New insights into the effects of NaCl and LiCl on the hydrogen storage behaviours of a 6LiBH4–Mg(AlH4)2 composite
Yuepeng Pangab,
Yongfeng Liu*a,
Xin Zhanga,
You Lia,
Mingxia Gaoa and
Hongge Pana
aState Key Laboratory of Silicon Materials, Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, Department of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, People's Republic of China. E-mail: mselyf@zju.edu.cn; Fax: +86 571 87952615; Tel: +86 571 87952615
bShanghai Key Laboratory of Modern Metallurgy and Materials Processing, Shanghai University, Shanghai 200072, China
First published on 9th January 2015
Abstract
The effects of the NaCl and LiCl by-products generated during the synthesis of Mg(AlH4)2 on the hydrogen storage properties of a 6LiBH4–Mg(AlH4)2 composite are investigated and clarified for the first time. The results indicate that the presence of NaCl and LiCl changes the dehydrogenation/hydrogenation kinetics of the 6LiBH4–Mg(AlH4)2 composite in addition to producing a distinct reduction in the hydrogen capacity. For the NaCl-containing sample, the chemical composition is changed due to the metathesis reaction between NaCl and LiBH4 during ball milling, which converts the NaCl and LiBH4 to LiCl and NaBH4. However, for the LiCl-containing system, the kinetic barriers of the dehydrogenation reaction were changed by the presence of LiCl, which is responsible for the change in the dehydrogenation temperature. These findings elucidate the effects of NaCl and LiCl, which are produced during the synthesis of Mg(AlH4)2, on the hydrogen storage behaviours of the 6LiBH4–Mg(AlH4)2 composite.
Introduction
The greatest challenge for the widespread use of hydrogen energy is how to store it in a safe, efficient and reversible manner.1,2 Compared to high pressure compression and liquefaction via cryogenics, storing hydrogen in solid state materials has potential advantages in terms of the volumetric density and safety, especially for mobile applications.1–3 Since Bogdanović and co-worker demonstrated that reversible hydrogen storage in NaAlH4 can be achieved at moderate temperature and hydrogen pressure by the addition of Ti-based catalysts in 1997, more and more attention has been paid to light-metal complex hydrides, including metal alanates and borohydrides, as promising hydrogen storage candidates due to their high gravimetric and volumetric hydrogen capacities.3–6Mg(AlH4)2 has a high theoretical hydrogen capacity of 9.3 wt% (on material basis,7,8 similarly hereinafter), and approximately 7 wt% of hydrogen can be released below 180 °C according to the following reaction:9
| Mg(AlH4)2 → MgH2 + 2Al + 3H2 | (1) |
Unfortunately, hydrogen desorption from reaction (1) is mildly exothermic in nature. It is therefore difficult for the Mg(AlH4)2 to be re-hydrogenated under moderate conditions, which is the key disadvantage of Mg(AlH4)2 as a reversible hydrogen storage material.10,11
As a typical borohydride, LiBH4 delivers a hydrogen capacity as high as 13.8 wt% via reaction (2).12
 | (2) |
However, reaction (2) proceeds at a rather high temperature above 400 °C due to the problematic thermodynamics with an enthalpy change of approximately 74 kJ mol−1-H2.13 In particular, the reverse of reaction (2) requires even harsher conditions of 600 °C and 150 atm of hydrogen pressure.14 These prevent LiBH4 from having practical applications as a hydrogen storage material.
