One-step, ultrasonication-mobilized, solvent-free extraction/synthesis of nanocurcumin from turmeric
Judy Gopal,
Manikandan Muthu and
Se-Chul Chun*
Department of Molecular Biotechnology, Konkuk University, Seoul 143-701, Korea. E-mail: scchun@konkuk.ac.kr
First published on 12th May 2015
Abstract
Curcumin's current solvent-based extraction and poor solubility in water are two unresolved obstacles that limit the harnessing of this medicinal resource. To date, the extraction of curcumin from turmeric in water remains a challenge. This work resolves both these problems via a simple, ultrasonication-based, one-step strategy. Two different sonication methods, water bath type and probe sonication, were employed (using varying variables such as sonication time and sonication frequencies) to develop this one-step, water-based technique for extracting curcumin directly from turmeric. The probe sonication technique with sonication time within 5 min and 20 kHz frequency led to 55% curcumin extraction yield in water. This yield is even higher than that achieved by solvent-based extraction methods using ethanol. The ultrasonic physical conversion of micro-curcumin to nano-curcumin is shown to be the reason for the enhanced solubility of curcumin in water, leading to effective extraction. The results of this study suggest the use of probe ultrasonication for water-based extraction of curcumin from turmeric in a one-step process. This study also provides a solution for the bioavailability problem of curcumin, owing to its insolubility in water through nano-sizing of the curcumin using ultrasonication methods. The results and validation of these findings are reported in this communication.
Introduction
Turmeric, which is designated as a ‘wonder drug’,1 is isolated from the rhizomes of the perennial herb Curcuma longa, a member of the Zingiberaceae family. Turmeric has been used in the Indian subcontinent for a long time and for various purposes, including as an anti-inflammatory and antimicrobial agent in wound healing and also in skin lightening.2 Interestingly, it is also a major ingredient in Indian/Asian cuisine, where it is used as a spice as well as a coloring agent in the Indian curries.Curcumin, or diferuloylmethane (1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione), is a major component (2–6%) of turmeric.3–5 A polyphenol compound, it is a yellow-orange dye that is usually termed ‘Indian solid gold’ because of its extensive medicinal properties, which include anti-oxidant, anti-inflammatory, antimicrobial, anti-cancer, anti-tumor and angiogenesis inhibitory6–9 activities. It is also reported to inhibit lipid peroxidation and scavenge superoxide anions, singlet oxygen, nitric oxide, and hydroxyl radicals.10–13
In addition, curcumin has shown potent anti-amyloidogenic effects for Alzheimer's amyloid fibrils.14,15 It is reported that the low molecular weight and the hydrophobic nature of curcumin results in its effective penetration into the blood–brain barrier and its binding with the beta amyloids.15 Reports establish a link between the relatively lower number of neurological diseases in the Indian subcontinent (such as Alzheimer's and Parkinson's disease)15,16 with their intake of surplus curcumin as part of their regular diet, in the form of Indian curries. Further, curcumin has been shown to down-regulate the activity of a growth factor receptor closely linked with cancer of the breast, lung, kidney and prostate gland.17 It is reported to possess cancer-preventing and cancer-curing properties.17–19 The therapeutic efficacy of curcumin against various human diseases, including cardiovascular diseases, diabetes, arthritis, and Crohn's disease, is well documented.20–27 Owing to its wondrous actions in protecting the human body, the molecule is being recently revisited using modern science and technological tools, with the aim of validating age-old practices in a scientific way.
Although clinical studies have shown that it is safe to use curcumin even at high doses, to date curcumin has not been established as a pharmacological drug due to its very low bioavailability. The extremely low solubility of curcumin in water is the reason for its poor bioavailability.28 Researchers have proven that in humans, after 1 h of oral administration of 4–8 g of curcumin, only 0.41–1.75 μM (ref. 29) was detected in the plasma, whereas in another study, after an oral dose of 3.6 g of curcumin, the peak plasma level of curcumin was at 11.1 nmol L−1.30 Also, studies by Wahlstrom et al.31 have shown that when rats were administered curcumin at a dose of 1 g kg−1, about 75% of curcumin was excreted in the feces, and only negligible amounts of curcumin were recorded in the urine. Measurements of blood plasma levels and biliary excretion showed that curcumin was poorly absorbed from the gut, and the quantity of curcumin that reached tissues outside the gut was pharmacologically insignificant. This indicated the insolubility of curcumin in water at physiological pH, its limited absorption, poor bioavailability, rapid metabolism, and excretion,31 which are major hurdles for the practical application of this compound.
