A pillar-layer MOF for detection of small molecule acetone and metal ions in dilute solution†
Hai-Ning Wanga,
Si-Quan Jianga,
Qing-Yun Lua,
Zi-Yan Zhou*a,
Shu-Ping Zhuo*a,
Guo-Gang Shanb and
Zhong-Min Su*b
aCollege of Chemical Engineering, Shandong University of Technology, Zibo, Shandong 255049, China. E-mail: zyzhou@sdut.edu.cn
bInstitute of Functional Material Chemistry, Faculty of Chemistry, Northeast Normal University, Changchun 130024, Jilin, P. R. China. E-mail: zmsu@nenu.edu.cn; Fax: +86-431-85684009
First published on 20th May 2015
Abstract
A pillar-layer MOFs Cd(5-aip)L·3DMA (1) has been obtained under solvothermal conditions. 1 possesses non-interpenetrating pillar-layer framework with 1D rectangular-shape channels. The experimental results show that the luminescent intensity of 1 is highly dependent on metal ions and small solvent molecules, particularly Ni2+/Cd2+ ions and acetone.
As an active research field of porous materials, metal–organic frameworks (MOFs) self-assembled from organic linkers and metal ions or clusters are powerful platforms for developing functional materials. MOFs integrate with both advantages of each component, and possess high surface area, intriguing and diverse architectures, tuneable pore sizes and compositions. Due to these excellent characteristics, over the past decade, research on the construction of such materials is shifting more and more, and MOFs have possessed a wide variety of applications such as gas storage and separation,1 catalysis,2 drug delivery,3 etc. In particular, microporous MOFs with good skeleton stability, appreciable internal surface areas and recognition capability for specific components have drawn particular attention because they can act as excellent candidates for the rapid recognition and sensing of cations, anions, small molecules, and so on.4
As an important member of this family, the pillar-layer MOF draw more and more attention owing to the following advantages: (i) varying the length of the pillar linkers can tune the pore size and shape without changing the structure topology,5 (ii) some features such as hydrophilic/hydrophobic character, hydrogen bonding, and Lewis acid sites/base sites can be obtained through straightforward functionalization of the pillar linkers.6 Owing to these advantages, this intriguing and challenging realm is worthy of our study. In the process of investigation, it is well established that the reasonable selection of the ligand can avoid interpenetration and maximize the accessible space existing in the pillar-layer MOFs.7 Among numerous organic ligands, the 5-aminoisophthalic acid (5-aip) is chosen because it can serve as excellent candidates for building frameworks due to its strong binding ability, variable coordination modes. In addition, L (L = 3,5-di(pyridin-4-yl)-4H-1,2,4-triazol-4-amine) is selected based on the following considerations: (i) L with conjugated π systems can give rise to photoluminescence upon excitation. (ii) L is rigid ligand with amino groups as well as nitrogen atoms possessing lone pair electrons, which may facilitate the generation of intraligand interaction and improve luminescent character; (iii) owing to its multiple bridging fragments, it can be used as an outstanding candidate for the construction of multiple structural moieties, which not only enriches the diverse geometric structures of MOFs, but also improves the electronic surface characteristics of MOFs.8 d10 metal ions, for example Cd(II), is selected to construct MOFs owing to that it exhibits varied coordination numbers and geometries as well as excellent luminescent properties.9
In previous work, we have constructed a series of pillar-layer metal–organic frameworks based on 5-aminoisophthalic acid (5-aip) and 4,4′-bipyridine.10 As a continuation and improvement of our work, we take advantage of L and have obtained another compound Cd(5-aip)L·3DMA (DMA = N,N-dimethylacetamide).
Single-crystal X-ray analysis reveals that compound 1 crystallizes in the monoclinic system with space group P21/c. Each CdII atom is heptacoordinated, adopting pentagonal bi-pyramid geometry, coordinated by five oxygen atoms from 5-aip ligands in the equatorial positions and two nitrogen atoms from two L ligands ligated in axial positions to give a CdO5N2 segment (Fig. 1a). With the help of carboxylate oxygen atoms from 5-aip ligands, a [Cd2(COO)4] dimetallic cluster with the non-bonding Cd⋯Cd separation of 3.898 Å generates.
