration. On a per mass basis, then, CNTs typically offer an order of magnitude increase in adsorption capacity over AC. Finally, the values of θ, corresponding to the fraction of the bed used at breakthrough, significantly increases when CNTs are packed in the column, comparing to AC (Table 2). This demonstrates the superior utilization efficiency of CNTs in a fixed-bed adsorption system.
From the aforementioned adsorption data (Table 2) and the characteristics of the different adsorbents reported in Table 1, it seems that the adsorption affinities of DqDb depend more on the degree of carbon graphitization than on the SSA of a given carbonaceous adsorbent. It has been reported that π–π interactions are mostly responsible for the adsorption of aromatic molecules, such as DqDb, onto graphitic materials.32 The π–π complexes are formed by electrostatic forces between sigma-π quadrupoles of opposing ring systems.33 Since sp2-bonding graphitic surfaces have high electronic polarizability, they are able to induce strong π–π stacking interaction. Hence, the degree of carbon graphitization of the adsorbent is a critical factor controlling the adsorption of aromatic chemicals like DqDb. Furthermore, the presence of oxygen containing functional groups on the adsorbent surface exhibits a strong influence on the uptake of DqDb. While the breakthrough curves of the pristine CNTs are steeper than those of the OH-functionalized CNTs (Fig. 3), the mass of DqDb adsorbed at exhaustion is much larger on SWCNTs-OH than on the pristine CNTs (Table 2). These observations reveal that the nature of DqDb adsorption is different on the oxidized materials. Since DqDb is an organic cation (Fig. 1 inset), it also can bind with the oxygen containing functional groups through ion exchange or electrostatic attraction. This is consistent with various studies reporting the adsorption of other organic cations on oxidized carbon nanostructures.33–35 Finally, the different pore structures of CNTs and AC may also influence the adsorption behavior of DqDb. From Fig. 2, it can be observed that CNTs exhibit an open pore network which facilitates fast molecular diffusion and promotes the accessibility of adsorption sites (Fig. 2b and c), as opposed to the closed, irregular shaped micropore structure of granular AC (Fig. 2a). Microporous granular AC are usually comprised of a large portion of very small micropores (<few nm), which is responsible for their large specific surface area. Whereas micropore filling can significantly affect the adsorption of aromatic compounds, its significance depends on how well the adsorbate molecules fit in the pores of the adsorbent.36 In the case of DqDb, its relative bulk geometry might lead to size-exclusion effects, thus preventing the DqDb molecules from reaching adsorption sites located in smaller micropores on the surface of AC. Therefore, the superior adsorption properties of CNTs compared with AC may be attributed to the higher degree of graphitization of the CNT carbon surface and its open pore structure. Furthermore, the differences in adsorption between SWCNT and MWCNT may be ascribed to the higher specific surface area of the former, thus providing a larger number of available sites for adsorption.
Conclusion
In summary, this study provides a comparison of the aqueous-phase adsorption of DqDb between carbonaceous nanostructures (i.e. MWCNTs, SWCNTs and SWCNTs-OH) and bulk carbon-based materials (i.e. AC) through continuous flow separation in a fixed bed column. While the uptake values in fixed bed adsorption are not quite as high as in batch process, the former allows for a complete removal of contaminant over a certain time that is not possible in a well-mixed batch process.† Not only does the breakthrough time increase for both types of CNT, but the adsorption capacity and the fraction of the bed that is utilized are also significantly improved when CNTs are used in the adsorption bed instead of AC. This indicates that larger volumes of solution can be treated in a more efficient way with CNTs under similar conditions. Among the different types of CNTs, SWCNTs-OH exhibit the best adsorption properties for each experimental condition studied. The nature of the uptake of DqDb on the surface of carbonaceous materials can be attributed to the combination of various mechanisms, including hydrophobicity, π–π interactions, and micropore filling, whose role may vary depending on the type of adsorbent. Moreover, the presence of oxygen containing functional groups on the adsorbent surface strongly affects the adsorption of DqDb.Acknowledgements
The authors thank S. J. Weinstein and N. S. Barlow for useful discussion. We also acknowledge the Kate Gleason Gift and the Office of the Vice President for Research at Rochester Institute of Technology for funding of work presented in this paper. S. Harlander thanks the KGCOE Dean's fund for undergraduate student support during this project.Notes and references
- R. I. L. Eggen, J. Hollender, A. Joss, M. Scharer and C. Stamm, Environ. Sci. Technol., 2014, 48, 7683 CrossRef CAS PubMed
.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301 CrossRef CAS PubMed
.
- I. Ali and V. K. Gupta, Nat. Protoc., 2007, 1, 2661 CrossRef PubMed
.
- M. Khajeh, S. Laurent and K. Dastafkan, Chem. Rev., 2013, 113, 7728 CrossRef CAS PubMed
.
- F. Perreault, A. Fonseca de Faria and M. Elimelech, Chem. Soc. Rev., 2015 10.1039/c5cs00021a
.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931 CrossRef CAS PubMed
.
- X. Liu, M. Wang, S. Zhang and B. Pan, J. Environ. Sci., 2013, 25, 1263 CrossRef CAS
.