The use of ultrasound as a laboratory-based technique for assisting extraction is well known. This technique has been applied to the extraction of metabolites of plant origin,32 flavonoids from foods33 and bioactives from herbs.34 Ultrasound-assisted extraction (UAE) is recognized for its widespread use in the edible oil industry to improve extraction efficiency and reduce extraction time.35 The proposed benefits of UAE include: (a) overall enhanced extraction yield or rate, (b) enhanced aqueous extraction, (c) opportunity to use alternative solvents, (d) cost effectiveness, (e) enhanced extraction of heat-sensitive components under conditions which would otherwise have low or unacceptable yields and (f) enhanced speed of extraction. Two different types of sonicators are in use: the water bath type and the probe type. Dhanalakshmi et al.36 and Kiani et al.37 compared the efficiency of water bath sonicators and probe sonicators and clearly established that although both techniques apply ultrasound to the sample, there are significant differences in effectiveness, efficiency and process capabilities. Their studies indicated that the water bath sonicator resulted in low-intensity sonication effect and was unevenly spread. The repeatability and scalability of the process was reported to be very poor. Dhanalakshmi et al. found in their study that probe-type ultrasonic devices have a high localized intensity compared to bath-type and hence greater localized effect. This means higher intensity and efficiency in the sonication process. Whilst an ultrasonic bath provides weak sonication with approx. 20–40 W L−1 and a very non-uniform distribution, ultrasonic probe-type devices can easily couple approximately 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W L−1 into the processed medium. Moreover, full control over the most important sonication parameters was observed to result in completely repeatable processes, and linear scalability of the process results in the case of the probe sonicator. Recently, the ultrasonication technique has been extended to nanoparticle synthesis. Nanomaterials are superior and exhibit enhanced physicochemical properties compared to their bulk counterparts, provoking interest in the area of nanotechnology. The quantum mechanical properties of the particles at nanoscale dimensions have a profound influence on the physical properties of the particles. By designing materials in nanoscale, it is possible to vary micro and macroscopic properties, such as charge capacity, magnetization, and melting temperature, without changing their chemical composition. The idea of employing the ultrasonication technique for nanosizing curcumin is used in the following study.
000 W L−1 into the processed medium. Moreover, full control over the most important sonication parameters was observed to result in completely repeatable processes, and linear scalability of the process results in the case of the probe sonicator. Recently, the ultrasonication technique has been extended to nanoparticle synthesis. Nanomaterials are superior and exhibit enhanced physicochemical properties compared to their bulk counterparts, provoking interest in the area of nanotechnology. The quantum mechanical properties of the particles at nanoscale dimensions have a profound influence on the physical properties of the particles. By designing materials in nanoscale, it is possible to vary micro and macroscopic properties, such as charge capacity, magnetization, and melting temperature, without changing their chemical composition. The idea of employing the ultrasonication technique for nanosizing curcumin is used in the following study.
The objective of the current study is to provide a solution to the insolubility issue of curcumin in water. In the present study, we report for the first time a single step, direct method for water-based extraction of curcumin from turmeric. The ultrasonication technique was used to successfully extract curcumin; the extracted curcumin was nanosized and highly soluble in water. The recovery of curcumin via sonication technology was found to yield results four times higher than the solvent-based extraction techniques. The proposed methodology solves the insolubility problem of curcumin through the sonication-based synthesis of nanocurcumin, which renders superior water solubility.
Materials and methods
Chemicals
Commercial turmeric powder (100% purity) was purchased from a supermarket in Seoul, Korea. All the chemicals used in the study, unless specified as otherwise, were of analytical grade. Millipore water was used for all experiments.Experimental procedures
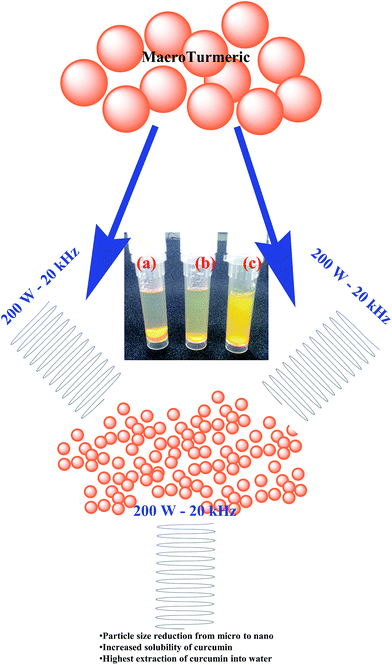
