Mn(OAc)3-mediated arylation–lactonization of alkenoic acids: synthesis of γ,γ-disubstituted butyrolactones†
Yuzhen Gaoa,
Jian Xua,
Pengbo Zhanga,
Hua Fangb,
Guo Tang*a and
Yufen Zhaoa
aDepartment of Chemistry, College of Chemistry and Chemical Engineering, The Key Laboratory for Chemical Biology of Fujian Province, Xiamen University, Xiamen, Fujian 361005, China. E-mail: t12g21@xmu.edu.cn; Fax: +86-592-2185780
bThird Institute of Oceanography, State Oceanic Administration, Xiamen, Fujian 361005, China
First published on 15th April 2015
Abstract
A general method for the oxidative cyclization of 4-alkenoic acids with arylboronic acids has been developed. The reactions described provide a novel access to γ,γ-disubstituted butyrolactones in moderate yields, and allow the direct formation of a C–C bond and the construction of a lactone ring in one reaction.
γ-Butyrolactones as a class of readily available compounds are pervasive in nature. Owing to their interesting biological activities, the γ,γ-disubstituted butyrolactones have received much attention.1 Consequently, the increasing demand for γ-butyrolactones has generated considerable interest in the development of efficient and flexible synthetic methods. In this context, classic methods for the synthesis of γ-butyrolactones include Baeyer–Villiger reaction of aryl cyclobutanones,2 arylation of unsaturated lactones,3 radical processes4 or the inter/intra-molecular reactions of alkenes with nucleophiles.5 Recently, difunctionalization of alkenes have provided a powerful strategy for the synthesis of various organic compounds, among which the carbolactonization of pent-4-enoic acids has been proved to be an efficient approach to benzyl-containing γ-lactones.6 Fagnoni's group reported a first ultraviolet light-induced synthesis of benzyl- and aryl-substituted γ-lactone from phenyl halides used as precursors of the cations, wherein only electron-donating substituted phenyl halides could give satisfactory yields.6a In 2010, the Zhang's group developed the carbolactonization of terminal alkenes via oxidative gold catalysis, which showed that the alkylgold intermediates could be readily functionalized by arylboronic acids in the presence of excess Selectfluor.6b
However, these pioneering works are difficult to be used in the synthesis of γ,γ-disubstituted butyrolactones. In 2014, Xiao's group developed a visible light photocatalytic arylation–lactonization cascade of 4-alkenoic acids with aryldiazonium salts. Xiao's method provides a rapid and straightforward access to various γ,γ-disubstituted butyrolactones under very mild conditions.6c However, the high reactivity of aryldiazonium salts and hazardous profile make them unsuitable for safe handling, especially on a large scale.
Arylboronic acids represent versatile building blocks in organic synthesis. More recently, the aryl radical generated from arylboronic acid in the presence of some oxidants have been identified as coupling partners in the formation of carbon–carbon bonds.7 Inspired by these results, we reasoned that generating directly the radical B by addition of phenyl radical A onto an olefin-acid would produce cationic intermediates through single-electron oxidation which ultimately afford γ,γ-disubstituted butyrolactone via a facile intramolecular nucleophilic addition. This transformation allows the direct formation of a C–C bond and the construction of a lactone ring in one reaction (Scheme 1).