Recently, a new strategy was proposed to tailor the thermodynamics of the dehydrogenation reactions of complex hydrides by forming a reactive composite with metals or metal hydrides. A successful example is the 2LiBH4–MgH2 reactive hydride composite developed by Vajo et al.15 It was reported that the dehydrogenation temperature was reduced by 250 °C because of the formation of MgB2. More attractively, the dehydrogenation product can be re-hydrogenated at 350 °C and 100 bar of hydrogen, which are more moderate conditions relative to those required for the pristine LiBH4. After that, a variety of reactive hydride composites have been investigated and developed, such as LiBH4–Mg(Al), LiBH4–CaH2, LiBH4–ScH2, LiBH4–LiAlH4, LiBH4–NaAlH4, LiBH4–Mg(AlH4)2, LiBH4–Ca(AlH4)2, NaBH4–LiAlH4, Mg(BH4)2–LiAlH4, Mg(BH4)2–NaAlH4, and so on.16–23 Among these studied materials, the 6LiBH4–Mg(AlH4)2 composite exhibited a significantly lower dehydrogenation temperature, faster kinetics and better reversibility than the MgH2– or Al–LiBH4 individually.19 It was reported that the 6LiBH4–Mg(AlH4)2 composite released 11.8 wt% of hydrogen within 200 min at 400 °C, and the dehydrogenated sample absorbed 6.4 wt% of hydrogen within 300 min at 400 °C. Here, it should be mentioned that Mg(AlH4)2 was synthesised by a mechanochemically activated metathesis reaction of LiAlH4 and MgCl2 without solvent, and the by-product LiCl was not removed, which induces a distinct reduction in the practical hydrogen capacity due to the dead weight. Moreover, several recent studies revealed that the chlorides may play important roles for hydrogen desorption from complex hydrides instead of being only inert species.24–26 Singh et al.24 reported that NaCl acted as the nucleation centre of NaH in TiCl3-doped NaAlH4 and thus accelerated the decomposition of Na3AlH6. A similar phenomenon was also observed in the TiF4-doped Na2LiAlH6.25 More recently, it was found that adding a small amount of lithium halides (LiCl, LiBr, and LiI) significantly improved the hydrogen storage properties of the LiNH2–MgH2 composite by forming fast Li-ion conductors.26 Consequently, a question raised is what is the real role played by the NaCl and LiCl by-products in the dehydrogenation/hydrogenation of the 6LiBH4–Mg(AlH4)2 composite.
In this work, the effects of NaCl and LiCl on the dehydrogenation/hydrogenation of the 6LiBH4–Mg(AlH4)2 composite were studied and systematically compared for the first time. The results showed that NaCl could react with LiBH4 to form LiCl and NaBH4 during ball milling, which changes the chemical composition of the 6LiBH4–Mg(AlH4)2 composite. As for LiCl, its presence affected the hydrogen desorption/absorption kinetics of the 6LiBH4–Mg(AlH4)2 composite even though it does not react with LiBH4 or Mg(AlH4)2 during the ball milling and heating processes. These findings indicate that there is a distinct effect from the NaCl and LiCl by-products generated during the synthesis of Mg(AlH4)2 on the hydrogen storage properties of the 6LiBH4–Mg(AlH4)2 composite, which elucidates the roles played by NaCl and LiCl.
Experimental
The commercial chemicals NaAlH4 (ACROS, 93%), LiAlH4 (Alfa Aesar, 97%), MgCl2 (Alfa Aesar, 99%) and LiBH4 (Sigma-Aldrich, 95%) were used as received without further purification. Pure Mg(AlH4)2 was synthesised by the method described in our previous work.27 The 6LiBH4–Mg(AlH4)2 composite was obtained by first ball milling Mg(AlH4)2 for 3 h and then ball milling the as-milled Mg(AlH4)2 with LiBH4 for 12 h. Ball milling was carried out on a planetary ball mill (QM-3SP4, Nanjing) rotated at 500 rpm. The ball-to-sample weight ratio was approximately 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mill was set to rotate for 0.2 h in one direction and then rotate in the reverse direction after a 0.1 h pause. For comparison, the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites were also prepared by means of a two-step method. First, the Mg(AlH4)2–2LiCl and Mg(AlH4)2–2NaCl composites were produced by ball milling LiAlH4 with MgCl2 or NaAlH4 with MgCl2 for 3 h. Then, the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites were obtained after ball milling the as-prepared Mg(AlH4)2–2LiCl and Mg(AlH4)2–2NaCl composites with LiBH4 for 12 h, respectively. All of the sample handing was performed in a glove box (MBRAUN, Germany) filled with pure argon (H2O, O2 < 1 ppm).
1. The mill was set to rotate for 0.2 h in one direction and then rotate in the reverse direction after a 0.1 h pause. For comparison, the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites were also prepared by means of a two-step method. First, the Mg(AlH4)2–2LiCl and Mg(AlH4)2–2NaCl composites were produced by ball milling LiAlH4 with MgCl2 or NaAlH4 with MgCl2 for 3 h. Then, the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites were obtained after ball milling the as-prepared Mg(AlH4)2–2LiCl and Mg(AlH4)2–2NaCl composites with LiBH4 for 12 h, respectively. All of the sample handing was performed in a glove box (MBRAUN, Germany) filled with pure argon (H2O, O2 < 1 ppm).