Concentrations of turmeric used were always maintained constant at 1 g L−1, since this is the most soluble concentration with respect to solvents such as methanol, acetone and ethanol. 1 g L−1 commercially purchased turmeric powder (from now on referred to as macro turmeric, MT) was dispersed in sterile distilled water. Similar concentrations were prepared in individual Falcon tubes for the sonication-based experiments. A JAC-2010 ultrasonic instrument (KODO Technical Research Co., Ltd, Hwaseong-City, Gyeonggi-Do, Korea), which is a water bath-type sonicator, equipped with an ultrasonic power of 300 W and frequency of 60 Hz, was used. MT (1 g L−1) dispersed in 10 mL of sterile water in Falcon tubes was subjected to ultrasonication in the waterbath-type sonicator (WBS) at 50 ± 5 °C at varying time intervals of 10 min (WBS 1), 30 min (WBS 2), 1 h (WBS 3), 3 h (WBS 4) and 4 h (WBS 5). After sonication, the contents were stirred at 400 rpm at room temperature for about 1 h. The Falcon tubes with the extracts were then covered with aluminium foil and stored in the dark (since they are reported to undergo photooxidation38) till further use.Another series of MT dispersed in Falcon tubes were prepared and subjected to probe-type sonication using a Bandelin Sonopuls HD 2200 (Bandelin GmbH & Co. KG, Heinrichstrasse, Berlin, Germany) probe ultrasonicator (PUS) with 200 W ultrasonic power and a frequency of 20 kHz. The samples were sonicated one after another, with the probe directly in contact with the sample solution held in falcon tubes, which were held on tube racks. Sonication frequency (SF) of 50% (10 kHz frequency) and 100% (20 kHz frequency) were respectively used, and the sonication time was varied from 1 min to 2 min, 3 min, 4 min and 5 min. These samples are designated in the text as follows: 1 min-50% SF (PUS 1), 1 min-100% (PUS 2), 2 min-50% SF (PUS 3), 2 min-100% SF (PUS 4), 3 min-50% SF (PUS 5), 3 min-100% SF (PUS 6), 4 min-50% SF (PUS 7), 4 min-100% SF (PUS 8), 5 min-50% SF (PUS 9) and 5 min-100% SF (PUS 10). Thus, for each sonication time, two different sonication energy variants, one at 50% sonication energy and the other at 100% sonication energy, were employed. The temperature was not attempted to be maintained constant for the PUS treatments, since the maximum temperature (in the case of the longest sonication PUS 10) was not more than 70 °C, which was of no concern in terms of curcumin's stability. These samples were also stored in similar fashion as mentioned above. Fig. 1 gives the schematic flow of the study.
The prepared solutions were characterized for the presence of curcumin using a Nanodrop ND-1000 v 3.3.1 spectrophotometer (Nanodrop Technologies, Inc., Wilmington, USA). The absorbance was scanned from 220–700 nm. Also, the absorbance of each solution was read at 425 nm (which is the absorbance wavelength of curcumin). A curcumin stock solution was prepared by dissolving 10 mg of curcumin (ALX-350-028-M010, purchased from Enzo, Life Sciences, Inc., USA) in ethanol to a concentration of 1 mg mL−1. Different concentrations (0.001–0.005 mg mL−1) were made by diluting the stock solution with absolute alcohol (100% ethanol). The absorbance was read at 425 nm and plotted against concentration to obtain a standard graph. The recovery of curcumin using the various sonication-based extraction methods was quantified using the standard graph. Curcumin yield39 was calculated using equation:
| Curcumin yield% = curcumin extracted (g) × 100/turmeric used (g) |
The prepared curcumin solutions were also characterized using a JEM-1400PLUS transmission electron microscope (TEM, JEOL USA, Inc., Peabody, MA, USA) and confocal laser scanning microscope (CLSM, Olympus FluoView™ FV1000, Olympus America Inc., Melville, NY, USA) to determine their particle sizes. The particle size distribution of curcumin was obtained using OPTIMAS 6.1 software (Optimas Corporation, Langham Creek, Houston, TX, USA) based on the TEM images. Further characterization to confirm the successful extraction of curcumin was done using FTIR (Shimadzu FTIR-8300 spectrometer, San Diego, CA, USA) with KBr pellets. For FTIR, the samples were dried in an oven and the powder was used for analysis. For comparison with the traditional solvent extraction process, curcumin was extracted from turmeric using ethanol, and the recovered curcumin was compared with the sonication-extracted curcumin in water.
A Tukey–Kramer multiple comparison test was performed to assess the statistical significance of the results using MYSTAT 1.0 software (Systat Software, Inc. 1735 Technology Drive, Suite 430, San Jose, CA, USA). A p-value <0.05 is considered statistically significant.