This idea was first examined by using 4-phenylpent-4-enoic acid (1a) and phenylboronic acid (2a) as reaction partners (Table 1). Manganese(III) salts has been considered as the most prominent single-electron oxidant in the field of free-radical chemistry which are commercially available, cheap and easily prepared.8 When Mn(OAc)3·2H2O and Mn(acac)3 were choose as the oxidants in the beginning, the targeted product 3a was obtained in 37% and 21% yield (entries 1 and 2). The reaction was then performed in a variety of solvents (entries 3–6) by using Mn(OAc)3·2H2O as the oxidant, such as 1,2-dichloroethane (DCE), N-methyl-2-pyrrolidone (NMP), acetonitrile (CH3CN), and acetic acid (CH3COOH), giving product 3a in 30%, 15%, 38% and 55% yield, respectively. Pleasingly, the yield of product 3a raised to 62% when the temperature was decreased to 60 °C (entry 7). Under this reaction condition, biphenyl was obtained as a by-product, and 10% of 1a was recovered. However, the yield did not change when the equivalent of 2a was increased (entry 8). Other oxidants such as AgNO3/K2S2O8, Mn(OAc)2/air, Fe(ii)/K2S2O8, Mn(OAc)3/t-BuOOH, Mn(OAc)3/KMnO4, and KMnO4 were also investigated, but the reaction did not work well under these conditions (entries 9–15). Decreasing the load of Mn(OAc)3 to 2 equiv. produced a lower yield of 41% (entry 16). No desired product was obtained when 2.0 equiv. of TEMPO was added into the reaction under the optimal conditions (entry 17). This result suggests that the radical was intercepted by TEMPO and this reaction might go through a radical pathway. After optimization of the reaction conditions, we established an efficient route to the carbolactonization of olefinic carboxylic acids. The optimal reaction conditions are: 3.0 equiv. of Mn(OAc)3·2H2O as the oxidant, 1.5 equiv. of arylboronic acids and HOAc as the solvent at 60 °C for 6 h under nitrogen atmosphere (entry 7).
| Entry | Additive (equiv.) | Solvent | T [°C] | Yield [%] |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.45 mmol), additive in solvent (2 mL) stirring under nitrogen for 6 h. Oil bath temperature. Yield of the isolated product.b 2a (0.6 mmol).c Under air. | ||||
| 1 | Mn(OAc)3·2H2O (3) | Toluene | 100 | 37 |
| 2 | Mn(acac)3 (3) | Toluene | 100 | 21 |
| 3 | Mn(OAc)3·2H2O (3) | DCE | 80 | 30 |
| 4 | Mn(OAc)3·2H2O (3) | NMP | 80 | 15 |
| 5 | Mn(OAc)3·2H2O (3) | CH3CN | 80 | 38 |
| 6 | Mn(OAc)3·2H2O (3) | HOAc | 80 | 55 |
| 7 | Mn(OAc)3·2H2O (3) | HOAc | 60 | 62 |
| 8b | Mn(OAc)3·2H2O (3) | HOAc | 60 | 62 |
| 9 | AgNO3 (0.2) + K2S2O8 (3) | CH3CN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | Trace |
| 10c | Mn(OAc)2·4H2O (0.05) | DMSO | 100 | Trace |
| 11 | FeS (0.5) + K2S2O8 (3) | DCM | rt | Trace |
| 12 | FeSO4 (0.2) + K2S2O8 (3) | PhCl–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
rt | n.d |
| 13 | Mn(OAc)3·2H2O (1) + t-BuOOH (4) | HOAc | 60 | n.d |
| 14 | Mn(OAc)3·2H2O (1) + KMnO4 (2) | HOAc | 60 | 26 |
| 15 | KMnO4 (3) | Toluene–HOAc (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
100 | 32 |
| 16 | Mn(OAc)3·2H2O (2) | HOAc | 60 | 41 |
| 17 | Mn(OAc)3·2H2O (3) + TEMPO (2) | HOAc | 60 | 0 |
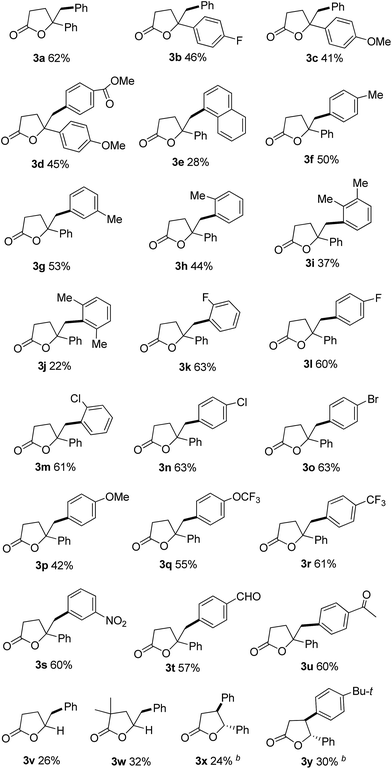