The macrometallocycle constructed by four dimmer units and four 5-aip ligands link together generating a 2D sheet, {Cd(5-aip)}n (Fig. 1b). Then, the neighbour sheets are pillared by the L in axial positions with the distance approximately 15 Å without van der Waals radius. Consequently, compound 1 possesses a non-interpenetrating pillar-layer network. Consequently, compound 1 built from the layered motif {M(5-aip)}n is bridged by the linear linker into a 3D porous framework, which is the primitive cubic network with Schläfli symbol (412·63) (Fig. S1†).11 Furthermore, this framework exhibits two kinds of interconnected 1D rectangular-shape channels, with the dimensions 4.929 × 10.736 Å and 3.028 × 10.736 Å with taking van der Waals radius in consideration (Fig. 1c and d). In these channels, the free nitrogen atoms of L as well as the amino groups point toward the interior of the channels. The solvent accessible volumes in this dehydrated structure is 55% as calculated by PLATON.12
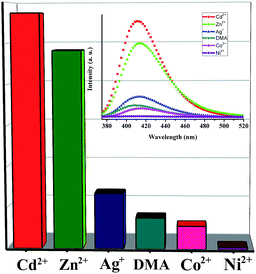
A wide range of sophisticated instruments like ion mobility spectroscopy (IMS), X-ray dispersion, Raman spectroscopy, etc., are currently being employed to detect metal ions or small molecules. The above techniques are not widespread use in the field because of limited portability, high cost, and these instruments need frequent careful calibrations. Photoluminescence-based sensors have been considered to be the most effective tool for detection due to their high sensitivity and affordability.13 In recent years, recognition and sensing of metal ions and small molecules by means of this method have attracted more and more attention.4,14 Compound 1 formed by d10 metal ions Cd2+ and conjugated organic linkers can be used as a candidate for potential luminescent materials, which inspire us to systematically explore its potential application in this field. The solid luminescent spectra of 1 are recorded at room temperature (Fig. S2†). To examine the potential applications of 1 for the sensing of common toxic metal ions, compound 1 is immersed in DMA solutions containing various metal ions (M = Cd2+, Co2+, Ag+, Ni2+ or Zn2+). The amount of loaded metal ions monitored by ICP analysis has been listed in Table S1.† The difference in loaded amount among metal ions perhaps can be assigned to not only the size and charge of cations, but also the preferred affinity between cations and nitrogen atoms from the framework (see ESI†). As shown in Fig. 2, the luminescence intensity of metal-ion included samples largely depends on the metal ions, and the maximum luminescent intensity decreases in the order Cd2+ > Zn2+ > Ag+ > DMA > Co2+ > Ni2+. Cd2+, Zn2+ and Ag+ ions, possessing closed-shell electron configuration, exhibit an enhancing effect on the luminescence intensity, whereas the others with different electron configurations show varying degrees of quenching, especially the Ni2+ ions. The luminescence intensities of 1–Cd2+, 1–Zn2+ and 1–Ag+ are approximately eight times, seven times, and twice that of metal-ion free 1 respectively. While, the luminescence intensity of 1–Co2+ is lower than that without metal cations, and the Ni2+ ion exhibits the most significant quenching effect. The 1–Cd2+ trapped in DMA solutions with different concentrations is used for further luminescent investigations. While, the luminescent intensity of metal-ion free 1 is about fourteen times compared with that of 1–Ni2+. Interestingly, the luminescence intensity of the 1–Ni2+ mainly depend on the concentration of the Ni2+ ion. Following the decrease of the concentration of DMA solution of Ni2+, the luminescence intensity of 1–Ni2+ is increasing. The luminescent intensity of 1–Ni2+ from a 10−3 mol L−1 Ni2+ solution is nearly three times that of 1 immersed in 10−2 mol L−1 Ni2+ solution (Fig. S4†). On the contrary, the luminescence