Results and discussion
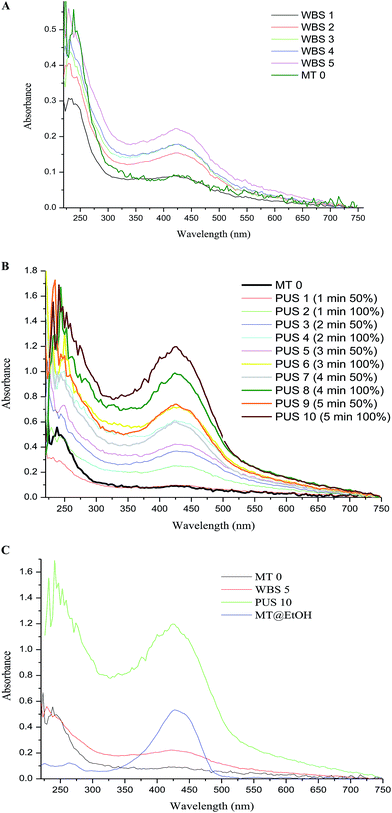
Using ultrasonication-based technology, efforts were made to develop a single-step extraction methodology for curcumin extraction from turmeric. Curcumin is reported to be insoluble in water; this property has been confirmed by various researchers40–42 and is the reason for its reduced bioavailability, preventing it from being used in biomedical applications. Generally, curcumin is extracted in solvents such as methanol, ethanol, acetone and most popularly, dimethyl sulfoxide (DMSO). Therefore, we have attempted to use ultrasonication to increase the solubility of curcumin in water. A WBS-based low frequency sonicator and a PUS were used in this study. WBS was employed combined with 50 °C temperature treatment. The photograph in Fig. 1 shows the insolubility of MT in water (a) and the increased solubility of turmeric following 4 h (WBS 5) of sonication (b) and 5 min-100% sonication energy (c) under probe sonication (PUS 10). The MT suspension initially appeared as a yellow solution, but when left undisturbed for a while, all the particles settled down, leaving a faint yellowish clear supernatant. The absorbance of this supernatant, which would depict the solubility of curcumin in water, is merely 0.18, confirming the poor solubility of curcumin in water. It is believed that the impartation of the yellowish color to the solution confirms the extraction of the yellowish curcumin dye; following WBS a more yellowish solution was obtained, and after PUS, a bright yellow solution was obtained.Bhawana et al.43 had conducted a study where they used a sonication-based method to prepare nanocurcumin from curcumin. In order to enable the direct extraction of curcumin from turmeric using water, both the WBS and PUS type of sonication techniques were employed, and as will be confirmed later, the results showed that the PUS method was more efficient and led to successful extraction of curcumin directly from water in 5 min.
Using a nanodrop spectrophotometer, the entire absorbance spectra from 220 to 700 nm was scanned, as observed in Fig. 2A. MT, which is the control or the sample prior to sonication, shows no absorbance in the curcumin absorbance region extending from 420–450 nm. However, a linear increase in the absorbance as a function of increasing sonication time from 10 min (WBS 1) to 30 min (WBS 2), 1 h (WBS 3), 3 h (WBS 4) and 4 h (WBS 5) is observed. Also, the sonication time of 10 min did not appear to contribute with respect to the WBS method, while sonication time above 30 min significantly contributed to the extraction of curcumin in water. The highest curcumin absorbance was obtained from WBS 5 following 4 h sonication. Fig. 2B gives the comparative absorbance spectra of the PUS method. As clearly evidenced from the figure, the PUS method is a far superior technique for the successful extraction of curcumin in water. The extraction efficiency observed at 4 h using the WBS method was obtained as early as 1 min (PUS 2) using 100% SF. It was observed that the extraction of curcumin increased linearly as a function of sonication time; the use of 100% SF contributed significantly to the extraction process. 50% SF with longer sonication time (above 4 min) yielded good results too. Thus, results based on the UV-vis absorbance spectra studies confirm the PUS technique as a superior methodology compared to WBS, showing higher extraction efficiency and less time consumption. The efficiency of these techniques was compared with the conventional solvent-based extraction method, using ethanol as solvent. Fig. 2C gives the comparative spectra of MT in water, MT in ethanol, WBS 5, and PUS 10. Compared to even the conventional solvent extraction process, the probe sonication method showed superior extraction with extended abilities of extracting curcumin directly in water. However, the WBS method (even with the longest sonication time of 4 h) showed lower extraction efficiency compared to the traditional ethanol-based solvent extraction process based on this comparative study. The efficiency of the PUS method is due to the fact that the probe sonicator is in direct contact with the sample and thereby can impart more concentrated energy to the sample than the bath sonicator.44 The increase in temperature (70 °C, PUS 10) during probe sonication is also understood to aid the successful extraction of curcumin. Hence, compared to the WBS method, the PUS method imposed temperatures higher than 50 °C; also, within the various PUS treatments, the temperature varied, with the highest temperatures recorded in the 100% SF samples. In terms of frequency, the probe sonicator is higher, and hence significant results were obtained within a short period of time. Also, the influence of sonication, be it WBS type or PUS type, on the extraction of curcumin is confirmed.