Having the optimal conditions in hand, we next examined the reactions of various arylboronic acids with olefinic carboxylic acids to understand the scope of the reaction (Table 2). Readily available 4-arylpent-4-enoic acids with fluoro or methoxy group on the benzene ring were investigated, and the reaction afforded the corresponding γ,γ-disubstituted butyrolactones (3a–3d) in moderate to good yields (62–41%). The arylation–lactonization process was compatible with various arylboronic acids. The bulky naphthylboronic acid only gave 3e in 28% yield. Moreover, the position of methyl group on the benzene ring did not affect this transformation significantly (3f–3h). 2,3-Dimethylphenylboronic acid gave 3i in 37% yield. More bulky substrate like 2,3-dimethylphenylboronic acid also reacted with 1a and afforded the corresponding product 3j in much lower yield. Halogen atoms such as fluoro, chloro and bromo at different positions on the aromatic ring (3k–3o) were unaffected under the present reaction conditions, providing potential possibility for further functionalization. The substituted phenyl group with electron-donating groups (3p and 3q) and electron-withdrawing groups (3r and 3s) were demonstrated to be applicable and converted into the desired products smoothly. The reaction also worked well with other functional groups such as ester (3d), formyl (3t) and acetyl (3u) resulting in 45%, 57% and 60% yield, respectively. It should be noted that the present carbolactonization is also applicable to unactivated 4-pentenoic acids, giving products 3v and 3w in 26% and 32% yield, respectively. Internal alkenoic acid was also investigated, generating the corresponding products 3x and 3y in low yields.
In conclusion, we have successfully developed a simple and general method for the preparation of γ,γ-disubstituted butyrolactones through Mn(OAc)3-mediated radical carbolactonization of olefinic carboxylic acids under relatively mild reaction conditions. As one of its notable features, the radical process allows the direct formation of a C–C bond and the construction of a butyrolactone ring in one reaction. Moreover, a variety of useful functional groups are also tolerated, which is attributed to the mild conditions. Finally, the use of a commercially available, cheap and easily prepared Mn(OAc)3 represents an added advantage of this method.
Acknowledgements
We acknowledge financial support from the Chinese National Natural Science Foundation (21173178, 21232005, 21375113), the National Basic Research Program of China (2012CB821600), the Public Science and Technology Research Funds Projects of the Ocean (201405017), GD2012-D01–001, and the Program for Changjiang Scholars and Innovative Research Team in University.Notes and references
- (a) M. Seitz and O. Reiser, Curr. Opin. Chem. Biol., 2005, 9, 285 CrossRef CAS PubMed; (b) H. C. Brown, S. V. Kulkarni and U. S. Racherla, J. Org. Chem., 1994, 59, 365 CrossRef CAS; (c) R. A. Pilli, G. B. Rosso and M. C. F. de Oliveira, Nat. Prod. Rep., 2010, 27, 1908 RSC; (d) Y. Ye, G. W. Qin and R. S. Xu, J. Nat. Prod., 1994, 57, 665 CrossRef CAS; (e) E. G. McMahon, Curr. Opin. Pharmacol., 2001, 1, 190 CrossRef CAS.
- S. I. Murahashi, S. Ono and Y. Imada, Angew. Chem., Int. Ed., 2002, 41, 2366 CrossRef CAS.
- C. Defieber, J. F. Paquin, S. Serna and E. M. Carriera, Org. Lett., 2004, 6, 3873 CrossRef CAS PubMed.
- (a) B. B. Snider, Chem. Rev., 1996, 96, 339 CrossRef CAS PubMed; (b) B. B. Snider, Tetrahedron, 2009, 65, 10738 CrossRef CAS PubMed; (c) H. Oumar-Mahamat, C. Moustrou, J. M. Surzur and M. P. Bertrand, J. Org. Chem., 1989, 54, 5684 CrossRef CAS; (d) L. H. Powell, P. H. Docherty, D. G. Hulcoop, P. D. Kemmitt and J. W. Burton, Chem. Commun., 2008, 2559 RSC; (e) W. Wang, M. H. Xu, X. S. Lei and G. Q. Lin, Org. Lett., 2000, 2, 3773 CrossRef CAS PubMed; (f) T. Iwahama, S. Sakaguchi and Y. Ishii, Chem. Commun., 2000, 613 RSC; (g) Y. Gao, X. Li, J. Xu, Y. Wu, W. Chen, G. Tang and Y. Zhao, Chem. Commun., 2015, 51, 1605 RSC.