intensity of 1–Cd2+ also closely depends on the concentration of the Cd2+ ion, and increases as the increment of the concentration of Cd2+ (Fig. S5†). The possible mechanism about the luminescent quenching effect is given here. N1s X-ray photoelectron spectroscopy (XPS) studies have been carried out on 1 and 1–Ni2+ (Fig. S6†). The N1s peak at 405.05 eV in 1 is shifted to 404.75 eV after the addition of Ni2+. There may exist a weak binding between nitrogen atoms from L and Ni2+ in 1–Ni2+,15 which reduces the intraligand luminescent efficiency and lead to the quenching effect. However, the obvious enhancing effect may result from the ligand chelating or bridging effect owing to the coordination of CdII,16 which improve the rigidity and conjugation,17 and facilitate ligand-centred charge transfer. It should be mentioned that, the skeletal structure of compound 1 remains intact after the encapsulation of metal ions in dilute aqueous solution, as demonstrated by powder X-Ray Diffraction (XRD) (Fig. S7†).
Meanwhile, the luminescent properties of 1 in different solvent emulsions are also investigated (Fig. 3). The finely ground sample of 1 (5 mg) is immersed in different organic solvents (5 mL), treated by ultrasonication for 90 min, and then aged for 3 h to generate stable suspensions before the fluorescence study. The solvents used are methanol (CH3OH), ethanol (CH3CH2OH), 1,3-propanediol, cyclohexane, tetrahydrofuran (THF), N,N-dimethylformamide (DMF), DMA, acetone and acetonitrile (CH3CN). The most interesting feature is that its luminescent spectrum is largely dependent on the solvent, particularly in the case of 1,3-propanediol and acetone, which exhibits the most significant enhancing and quenching effects, respectively (Fig. 3). Such solvent-dependent luminescent properties are of interest for the sensing of acetone solvent molecules. Subtle shifts in emission peaks can be observed with different solvents and the maximum luminescence intensity decreases in the order 1,3-propanediol > CH3CH2OH > DMF > DMA > CH3OH > THF > CH3CN > cyclohexane > acetone (Fig. 3).
 | ||
| Fig. 3 Comparisons of the luminescent intensity of 1-solvent at room temperature. Inset: corresponding emission spectra. | ||
To carefully examine the sensitivity of sensing acetone, the acetone content is gradually introduced into 1-DMA emulsion. The fluorescence intensity of the emulsion gradually decreases, while the acetone content gradually increases (Fig. S8†). Finally, this system reaches an equilibrium state. To explain the reason for the enhancing and quenching effects, the absorption spectra of 1,3-propanediol and acetone are investigated, as shown in Fig. S9.† It reveals that acetone has a wide absorption range from 307 to 360 nm, while 1,3-propanediol exhibits no absorption. The absorption band of 1-DMA is between 300 and 400 nm, which is partly overlaid by the absorbing band of acetone. Combined with the absorption and luminescence spectra, it is suggested that energy exchange occurs between 1-DMA and acetone molecules. Due to the physical intermolecular solute–solvent interaction forces between 1-DMA and acetone, the energy absorbed by 1-DMA is transferred to acetone molecules, resulting in a decrease in the luminescence intensity, even quenching of 1.9a,18 The encouraging results reveal that 1 could be promising luminescent probes for detecting small molecules acetone.
Up to now, a number of MOFs used for chemosensors have been exploited by us. Most of our works are focus on sensing of small organic molecules.19 Inspired by our pioneering works, we attempt to employ L to synthesize a MOF for sensing metal ions via the weak interaction between nitrogen atoms and metal ions. The results show that 1 is sensitive to metal ions, particularly Ni2+ and Cd2+ ions. In addition, the detection limit for acetone is also calculated (Fig. S10†).