FTIR spectroscopy was used to confirm the successful extraction of curcumin and to discover the changes occurring on the surface owing to sonication. The FTIR spectra of curcumin show vibration of the phenolic group at 3504 cm−1. The peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, belonging to aromatic and allopathic rings, appeared at 1610 and 1560 cm−1. Curcumin contains two carbonyl groups showing bands at around 1640 cm−1.45 Modi and Pitre46 have also elaborately studied the FTIR spectra of curcumin; they have reported characteristic peaks for curcumin at (a) 1627 cm−1, which is a characteristic peak for C
C stretching, belonging to aromatic and allopathic rings, appeared at 1610 and 1560 cm−1. Curcumin contains two carbonyl groups showing bands at around 1640 cm−1.45 Modi and Pitre46 have also elaborately studied the FTIR spectra of curcumin; they have reported characteristic peaks for curcumin at (a) 1627 cm−1, which is a characteristic peak for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (enolic); (b) 1520 cm−1, showing the presence of the C
O (enolic); (b) 1520 cm−1, showing the presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C group; (c) 1250 cm−1, showing the C–O stretching; and (d) 3547 cm−1, revealing the presence of the OH group present in the molecule. Fig. 3 reveals the results of the FTIR analysis of curcumin extracted using the WBS 1–5 and PUS 1–10 experimental sets. As observed in the figure, all the major peaks characteristic of curcumin were obtained from the sonicated samples. It was observed that the MT 0 showed no significant curcumin peaks, confirming the fact that curcumin was not being extracted in water without any ultrasonic involvement. Also, an increase in the sharpness and intensity of the peaks with increasing sonication was observed.
C group; (c) 1250 cm−1, showing the C–O stretching; and (d) 3547 cm−1, revealing the presence of the OH group present in the molecule. Fig. 3 reveals the results of the FTIR analysis of curcumin extracted using the WBS 1–5 and PUS 1–10 experimental sets. As observed in the figure, all the major peaks characteristic of curcumin were obtained from the sonicated samples. It was observed that the MT 0 showed no significant curcumin peaks, confirming the fact that curcumin was not being extracted in water without any ultrasonic involvement. Also, an increase in the sharpness and intensity of the peaks with increasing sonication was observed.
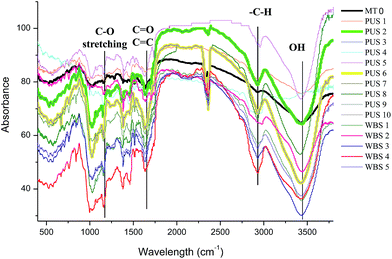 | ||
| Fig. 3 FTIR spectra of the various samples, showing changes in the spectra with sonication treatment compared to the control (MT 0). | ||
The quantity of curcumin extracted using the sonication variables was determined by measuring the absorbance at 425 nm and correlating the obtained optical density (OD) values with the standard graph plotted using the curcumin standard. Also, in order to compare the efficiency of the ultrasonication-based extraction technique developed in the current work with the existing conventional solvent extraction method, the quantity of curcumin extracted from turmeric in ethanol was also measured spectrophotometrically. Fig. 4 displays the results of these correlations. As can be observed from the graph, the PUS technique led to significantly enhanced extraction of curcumin, exceeding the conventional solvent extraction method (MT@EtOH) even as early as 2 min of ultrasonication time at 100% sonication frequency (PUS 4). PUS 5, 6, 7, 8, 9, and 10 all show an increasing trend of curcumin levels with increasing sonication time. Also, as observed from the graph, the sonication frequency increase from 50% to 100% was found to have a profound role in enhancing the curcumin extraction levels. The poor extraction of curcumin in water (MT 0) is reflected in Fig. 4. The PUS technique was thus highly effective compared to both the untreated control and the solvent extraction experimental set. However, the WBS technique showed comparable extraction to the solvent extract after long sonication times (>3 h). However, it was interesting to observe that even the WBS technique showed higher extraction efficiency of curcumin in water compared to the control. Thus, the curcumin extraction efficiency can be described in the order PUS > MT@EtOH > WBS > MT 0. Curcumin recovery, calculated using the equation, gives the yield (%) of curcumin using the various methods used. The slope was calculated using the following equation Y = 0.0955x with the regression coefficient (R2) value of 0.9145. Table 1 summarizes these results, as evident from the tabulated results; yield (%) of 56% was achieved using PUS 10 method for the extraction of curcumin in water; the WBS method recorded a highest of 22%, while conventional solvent extraction method gave 20% and the control (turmeric in water) 2% yield. The current methodology delivered better results compared to the traditional Soxhlet extraction method. Soxhlet method using acetone yielded 42% curcumin in 4 to 5 h.47 The other major extraction technique reported was the microwave-assisted extraction method (MAE), where a variety of solvents ranging from non-polar to polar ones, i.e. n-hexane, dichloromethane (DCM), ethyl acetate (EtOAc), acetone, ethanol and methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (60
water (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) are used. The efficiency of this technique is reported to be 60–70% but is limited to the use of these organic solvents.48