The temperature dependence of hydrogen desorption from the samples was measured using a homemade temperature-programmed desorption (TPD) system with an online mass spectrometer (Hiden QIC-20, England). Approximately 60 mg of sample was loaded into a stainless steel microreactor and gradually heated from room temperature to 500 °C at a rate of 2 °C min−1 with a continuous flow of pure Ar as the carrier gas. The quantitative dehydrogenation/hydrogenation performances were determined by a volumetric method using a Sieverts-type apparatus. The pressure and temperature in the reactor and gas reservoir were monitored and recorded automatically. The quantities of hydrogen desorbed/absorbed were determined by calculating the pressure and temperature changes in the reactor using the equation of state. The heat effect of hydrogen desorption was determined by differential scanning calorimetry (DSC) on a Netzsch DSC 200 F3 unit (Germany). Pure Ar was used as the carrier gas, and the heating rate was set to 10 °C min−1.
The phase structures of the samples were characterised by an X'Pert PRO diffractometer (PANalytical, The Netherland) with Cu-Kα radiation at 40 kV and 40 mA. The XRD data were collected in the 2θ range of 10–90° at room temperature. A homemade container was applied to protect the powdery samples from air and moisture contamination during transfer and scanning. Fourier transform infrared (FTIR) spectra were acquired using a Bruker Tensor 27 unit (Germany) in the transmission mode. The sample being tested was prepared by cold-pressing a mixture of the target powder and KBr (Alfa Aesar, 99%) at a weight ratio of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Results and discussion
Fig. 1 shows the FTIR spectra and XRD patterns of the as-prepared 6LiBH4–Mg(AlH4)2, 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites. As shown in Fig. 1a, it can be observed that the three composites with and without LiCl and NaCl exhibited identical FTIR spectra as the B–H vibrations at 2386, 2291, 2223 and 1125 cm−1 and Al–H vibrations at 1830 cm−1 were clearly observed. Further XRD examinations revealed that the characteristic reflections of LiBH4 and Mg(AlH4)2 dominated the XRD profiles of all three of the composites (Fig. 1b). For the 6LiBH4–Mg(AlH4)2–2LiCl composite, the typical diffraction peaks of LiCl were also unambiguously detected with considerable intensities. Therefore, we believe that the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites are only the physical mixtures of the corresponding chemicals after ball milling at room temperature. In other words, no chemical reaction occurred between LiBH4, Mg(AlH4)2 and LiCl during ball milling. However, NaCl is invisible in the 6LiBH4–Mg(AlH4)2–2NaCl composite, and two new phases of LiCl and NaBH4 were identified in addition to the LiBH4 and Mg(AlH4)2. This result suggests that NaCl reacts with LiBH4 and is converted to LiCl and NaBH4 during ball milling as described by the following reaction:
 | (3) |
 | ||
| Fig. 1 FTIR spectra (a) and XRD patterns (b) of the 6LiBH4–Mg(AlH4)2 composite with and without LiCl and NaCl. | ||
It is known that the standard enthalpies of formation of LiBH4, NaCl, NaBH4, and LiCl are −190.799, −411.120, −408.266, and −191.841 kJ mol−1, and their standard entropies are 75.902, 72.132, 59.300, and 101.391 J mol−1, respectively.28 Thus, the Gibbs free energy change of reaction (3) was determined to be −1.99 kJ mol−1 (27 °C), which indicates that the metathesis reaction between LiBH4 and NaCl is thermodynamically favourable, which can be initialised by the collision of grinding balls during energetic ball-milling. As a result, the 6LiBH4–Mg(AlH4)2–2NaCl composite converted to 4LiBH4–2NaBH4–Mg(AlH4)2–2LiCl after ball milling.