In summary, compound 1 possesses non-interpenetrating pillar-layer framework, which can be used as luminescent probes to detect metal ions and small molecules. The experimental results show that the luminescence intensity of 1 highly depends on Ni2+ and Cd2+ ions and small molecules acetone. Studies are going on for producing larger quantities of these new MOFs to thoroughly explore their properties for gas sense applications.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China 21303012.Notes and references
-
(a) B. V. de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766 RSC
; (b) S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116 RSC
; (c) E. Barea, C. Montoro and J. A. R. Navarro, Chem. Soc. Rev., 2014, 43, 5419 RSC
; (d) N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 300, 1127 CrossRef CAS PubMed
; (e) H. Hayashi, A. P. Côté, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Nat. Mater., 2007, 6, 501 CrossRef CAS PubMed
.
-
(a) J. Liu, L. Chen, H. Cui, J. Zhang, L. Zhang and C. Y. Su, Chem. Soc. Rev., 2014, 43, 6011 RSC
; (b) A. Dhakshinamoorthy and H. Garcia, Chem. Soc. Rev., 2014, 43, 5750 RSC
; (c) T. Zhang and W. Lin, Chem. Soc. Rev., 2014, 43, 5982 RSC
; (d) L. Ma, J. M. Falkowski, C. Abney and W. Lin, Nat. Chem., 2010, 2, 838 CrossRef CAS PubMed
.
-
(a) H. N. Wang, X. Meng, G. S. Yang, X. L. Wang, K. Z. Shao, Z. M. Su and C. G. Wang, Chem. Commun., 2011, 47, 7128 RSC
; (b) H. N. Wang, X. Meng, X. L. Wang, G. S. Yang and Z. M. Su, Dalton Trans., 2012, 2231 RSC
; (c) A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260 CrossRef CAS PubMed
; (d) Z. B. Ma and B. Moulton, Coord. Chem. Rev., 2011, 255, 1623 CrossRef CAS PubMed
.
-
(a) L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. V. Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105 CrossRef CAS PubMed
; (b) Z. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC
.
-
(a) J. Y. Lee, L. Pan, X. Huang, T. J. Emge and J. Li, Adv. Funct. Mater., 2011, 21, 993 CrossRef CAS PubMed
; (b) H. Chun, D. N. Dybtsev, H. Kim and K. Kim, Chem.–Eur. J., 2005, 11, 3521 CrossRef CAS PubMed
.
- Y. Zhao, H. Wu, T. J. Emge, Q. Gong, N. Nijem, Y. J. Chabal, L. Kong, D. C. Langreth, H. Liu, H. Zeng and J. Li, Chem.–Eur. J., 2011, 17, 5101 CrossRef CAS PubMed
.
-
(a) Y. He, B. Li, M. O'Keeffe and B. Chen, Chem. Soc. Rev., 2014, 43, 5618 RSC
; (b) H. L. Jiang, T. A. Makal and H. C. Zhou, Coord. Chem. Rev., 2013, 257, 2232 CrossRef CAS PubMed
; (c) V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, Chem. Soc. Rev., 2014, 43, 6141 RSC
.
- A. K. Chaudhari, S. S. Nagarkar, B. Joarder and S. K. Ghosh, Cryst. Growth Des., 2013, 13, 3716 CAS
.
-
(a) F. Y. Yi, W. T. Yang and Z. M. Sun, J. Mater. Chem., 2012, 22, 23201 RSC
; (b) M. Guo and Z. M. Sun, J. Mater. Chem., 2012, 22, 15939 RSC
; (c) H. Wang, W. T. Yang and Z. M. Sun, Chem.–Asian J., 2013, 8, 982 CrossRef CAS PubMed
.
- H. N. Wang, X. Meng, C. Qin, X. L. Wang, G. S. Yang and Z. M. Su, Dalton Trans., 2012, 1047 RSC
.