40, v/v) are used. The efficiency of this technique is reported to be 60–70% but is limited to the use of these organic solvents.48
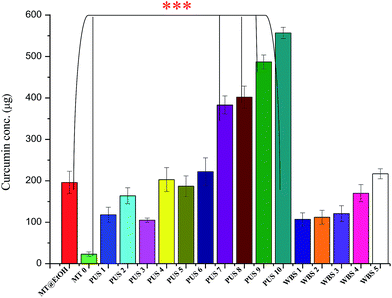 | ||
| Fig. 4 Graph showing the quantification of curcumin recovered by the respective methods. *** indicate statistically significant results. | ||
| Sample | Extraction details | Curcumin yield (%) |
|---|---|---|
| MT@EtOH | Curcumin extracted using conventional method (ethanol extraction) | 19.6 |
| MT 0 | Unsonicated turmeric in water (control) | 2.3 |
| WBS 1 | Water bath sonication for 10 min | 10.7 |
| WBS 2 | Water bath sonication for 30 min | 11.2 |
| WBS 3 | Water bath sonication for 1 h | 12.1 |
| WBS 4 | Water bath sonication for 3 h | 17 |
| WBS 5 | Water bath sonication for 4 h | 21.7 |
| PUS 1 | Probe ultrasonication 1 min-50% SF | 11.8 |
| PUS 2 | Probe ultrasonication 1 min-100% SF | 16.4 |
| PUS 3 | Probe ultrasonication 2 min-50% SF | 10.5 |
| PUS 4 | Probe ultrasonication 2 min-100% SF | 20.3 |
| PUS 5 | Probe ultrasonication 3 min-50% SF | 18.7 |
| PUS 6 | Probe ultrasonication 3 min-100% SF | 22.2 |
| PUS 7 | Probe ultrasonication 4 min-50% SF | 38.3 |
| PUS 8 | Probe ultrasonication 4 min-100% SF | 40.2 |
| PUS 9 | Probe ultrasonication 5 min-50% SF | 48.7 |
| PUS 10 | Probe ultrasonication 5 min-100% SF | 55.7 |
The results were statistically analyzed, and the curcumin extraction in water using both WBS and PUS sonication methods compared to the control (unsonicated turmeric in water) was found to be extremely significant (p value <0.001). However, the solvent-based extraction compared with the WBS extraction was found to be statistically insignificant (p value >0.05). On the other hand, the WBS extraction compared to the control (MT 0) was found to be statistically significant with p-value <0.01, depicting significant extraction capability of curcumin in water compared to the unsonicated control.
Mechanism of sonication-based extraction of curcumin from turmeric
The results unequivocally support the role of sonication in the successful extraction of curcumin in water. The mechanism behind this result has been probed into. A report by Bhawana et al. has shown that when curcumin was sonicated using a high frequency of 30 kHz, nanocurcumin was formed, which showed high solubility in water.43 In the current study, we have sonicated turmeric in water using high frequencies; this could have also led to the breaking of curcumin to nanocurcumin, thereby enhancing its solubility in water, leading to its extraction in water. In order to confirm the nature of the curcumin, we used TEM to analyze the turmeric powder (MT 0), WBS 5 and PUS 10 samples. The TEM micrographs confirm that the MT 0 (a) existed predominantly in sizes ranging from 0.4–0.7 μm. WBS 5 (b), which is the highest sonication time (4 h) and showed the maximum (amidst WBS variables) curcumin extraction ability, possessed particle sizes in the range of 200–500 nm, while PUS 10 (c), which showed the highest curcumin yield, showed particle sizes in the range of 30–70 nm. CLSM was also used to view the fluorescing curcumin particles, as shown by the insets in Fig. 5a–c; the trend observed in the TEM (Fig. 5) is also confirmed, whereby the nanocurcumin production due to high frequency ultrasonication is confirmed. Thus, as speculated in Fig. 6, the mechanism for the water-based extraction of curcumin from turmeric is due to the size reduction of curcumin, rendering it soluble in water, enabling extraction via sonication methods. Also, as shown in Fig. 6c, the PUS 10 sample showed prolonged solubility even beyond 48 h compared to the WBS method (b). The control resulted in immediate precipitation, leaving an almost clear supernatant, while WBS samples precipitated after 24 h. This result also confirms that the PUS method resulted in nanocurcumin that failed to precipitate even after prolonged standing. | ||
| Fig. 5 TEM micrographs of (a) MT 0, (b) WBS 5 and (c) PUS 10, showing morphology and size of curcumin particles, inset shows fluorescence image of respective samples. | ||
 | ||
| Fig. 6 Schematic representation of the PUS effect and sustained solubility in PUS (c) method compared to (b) WBS and the control (a) even after 48 h. | ||
Particle size and surface area play a major role in the interaction of materials with biological systems. Seemingly, decreasing the size of the materials leads to an exponential increase in surface area relative to volume, thereby making the nanomaterial surface more reactive on itself and to its contiguous milieu. Of note, particle size and surface area dictate how the system responds to, distributes, and eliminates the materials.49 One of the most important physical properties that will affect materials' solubility is particle size.50 The downsizing of the microcurcumin to nano scale is thus believed to be responsible for the enhanced solubility of curcumin in water.44,50 Researchers have demonstrated an increase in the saturation solubility and surface area through the reduction of particle size to less than 1 μm.51–54
Conclusion
The study confirmed the successful extraction of curcumin in water by a one-step, rapid, probe sonication-based technology directly from turmeric, yielding enhanced recovery compared to the conventional ethanol-based extraction method. This study solves the insolubility problem of curcumin in water.Acknowledgements
This work was supported by the KU Research Professor Program of Konkuk University.References
- C. K. B. Ishita, U. Bandyopadhyay and R. K. Banerjee, Curr. Sci., 2004, 87, 44 Search PubMed
.