Hydrogen desorption properties of the as-prepared 6LiBH4–Mg(AlH4)2 composites with and without LiCl and NaCl were measured as a function of temperature by TPD and volumetric methods. Fig. 2 shows the TPD curves of the as-prepared 6LiBH4–Mg(AlH4)2, 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites. It is observed that the as-prepared 6LiBH4–Mg(AlH4)2 composite roughly exhibits a four-step dehydrogenation behaviour in the tested temperature range. The four hydrogen desorption peaks appeared at 130, 295, 407 and 435 °C as the temperature was increased. For the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites, the first peak of dehydrogenation remains at 130 °C, and the second dehydrogenation step exhibits a shift to a lower temperature relative to the 6LiBH4–Mg(AlH4)2 composite. More interestingly, the dehydrogenation behaviours of 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl become more complicated above 350 °C. It is observed that at above 350 °C, the 6LiBH4–Mg(AlH4)2–2LiCl composite exhibits three dehydrogenation peaks, and there are four peaks for the 6LiBH4–Mg(AlH4)2–2NaCl composite above 350 °C. It is noteworthy that the temperatures of these dehydrogenation peaks are distinctly higher than those of the halide-free sample. Therefore, we believe that the presence of LiCl and NaCl affects the dehydrogenation behaviour of the 6LiBH4–Mg(AlH4)2 composite. This conjecture is further confirmed by the volumetric release measurements. As shown in Fig. 3, a slight shift to a lower temperature was detected for the second dehydrogenation step of the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites. However, a shift to a higher temperature was observed for the third and fourth dehydrogenation steps. This is in excellent agreement with the TPD results. Quantitative analyses revealed that the 6LiBH4–Mg(AlH4)2 composite delivered 2.6, 0.9, 8.0 and 0.3 wt% hydrogen at 70–150 °C, 250–300 °C, 300–420 °C and 420–500 °C as shown in Table 1. The total dehydrogenation amounted to 11.8 wt%, which is very close to the theoretical hydrogen capacity of 12.4 wt%. For the 6LiBH4–Mg(AlH4)2–2LiCl composite, the dehydrogenation amounts of the four steps were calculated to be 1.9, 0.7, 5.9 and 0.2 wt% at 106–160 °C, 220–290 °C, 290–450 °C and 450–500 °C, respectively, and the dehydrogenation amounts were 1.8, 0.6, 5.1 and 0.2 wt% of hydrogen at 100–160 °C, 220–290 °C, 290–480 °C and 480–500 °C for the 6LiBH4–Mg(AlH4)2–2NaCl composite. The total dehydrogenation amounts were determined to be 8.7 wt% and 7.7 wt% for the 6LiBH4–Mg(AlH4)2–2LiCl and 6LiBH4–Mg(AlH4)2–2NaCl composites, respectively. Specifically, the practical hydrogen capacity was distinctly reduced because no hydrogen atoms were included in LiCl and NaCl. In addition, it should be noted that the 6LiBH4–Mg(AlH4)2–2NaCl composite exhibited the lowest hydrogen capacity and much higher dehydrogenation temperatures for the third and fourth steps among the three studied samples. This can be attributed to the larger molar weight of NaCl than that of LiCl and the formation of NaBH4 caused by the metathesis reaction between LiBH4 and NaCl because NaBH4 is more thermodynamically stable than LiBH4 leading to dehydrogenation occurring at higher temperatures as extensively reported previously.3 Because NaCl is converted to LiCl after ball milling, the follow-up in-depth investigations on the hydrogen storage thermodynamics and kinetics were concentrated on 6LiBH4–Mg(AlH4)2–2LiCl.
| Samples | Hydrogen desorption amount (wt%) | ||||
|---|---|---|---|---|---|
| 1st step | 2nd step | 3rd step | 4th step | Total | |
| 6LiBH4–Mg(AlH4)2 | 2.6 | 0.9 | 8.0 | 0.3 | 11.8 |
| 6LiBH4–Mg(AlH4)2–2LiCl | 1.9 | 0.7 | 5.9 | 0.2 | 8.7 |
| 6LiBH4–Mg(AlH4)2–2NaCl | 1.8 | 0.6 | 5.1 | 0.2 | 7.7 |
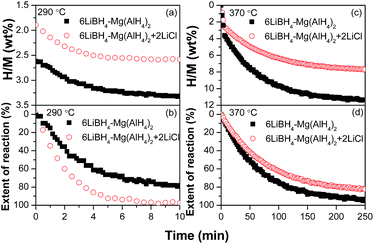
Fig. 4 presents the isothermal dehydrogenation curves of the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites. It is observed that approximately 3.3 and 2.6 wt% of hydrogen were quickly released from the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites, respectively, within 10 min at 290 °C. Such dehydrogenation amounts are close to the capacities of the initial two steps of dehydrogenation. To compare the dehydrogenation kinetics, the isothermal dehydrogenation curves were re-plotted by calculating the extent of reaction as shown in Fig. 4b. It is clear that a faster dehydrogenation rate was observed for the 6LiBH4–Mg(AlH4)2–2LiCl composite in comparison with the 6LiBH4–Mg(AlH4)2 composite, indicating that the presence of LiCl improves the dehydrogenation kinetics of the 6LiBH4–Mg(AlH4)2 composite at low temperatures. When the dehydrogenation temperature was elevated to 370 °C, hydrogen desorption amounts increased to 11.3 wt% and 7.7 wt% for the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites, respectively, within 250 min (Fig. 4c). Re-plotting the isothermal dehydrogenation curves by using the extent of reaction against time (Fig. 4d) shows a slightly slower overall dehydrogenation rate for the LiCl-containing composite, which can be attributed to that the majority of dehydrogenation occurred at higher temperatures for the 6LiBH4–Mg(AlH4)2–2LiCl composite as shown in Fig. 3. These results indicate that the presence of LiCl accelerates the dehydrogenation rate of the second step of the 6LiBH4–Mg(AlH4)2 composite but retards the dehydrogenation rate of the third step, which is consistent with the non-isothermal results (Fig. 2 and 3).