-
(a) S. R. Batten, N. R. Champness, X. M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715 CrossRef CAS
; (b) B. F. Abrahams, B. F. Hoskins, R. Robson and D. A. Slizys, CrystEngComm, 2002, 4, 478 RSC
.
- A. L. J. Spek, Appl. Crystallogr., 2003, 36, 7 CrossRef CAS
.
-
(a) A. K. Chaudhari, S. S. Nagarkar, B. Joarder and S. K. Ghosh, Cryst. Growth Des., 2013, 13, 3716 CrossRef CAS
; (b) Y. Salinas, R. Martínez-Máñez, M. D. Marcos, F. Sancenón, A. M. Costero, M. Parra and S. Gil, Chem. Soc. Rev., 2012, 41, 1261 RSC
; (c) M. E. Germain and M. J. Knapp, Chem. Soc. Rev., 2009, 38, 2543 RSC
; (d) S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC
.
- Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed
.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, Angew. Chem., Int. Ed., 2009, 48, 500 CrossRef CAS PubMed
.
-
(a) G. Wu, X. F. Wang, T. Okamura, W. Y. Sun and N. Ueyama, Inorg. Chem., 2006, 45, 8523 CrossRef CAS PubMed
; (b) R. Sun, Y. Li, J. Bai and Y. Pan, Cryst. Growth Des., 2007, 7, 890 CrossRef CAS
; (c) J. C. Jin, Y. Y. Wang, W. H. Zhang, A. S. Lermontov, E. K. Lermontova and Q. Z. Shi, Dalton Trans., 2009, 10181 RSC
.
- S. M. Fang, M. Hu, Q. Zhang, M. Du and C. S. Liu, Dalton Trans., 2011, 4527 RSC
.
-
(a) Z. Guo, H. Xu, S. Su, J. Cai, S. Dang, S. Xiang, G. Qian, H. Zhang, M. O'Keeffe and B. Chen, Chem. Commun., 2011, 47, 5551 RSC
; (b) W. Yang, J. Feng and H. Zhang, J. Mater. Chem., 2012, 22, 6819 RSC
.
-
(a) X. L. Hu, F. H. Liu, C. Qin, K. Z. Shao and Z. M. Su, Dalton Trans., 2015, 7822 RSC
; (b) F. H. Liu, C. Qin, Y. Ding, H. Wu, K. Z. Shao and Z. M. Su, Dalton Trans., 2015, 1754 RSC
; (c) S. R. Zhang, D. Y. Du, J. S. Qin, S. L. Li, W. W. He, Y. Q. Lan and Z. M. Su, Inorg. Chem., 2014, 53, 8105 CrossRef CAS PubMed
; (d) S. R. Zhang, D. Y. Du, J. S. Qin, S. J. Bao, S. L. Li, W. W. He, Y. Q. Lan, P. Shen and Z. M. Su, Chem.–Eur. J., 2014, 20, 3589 CrossRef CAS PubMed
; (e) W. Xie, S. R. Zhang, D. Y. Du, J. S. Qin, S. J. Bao, J. Li, Z. M. Su, W. W. He, Q. Fu and Y. Q. Lan, Inorg. Chem., 2015, 54, 3290 CrossRef CAS PubMed
; (f) C. Y. Sun, X. L. Wang, C. Qin, J. L. Jin, Z. M. Su, P. Huang and K. Z. Shao, Chem.–Eur. J., 2013, 19, 3639 CrossRef CAS PubMed
; (g) E. L. Zhou, P. Huang, C. Qin, K. Z. Shao and Z. M. Su, J. Mater. Chem. A, 2015, 3, 7224 RSC
; (h) H. N. Wang, G. G. Shan, H. B. Li, X. L. Wang, H. T. Cao and Z. M. Su, CrystEngComm, 2014, 16, 2754 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, PXRD, TGA plot, and experimental details of sensing, and additional figures for 1. CCDC 1040849. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra08347h |
| This journal is © The Royal Society of Chemistry 2015 |