- H. Wang, H. Ma, W. Zheng, D. An and C. Na, ACS Appl. Mater. Interfaces, 2014, 6, 9426 CAS
.
- A. B. Dichiara, T. J. Sherwood and R. E. Rogers, J. Mater. Chem. A, 2013, 1, 14480 CAS
.
- R. E. Rogers, T. L. Bardsley, S. J. Weinstein and B. J. Landi, Chem. Eng. J., 2011, 173, 486 CrossRef CAS PubMed
.
- A. B. Dichiara, T. J. Sherwood, J. Benton-Smith, J. C. Wilson, S. J. Weinstein and R. E. Rogers, Nanoscale, 2014, 6, 6322 RSC
.
- A. B. Dichiara, J. Benton-Smith and R. E. Rogers, Environ. Sci.: Nano, 2014, 1, 113 RSC
.
- M. Li, C. Wang, M. J. O'Connell and C. K. Chan, Environ. Sci.: Nano, 2015, 2, 245 RSC
.
- S. Chowdhury and R. Balasubramanian, Adv. Colloid Interface Sci., 2014, 204, 35 CrossRef CAS PubMed
.
- Y. Tian, B. Gao, V. L. Morales, H. Chen, Y. Wang and H. Li, Chemosphere, 2013, 90, 2597 CrossRef CAS PubMed
.
- A. Naghizadeh, S. Nasseri, A. H. Mahvi, R. Nabizadeh, R. R. Kalantary and A. Rashidi, J. Environ. Health Sci. Eng., 2013, 11, 14 CrossRef PubMed
.
- E. L. Cussler, Diffusion: mass transfer in fluid systems, Cambridge university press, 2009, p. 436 Search PubMed
.
- A. Olgun, N. Atar and S. Wang, Chem. Eng. J., 2013, 222, 108 CrossRef CAS PubMed
.
- K. Z. Setshedi, M. Bhaumik, M. S. Onyango and A. Maity, Chem. Eng. J., 2015, 262, 921 CrossRef CAS PubMed
.
- L. Tofan, C. Teodosiu, C. Paduraru and R. Wenkert, Appl. Surf. Sci., 2013, 285P, 33 CrossRef PubMed
.
- M. Auta and B. H. Hameed, Chem. Eng. J., 2014, 237, 352 CrossRef CAS PubMed
.
- F. J. García-Mateos, R. Ruiz-Rosas, M. D. Marqués, L. M. Cotoruelo, J. Rodríguez-Mirasol and T. Cordero, Chem. Eng. J., 2015, 279, 18 CrossRef PubMed
.
- A. M. Kennedy and R. S. Summers, Environ. Sci. Technol., 2015, 49, 6617 CrossRef CAS PubMed
.
- D. P. Sounthararajah, P. Loganathan, J. Kandasamy and S. Vigneswaran, J. Hazard. Mater., 2015, 287, 306 CrossRef CAS PubMed
.
- J. Deng, Y. S. Shao, N. Y. Gao, Y. Deng, C. Q. Tan, S. Q. Zhou and X. H. Hu, Chem. Eng. J., 2012, 193, 339 CrossRef PubMed
.
- A. De Martino, M. Iorio, B. S. Xing and R. Capasso, RSC Adv., 2012, 2, 5693 RSC
.
- N. Rambabu, C. A. Guzman, J. Soltan and V. Himabindu, Chem. Eng. Technol., 2012, 35, 272 CrossRef CAS PubMed
.
- C. Lewis, Drinking water health advisory: pesticides, USEPA, Chelsea, MI, 1989 Search PubMed
.
- S. Murphy, Casarett and Doull’s toxicology, New York, Macmillan, 1986, p. 519 Search PubMed
.
- M. S. Dresselhaus, A. Jorio and R. Saito, Annu. Rev. Condens. Matter Phys., 2010, 1, 89 CrossRef CAS
.
- J.-L. Gong, Y.-L. Zhang, Y. Jiang, G.-M. Zeng, Z.-H. Cui, K. Liu, C.-H. Deng, Q.-Y. Niu, J.-H. Deng and S.-Y. Huan, Appl. Surf. Sci., 2015, 330, 148 CrossRef CAS PubMed
.
- B. Pan and B. Xing, Environ. Sci. Technol., 2008, 42, 9005 CrossRef CAS
.
- D. Zhu and J. J. Pignatello, Environ. Sci. Technol., 2005, 39, 2033 CrossRef CAS
.
- J. Gong, J. Liu, X. Chen, Z. Jiang, X. Wen, E. Mijowskac and T. Tang, J. Mater. Chem. A, 2015, 3, 341 CAS
.
- J.-G. Yu, X.-H. Zhao, Hua Yang, X.-H. Chen, Q. Yang, L.-Y. Yu, J.-H. Jiang and X.-Q. Chen, Sci. Total Environ., 2014, 482, 241 CrossRef PubMed
.
- B. Wang, W. Chen, H. Fu, X. Qu, S. Zheng, Z. Xu and D. Zhu, Carbon, 2014, 79, 203 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details related to adsorbate preparation, adsorbents purification and characterization, and fixed bed adsorption studies. See DOI: 10.1039/c5ra11167f |
| This journal is © The Royal Society of Chemistry 2015 |