To understand the chemical events occurring in the hydrogen desorption process, FTIR and XRD examinations were carried out on the dehydrogenated 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl samples at different temperatures. Fig. 5 presents the results of the FTIR and XRD measurements of the 6LiBH4–Mg(AlH4)2 composite. FTIR analyses (Fig. 5a) reveal that after dehydrogenation at 160 and 310 °C, only the absorbances of the B–H vibration were observed, and the Al–H vibration was invisible. When the samples were dehydrogenated at 420 and 460 °C, no apparent absorbance was detected in the FTIR spectrum, indicating the disappearance of the B–H vibration due to the consumption of LiBH4. In a further XRD experiment (Fig. 5b), the reflections of MgH2 and Al were identified along with the absence of Mg(AlH4)2 after dehydrogenation at 160 °C. When the sample was heated to 310 °C, the newly developed MgH2 and Al disappeared, and an Al(Mg) solid solution phase and Al3Mg2 were discernible. At 420 °C, LiBH4, Al3Mg2 and the Al(Mg) solid solution were completely consumed while LiH, AlB2 and MgAlB4 were formed. When the temperature was further increased to 460 °C, LiAl, LiH and MgAlB4 are the only three phases detected in the dehydrogenated sample. According to the above discussion, we believe that upon heating, Mg(AlH4)2 in the 6LiBH4–Mg(AlH4)2 composite first decomposes to form MgH2, Al and H2. Then, MgH2 reacts with Al to form the Al(Mg) solid solution, Al3Mg2 and H2 as the temperature is increased. After that, the chemical reaction between LiBH4, the Al(Mg) solid solution and Al3Mg2 occurs producing LiH, AlB2, MgAlB4 and H2. Finally, LiH reacts with AlB2 to form LiAl, B and H2. The overall reaction process can be described by the following reactions:
 | (4) |
 | ||
| Fig. 5 FTIR spectra (a) and XRD patterns (b) of the 6LiBH4–Mg(AlH4)2 composite dehydrogenated at different temperatures. | ||
Here, it is deduced that B should exist in the final dehydrogenation product according to the chemical balance; however, it is rather hard to detect only by XRD due to its poor crystallinity. Theoretically, the dehydrogenation amounts of the four steps of eqn (4) were calculated to be 2.8, 0.9, 8.3 and 0.4 wt%, respectively, which offers a total dehydrogenation capacity of 12.4 wt%. This is in good agreement with the experimental results shown in Fig. 3 and Table 1.
Fig. 6 shows the FTIR spectra and XRD patterns of the dehydrogenated 6LiBH4–Mg(AlH4)2–2LiCl composite at different temperatures. It can be observed that the LiCl remained nearly constant throughout the dehydrogenation process, suggesting that it did not take part in the dehydrogenation reaction. Moreover, the structural changes of the 6LiBH4–Mg(AlH4)2–2LiCl composite are very similar to those of the 6LiBH4–Mg(AlH4)2 composite upon heating, representing the occurrence of identical chemical events during dehydrogenation.
 | ||
| Fig. 6 FTIR spectra (a) and XRD patterns (b) of the 6LiBH4–Mg(AlH4)2–2LiCl composite dehydrogenated at different temperatures. | ||
It should be noted that in the present study, the dehydrogenation pathway is slightly different from a previous report,19 in which only the first three hydrogen desorption steps occurred, and the reaction between LiH and AlB2 did not take place in the temperature range of 25–500 °C. This is potentially due to the difference in the hydrogen pressure within the reactor after dehydrogenation. As a hydrogen desorption reaction, the reaction temperature of LiH and AlB2 is closely related to the hydrogen pressure. Therefore, we believe that the different hydrogen pressure inside the reactor after dehydrogenation should be responsible for the different dehydrogenation pathways.