- (a) L. Huang, H. Jiang, C. Qi and X. Liu, J. Am. Chem. Soc., 2010, 132, 17652 CrossRef CAS PubMed; (b) X. Xie, Y. Li and J. M. Fox, Org. Lett., 2013, 15, 1500 CrossRef CAS PubMed.
- (a) S. Protti, M. Fagnoni and A. Albini, J. Am. Chem. Soc., 2006, 128, 10670 CrossRef CAS PubMed; (b) G. Zhang, L. Cui, Y. Wang and L. Zhang, J. Am. Chem. Soc., 2010, 132, 1474 CrossRef CAS PubMed; (c) W. Guo, H. G. Cheng, L. Y. Chen, J. Xuan, Z. J. Feng, J. R. Chen, L. Q. Lu and W. J. Xiao, Adv. Synth. Catal., 2014, 356, 2787 CrossRef CAS PubMed.
- (a) G. Yan, M. Yang and X. Wu, Org. Biomol. Chem., 2013, 11, 7999 RSC; (b) M. Tobisu, K. Koh, T. Furukawa and N. Chatani, Angew. Chem., Int. Ed., 2012, 51, 11363 CrossRef CAS PubMed; (c) A. Dickschat and A. Studer, Org. Lett., 2010, 12, 3972 CrossRef CAS PubMed; (d) A. S. Demir, Ö. Reis and M. Emrullahoglu, J. Org. Chem., 2003, 68, 578 CrossRef CAS PubMed; (e) X. Li, J. Xu, Y. Gao, H. Fang, G. Tang and Y. Zhao, J. Org. Chem., 2015, 80, 2621 CrossRef CAS PubMed; (f) Y. Fujiwara, V. Domingo, I. B. Seiple, R. Gianatassio, M. Del Bel and P. S. Baran, J. Am. Chem. Soc., 2011, 133, 3292 CrossRef CAS PubMed; (g) A. S. Demir and H. Findik, Tetrahedron, 2008, 64, 6196 CrossRef CAS PubMed; (h) J. Wang, S. Wang, G. Wang, J. Zhang and X.-Q. Yu, Chem. Commun., 2012, 48, 11769–11771 RSC.
- (a) T. D. Lash, Chem.–Asian J., 2014, 9, 682 CrossRef CAS PubMed; (b) J. M. Concellón, H. Rodríguez-Solla and V. Del Amo, Chem.–Eur. J., 2008, 14, 10184 CrossRef PubMed; (c) X. Q. Pan, L. Wang, J. P. Zou and W. Zhang, Chem. Commun., 2011, 47, 7875 RSC; (d) X. J. Mu, J. P. Zou, Q. F. Qian and W. Zhang, Org. Lett., 2006, 8, 5291 CrossRef CAS PubMed; (e) T. Kagayama, A. Nakano, S. Sakaguchi and Y. Ishii, Org. Lett., 2006, 8, 407 CrossRef CAS PubMed; (f) O. Tayama, A. Nakano, T. Iwahama, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2004, 69, 5494 CrossRef CAS PubMed; (g) Y. Gao, J. Wu, J. Xu, X. Wang, G. Tang and Y. Zhao, Asian J. Org. Chem., 2014, 3, 691 CrossRef CAS PubMed; (h) H. C. Fisher, O. Berger, F. Gelat and J. L. Montchamp, Adv. Synth. Catal., 2014, 356, 1199 CrossRef CAS PubMed; (i) Y. Gao, J. Wu, J. Xu, P. Zhang, G. Tang and Y. Zhao, RSC Adv., 2014, 4, 51776 RSC; (j) J. Bush, B. John and H. Finkbeiner, J. Am. Chem. Soc., 1968, 90, 5903 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for the synthesis, spectral data and NMR spectra of compounds 3a–3y. See DOI: 10.1039/c5ra04429d |
| This journal is © The Royal Society of Chemistry 2015 |