- M. S. Valianthan, The Legacy of Caraka, Orient Longman, 2003 Search PubMed
.
- H. Ohori, H. Yamakoshi, M. Tomizawa, M. Shibuya, Y. Kakudo, A. Takahashi, S. Takahashi, S. Kato, T. Suzuki, C. Ishioka, Y. Iwabuchi and H. Shibata, Mol. Cancer Ther., 2006, 5, 2563 CrossRef CAS PubMed
.
- K. E. Dunsmore, P. G. Chen and H. R. Wong, Crit. Care Med., 2001, 29(11), 2199 CrossRef CAS PubMed
.
- A. Kuhad, S. Pilkhwal, S. Sharma, N. Tirkey and K. Chopra, J. Agric. Food Chem., 2007, 55(25), 10150 CrossRef CAS PubMed
.
- R. A. Calderone and W. A. Fonzi, Trends Microbiol., 2001, 9, 327 CrossRef CAS
.
- K. C. Das and C. K. Das, Biochem. Biophys. Res. Commun., 2002, 295, 62 CrossRef CAS
.
- J. C. Tilak, M. Banerjee, H. Mohan and T. P. A. Devasagaym, Phytother. Res., 2004, 18, 798 CrossRef PubMed
.
- M. Balasubramanyam, A. A. Koteswari, R. S. Kumar, S. F. Monickaraj, J. U. Maheswari and V. Mohan, J. Biosci., 2003, 28, 715 CrossRef CAS
.
- S. Wood, B. Nattress, J. Kirkham, R. Shore, S. Brookes and J. Griffiths, et al., J. Photochem. Photobiol., B, 1999, 50, 1 CrossRef CAS
.
- R. A. Sharma, A. J. Gescher and W. P. Steward, Eur. J. Cancer, 2005, 41, 1955 CrossRef CAS PubMed
.
- H. K. Koon, A. W. N. Leung, K. K. M. Yue and N. K. Mak, J. Environ. Pathol., Toxicol. Oncol., 2006, 25, 205 CrossRef CAS PubMed
.
- W. J. Silva, J. Seneviratne, N. Parahitiyawa, E. A. Rosa, L. P. Samaranayake and A. A. Del Bel Cury, Braz. Dent. J., 2008, 19, 364 Search PubMed
.
- K. Ono, K. Hasegawa, H. Naiki and M. J. Yamada, Neurosci. Res., 2004, 75(6), 742 CrossRef CAS PubMed
.
- F. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy and G. M. Cole, J. Biol. Chem., 2005, 280(7), 5892 CrossRef CAS PubMed
.
- K. Balasubramanian, J. Agric. Food Chem., 2006, 54, 3512 CrossRef CAS
.
- P. Anand, C. Sundaram, S. Jhurani, A. B. Kunnumakkara and B. B. Aggarwal, Cancer Lett., 2008, 267(1), 133 CrossRef CAS PubMed
.
- J. K. Lin, et al., Molecular Mechanism of Action of Curcumin, in Food Phytochemicals for Cancer Prevention II, 2009, American Chemical Society, pp. 196–203 Search PubMed
.
- A. M. Anderson, M. S. Mitchell and R. S. Mohan, J. Chem. Educ., 2000, 77, 359 CrossRef CAS
.
- P. Pizzo, C. Scapin, M. Vitadello, C. Florean and L. Gorza, J. Cell. Mol. Med., 2010, 14, 970 CrossRef CAS PubMed
.
- Y. Sugiyama, S. Kawakishi and T. Osawa, Biochem. Pharmacol., 1996, 52, 519 CrossRef CAS
.
- R. C. Srimal and B. N. Dhawan, J. Pharm. Pharmacol., 1973, 25, 447 CrossRef CAS PubMed
.
- B. B. Aggarwal and K. B. Harikumar, Int. J. Biochem. Cell Biol., 2009, 41, 40 CrossRef CAS PubMed
.
- Y. K. Lee, W. S. Lee, J. T. Hwang, D. Y. Kwon, Y. J. Surh and O. J. Park, J. Agric. Food Chem., 2009, 57, 305 CrossRef CAS PubMed
.
- W. C. Jordan and C. R. Drew, J. Natl. Med. Assoc., 1996, 88, 333 CAS
.
- R. De, P. Kundu, S. Swarnakar, T. Ramamurthy, A. Chowdhury, G. B. Nair and A. K. Mukhopadhyay, Antimicrob. Agents Chemother., 2009, 53, 1592 CrossRef CAS PubMed
.