To elucidate the role of LiCl in lowering the temperature of the second dehydrogenation step and raising the temperature of the third dehydrogenation step of the 6LiBH4–Mg(AlH4)2 composite, the thermodynamic and kinetic parameters of the dehydrogenation of the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites were examined and compared. Fig. 7 shows the DSC curves of the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites at a heating rate of 10 °C min−1. Six heat-flow peaks were observed one after another in the DSC curve of the 6LiBH4–Mg(AlH4)2 composite upon heating, representing a complicated heat effect during dehydrogenation. The endothermic peak at 100–140 °C should be attributed to the phase transformation of LiBH4 (Pnma to P63mc) according to a previous report.14 At 150–170 °C, the weak exothermic peak corresponds to the decomposition of Mg(AlH4)2 to MgH2, Al and H2.29 In the temperature range of 260–340 °C, the DSC curve of the 6LiBH4–Mg(AlH4)2 composite exhibits two strong endothermic peaks at 289 and 323 °C, which can be assigned to the melting of LiBH4 and the dehydrogenation reaction between MgH2 and Al.12,27 The large endothermic peak at 451 °C originates from the dehydrogenation reaction between LiBH4, the Al(Mg) solid solution and Al3Mg2. Finally, a weak endothermic peak at 478 °C was observed, which can be attributed to the reaction between LiH and AlB2. For the 6LiBH4–Mg(AlH4)2–2LiCl composite, it is observed that there is a shift to a lower temperature for the phase transformation of LiBH4 at 100–140 °C, but the melting temperature of LiBH4 moved to a higher temperature with respect to the 6LiBH4–Mg(AlH4)2 composite, which induces an overlap with the chemical reaction process of MgH2 and Al (the inset of Fig. 7). It was reported that LiBH4 and LiCl could form a solid solution of LiBH4(Cl) and stabilise the P63mc phase of LiBH4,30 which should be the most important reason for the lowered phase transformation temperature and elevated melting temperature of LiBH4. In addition, it is noted that the endothermic peak corresponding to dehydrogenation by reacting MgH2 with Al was moved to a lower temperature. However, there is an apparent shift to a higher temperature for the heat flow peaks of the third and fourth dehydrogenation steps. Moreover, this step can be divided into the following two overlapping parts: a wide endothermic peak at the temperature range of 370–500 °C and a severe fluctuation at 450–470 °C. According to eqn (4), the wide endothermic peak corresponds to the solid-state reaction between LiBH4, the Al(Mg) solid solution and Al3Mg2 to finally form LiAl, MgAlB4 and AlB2. The sharp endothermic peak in the severe fluctuation can be attributed to the melting of the Al–Mg phases because this process occurs at 450 °C.27 The following sharp exothermic peak is then believed to be the reaction between the liquid Al–Mg phase and LiBH4. A similar phenomenon was also observed in the DSC measurement of pristine LiAlH4.31 These results agree well with the TPD and volumetric release experiments. More interestingly, by normalising the DSC curves, it is found that the integrated intensities of the heat-flow peaks were roughly identical for the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites, suggesting that the presence of LiCl does not appreciably change the thermodynamic properties of the dehydrogenation reaction of the 6LiBH4–Mg(AlH4)2 composite.
 | ||
| Fig. 7 DSC curves of the 6LiBH4–Mg(AlH4)2 composite with and without LiCl. The inset shows an enlarged view of the overlapping peaks. | ||
The apparent activation energy (Ea) of the dehydrogenation reaction was determined by Kissinger's method using the following equation:32
 | (5) |
 | ||
| Fig. 8 Kissinger plots of the 6LiBH4–Mg(AlH4)2 composite (a) and the 6LiBH4–Mg(AlH4)2–2LiCl composite (b). | ||
The effects of the LiCl on the hydrogen storage reversibility of the 6LiBH4–Mg(AlH4)2 composite were further investigated by re-hydrogenating the dehydrogenated products under 100 atm of hydrogen pressure. Fig. 9a shows the hydrogenation curves of the dehydrogenated 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites as a function of temperature. It can be observed that the dehydrogenated samples started absorbing hydrogen at approximately 300 °C, and the hydrogen uptakes amounted to 8.3 and 6.0 wt% for the 6LiBH4–Mg(AlH4)2 and 6LiBH4–Mg(AlH4)2–2LiCl composites while dwelling at 450 °C for 24 hours, which correspond to 70% and 69% of the respective dehydrogenation amounts, representing an analogous hydrogenation behaviour. In addition, it should be noted that only partial hydrogen was recharged into the dehydrogenated samples potentially due to the insufficient hydrogen pressure applied in the present study. Fig. 9b presents the XRD patterns of the hydrogenated samples with and without LiCl. The characteristic reflections of LiBH4, MgH2, Al(Mg) and Al3Mg2 were detected in the two hydrogenated samples. In addition, the LiCl phase persisted in the XRD profile of the hydrogenated 6LiBH4–Mg(AlH4)2–2LiCl sample. As a result, we deduce that the following reactions took place during hydrogenation in the present study:
 | (6) |
 | (7) |
 | (8) |
 | ||
| Fig. 9 (a) Hydrogenation curves of dehydrogenated 6LiBH4–Mg(AlH4)2 with and without LiCl under 100 atm of hydrogen pressure; (b) XRD patterns of the hydrogenated samples. | ||
It was reported that Al3Mg2 and Al(Mg) were fully hydrogenated at 210 °C and 100 atm of hydrogen pressure.27 However, in the present study, the hydrogenation temperature was as high as 450 °C, which possibly induces the occurrence of a partial reversal of reaction (8). This is responsible for the insufficient hydrogenation as mentioned above. Moreover, it should be noted that approximately 75% of the reversible capacity was achieved for the 6LiBH4–Mg(AlH4)2 composite when it was only heated to 450 °C, while the reversible capacity was found to be only 56% for the 6LiBH4–Mg(AlH4)2–2LiCl composite under identical conditions by calculating the extent of reaction. These results indicate that the presence of LiCl in the 6LiBH4–Mg(AlH4)2 composite not only decreases the available hydrogen capacity but also slows down the hydrogenation rate, which is rather unfavourable for practical applications.
Conclusions
The effects of NaCl and LiCl on the hydrogen storage behaviours of a 6LiBH4–Mg(AlH4)2 composite were systematically investigated. It was determined that the presence of NaCl and LiCl significantly reduced the practical hydrogen storage capacity because no hydrogen is included in these two compounds. In addition, NaCl could react with LiBH4 to form LiCl and NaBH4 during ball milling, which changes the chemical composition of the 6LiBH4–Mg(AlH4)2–2NaCl composite. However, the presence of LiCl changed the apparent activation energy of the dehydrogenation reaction of the 6LiBH4–Mg(AlH4)2 composite even though it did not participate in the dehydrogenation reaction. This is the most important reason for the change in the dehydrogenation temperature of the 6LiBH4–Mg(AlH4)2–2LiCl composite. We therefore believe that the presence of the by-products NaCl and LiCl, which were produced during the synthesis of Mg(AlH4)2, is quite unfavourable for practical applications.Acknowledgements
We gratefully acknowledge the financial support received from the National Natural Science Foundation of China (51171170, 51222101, 51025102), the Research Fund for the Doctoral Program of Higher Education of China (20130101110080, 20130101130007), the Program for Innovative Research Team in the University of Ministry of Education of China (IRT13037), and the Fundamental Research Funds for the Central Universities (2014XZZX003-08).Notes and references
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- P. M. Grant, Nature, 2003, 424, 129–130 CrossRef CAS PubMed.
- S. Orimo, Y. Nakamori, J. R. Eliseo, A. Züttel and C. M. Jensen, Chem. Rev., 2007, 107, 4111–4132 CrossRef CAS PubMed.
- B. Bogdanović, U. Eberle, M. Felderhoff and F. Schüth, Scr. Mater., 2007, 56, 813–816 CrossRef PubMed.
- B. Bogdanović and M. Schwickardi, J. Alloys Compd., 1997, 253, 1–9 CrossRef.
- B. Bogdanović, R. A. Brand, A. Marjanović, M. Schwickardi and J. Tölle, J. Alloys Compd., 2000, 302, 36–58 CrossRef.
- Targets for Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles, US Department of Energy, accessed 10 October 2009, http://www.energy.gov/sites/prod/files/2014/03/f12/targets_onboard_hydro_storage.pdf.
- U. Eberle, M. Felderhoff and F. Schüth, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS PubMed.
- M. Fichtner and O. Fuhr, J. Alloys Compd., 2002, 345, 286–296 CrossRef CAS.
- Y. P. Pang, Y. F. Liu, X. Zhang, M. X. Gao and H. G. Pan, Int. J. Hydrogen Energy, 2013, 38, 13343–13351 CrossRef CAS PubMed.
- Y. P. Pang, Y. F. Liu, M. X. Gao, L. Z. Ouyang, J. W. Liu, H. Wang, M. Zhu and H. G. Pan, Nat. Commun., 2014, 5, 4519 Search PubMed.