- Y. Wang, Z. Lu, H. Wu and F. Lv, Int. J. Food Microbiol., 2009, 136, 71 CrossRef CAS PubMed
.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4(6), 807 CrossRef CAS PubMed
.
- A. L. Cheng, C. H. Hsu, J. K. Lin, M. M. Hsu, Y. F. Ho, T. S. Shen, J. Y. Ko, J. T. Lin, B. R. Lin, W. Ming-Shiang, H. S. Yu, S. H. Jee, G. S. Chen, T. M. Chen, C. A. Chen, M. K. Lai, Y. S. Pu, M. H. Pan, Y. J. Wang, C. C. Tsai and C. Y. Hsieh, Anticancer Res., 2001, 21(4B), 2895 CAS
.
- R. A. Sharma, S. A. Euden, S. L. Platton, D. N. Cooke, A. Shafayat, H. R. Hewitt, T. H. Marczylo, B. Morgan, D. Hemingway, S. M. Plummer, M. Pirmohamed, A. J. Gescher and W. P. Steward, Clin. Cancer Res., 2004, 10(20), 6847 CrossRef CAS PubMed
.
- B. Wahlstrom and G. Blenow, Pharmacol. Toxicol., 1978, 43, 86 CAS
.
- D. Knorr, J. Food Eng., 2003, 56, 131 CrossRef
.
- R. Zhang, Y. Xu and Y. Shi, Food and Machinery, 2003, 1, 21 Search PubMed
.
- M. Vinatoru, Ultrason. Sonochem., 2001, 8, 303 CrossRef CAS
.
- R. Babaei, A. Jabbari and Y. Yamini, Asian J. Chem., 2006, 18, 57 Search PubMed
.
- N. P. Dhanalakshmi and R. Nagarajan, World Academy of Science, Engineering and Technology, 2011, 5, 11–29 Search PubMed
.
- H. Kiani, Z. Zhang, A. Delgado and D.-W. Sun, Food Res. Int., 2011, 44(9), 2915–2921, DOI:10.1016/j.foodres.2011.06.051
.
- S. D. Kumavat, Y. S. Chaudhari, P. Borole, P. Mishra, K. Shenghani and P. Duvvuri, Int. J. Pharm. Rev. Res., 2013, 3(2), 50 Search PubMed
.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharmaceutics, 2007, 4, 807 CrossRef CAS PubMed
.
- D. S. Sogi, S. Sharma, D. P. S. Oberoi and I. A. Wani, J. Food Sci. Technol., 2010, 47, 300 CrossRef CAS PubMed
.
- G. Garcea, D. J. Jones, R. Singh, A. R. Dennison, P. B. Farmer, R. A. Sharma, W. P. Steward, A. J. Gescher and D. P. Berry, Br. J. Cancer, 2004, 90(5), 1011 CrossRef CAS PubMed
.
- P. Anand, A. B. Kunnumakkara, R. A. Newman and B. B. Aggarwal, Mol. Pharm., 2007, 4(6), 807 CrossRef CAS PubMed
.
- R. K. Bhawana Basniwal, H. S. Buttar, V. V. K. Jain and N. Jain, J. Agric. Food Chem., 2011, 59, 2056 CrossRef PubMed
.
- S. Julian, A. Vincent and R. Mark Wiesner, Nanotoxicology, 2011, 5(4), 711 CrossRef PubMed
.
- A. Shrivastava, J. Sharma, S. Jain and K. L. Aggrawal, Asian J. Pharm., 2013, 7, 103 CrossRef CAS PubMed
.
- G. Modi and K. S. Pitre, Def. Sci. J., 2010, 60(3), 255 CrossRef CAS
.
- V. S. Govindarjan, CRC Crit. Rev. Food Sci. Nutr., 1980, 12(3), 199 CrossRef PubMed
.
- S. C. Verma, World J. Pharm. Pharm. Sci., 2014, 3(7), 752–761 CAS
.
- K. W. Powers, M. Palazuelos, B. M. Moudgil and S. M. Roberts, Nanotoxicology, 2007, 1(1), 42 CrossRef CAS PubMed
.
- J. Leszczynski and T. Puzyn, Towards Efficient Designing of Safe Nanomaterials, RSC Nanoscience and nanotechnology, 2012, p. 277 Search PubMed.
- R. H. Muller, K. Peters, R. Becker and B. Kruss, 1st world meeting APGI/APV, Budapest, 1995 Search PubMed
.
- R. H. Muller, K. Peters, R. Becker and B. Kruss, Nanosuspensions for the i.v. administration of poorly soluble drugs-stability during sterilization and long-term storage, 22nd Int Sym of Control Rel of Bioactive materials, Washington DC, 1995 Search PubMed
.
- T. Gershanik and S. Benita, Eur. J. Pharm. Biopharm., 2000, 50, 179 CrossRef CAS
.
- N. Rawat, M. S. Kumar and N. Mahadevan, International Journal of Recent Advances in Pharmaceutical Research, 2011, 1, 8 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2015 |