- A. Züttel, S. Rentsch, P. Fischer, P. Wenger, P. Sudan, P. Mauron and C. Emmenegger, J. Alloys Compd., 2003, 356, 515–520 CrossRef.
- P. Mauron, F. Buchter, O. Friedrichs, A. Remhof, M. Bielmann, C. N. Zwicky and A. Züttel, J. Phys. Chem. B, 2008, 112, 906–910 CrossRef CAS PubMed.
- A. Züttel, A. Borgschulte and S. Orimo, Scr. Mater., 2007, 56, 823–828 CrossRef PubMed.
- J. J. Vajo, S. L. Skeith and F. Mertens, J. Phys. Chem. B, 2005, 109, 3719–3722 CrossRef CAS PubMed.
- J. Yang, A. Sudik and C. Wolverton, J. Phys. Chem. C, 2007, 111, 19134–19140 CAS.
- S. A. Jin, J. H. Shim, Y. W. Cho, K. W. Yi, O. Zabara and M. Fichtner, Scr. Mater., 2008, 58, 963–965 CrossRef CAS PubMed.
- Q. Shi, X. Yu, R. Feidenhans'l and T. Vegge, J. Phys. Chem. C, 2008, 112, 18244–18248 CAS.
- D. M. Liu, Q. Q. Liu, T. Z. Si, Q. A. Zhang, F. Fang, D. L. Sun, L. Z. Ouyang and M. Zhu, Chem. Commun., 2011, 47, 5741–5743 RSC.
- R. Mohtadi, P. Sivasubramanian, S. J. Hwang, A. Stonwe, J. Gray, T. Matsunaga and R. Zidan, Int. J. Hydrogen Energy, 2012, 37, 2388–2396 CrossRef CAS PubMed.
- J. F. Mao, X. B. Yu, Z. P. Guo, C. K. Poh, H. K. Liu, Z. Wu and J. Ni, J. Phys. Chem. C, 2009, 113, 10813–10818 CAS.
- Y. J. Yang, M. X. Gao, Y. F. Liu, J. H. Wang, J. Gu, H. G. Pan and Z. X. Guo, Int. J. Hydrogen Energy, 2012, 37, 10733–10742 CrossRef CAS PubMed.
- Y. F. Liu, Y. J. Yang, Y. F. Zhou, Y. Zhang, M. X. Gao and H. G. Pan, Int. J. Hydrogen Energy, 2012, 37, 17137–17145 CrossRef CAS PubMed.
- S. Singh and S. Eijt, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 224110 CrossRef.
- Y. F. Liu, F. H. Wang, Y. H. Cao, M. X. Gao, H. G. Pan and Q. D. Wang, Energy Environ. Sci., 2010, 3, 645–653 CAS.
- B. Li, Y. F. Liu, C. Li, M. X. Gao and H. G. Pan, J. Mater. Chem. A, 2014, 2, 3155–3162 CAS.
- Y. F. Liu, Y. P. Pang, X. Zhang, Y. F. Zhou, M. X. Gao and H. G. Pan, Int. J. Hydrogen Energy, 2012, 37, 18148–18154 CrossRef CAS PubMed.
- D. R. Lide, CRC Handbook of Chemistry and Physics, CRC Press/Taylor and Francis, Boca Raton, FL, 2011 Search PubMed.
- Y. P. Pang, Y. F. Liu, X. Zhang, M. X. Gao and H. G. Pan, Int. J. Hydrogen Energy, 2013, 38, 1460–1468 CrossRef CAS PubMed.
- L. M. Arnbjerg, D. B. Ravnsbæk, Y. Filinchuk, R. T. Vang, Y. Cerenius, F. Besenbacher, J.-E. Jørgensen, H. J. Jakobsen and T. R. Jensen, Chem. Mater., 2009, 21, 5772–5782 CrossRef CAS.
- F. Zhai, P. Li, A. Sun, S. Wu, Q. Wan, W. Zhang, Y. Li, L. Cui and X. Qu, J. Phys. Chem. C, 2012, 116, 11939–11945 CAS.
- H. E. Kissinger, Anal. Chem., 1957, 29, 1702–1706 CrossRef CAS.
- J. Wang, Y. Du, L. Sun and X. Li, Int. J. Hydrogen Energy, 2014, 39, 877–883 CrossRef CAS PubMed.
- B. Li, Y. F. Liu, Y. Zhang, M. X. Gao and H. G. Pan, J. Phys. Chem. C, 2012, 116, 13551–13558 CAS.
| This journal is © The Royal Society of Chemistry 2015 |