Fluorescence resonance energy transfer between ZnO/MgO/carboxymethyl-β-cyclodextrin and Nile Red in HeLa cells – biosensing applications†
B. Sikora‡
*a,
K. Fronc‡a,
I. Kamińska‡a,
K. Koper‡bc,
M. Chwastyk‡a,
P. Stępień‡bc,
W. Paszkowicz‡a,
T. Wojciechowski‡a,
K. Sobczak‡a and
D. Elbaum‡a
aInstitute of Physics, Polish Academy of Sciences, al Lotników 32/46, 02-668 Warsaw, Poland
bInstitute of Genetics and Biotechnology, Faculty of Biology, University of Warsaw, ul. Pawińskiego 5a, 02-106 Warsaw, Poland
cInstitute of Biochemistry and Biophysics PAN, ul. Pawińskiego 5a, 02-106 Warsaw, Poland. E-mail: sikorab@ifpan.edu.pl
First published on 27th November 2014
Abstract
Early diagnosis remains an important problem of cancer treatment strategies. There is therefore a great need for cancer detection tests that are fast, inexpensive and do not require sophisticated laboratory equipment. For this purpose, we synthesized the ZnO/MgO core/shell nanoparticles which are relatively nontoxic, inexpensive and simple to create. We constructed biosensors based on ZnO/MgO nanoparticles which employ the fluorescence resonance energy transfer from ZnO/MgO nanoparticles (donor) to Nile Red (acceptor). Characteristic features of Nile Red luminescence are its solvatochromic and thermochromic properties. In the physiologically relevant temperature range (20–45 °C), the shift of Nile Red luminescence in the ZnO/MgO/CMCD/Nile Red complex is linear with temperature. In our experiment, thermochromic shift was 5.7 ± 1.5 cm−1 K−1. Nile Red thermochromism observed in the complex will allow us to construct a sensor capable of detecting exothermic changes and local environmental differences between normal and pathological cells. Subsequently, we studied ZnO/MgO/CMCD/Nile Red complex in vivo in biological samples. We present here, for the first time that the donor–acceptor energy transfer is affected by the intracellular or extracellular locations of the nanoparticles.
Introduction
The major aim of nanomedicine is to design diagnostic, therapeutic and pharmaceutical agents. Nanodrugs can exhibit different responses to light, magnetic fields and electronic irradiation. These nanomaterials overcome several limitations of unstable organic probes, which make them useful for molecular imaging.1Imaging of heat production in single cells has been a focus of our interest since 1998.2 In the quest to develop a new generation of nanostructures-based sensors we focused on Fluorescence Resonance Energy Transfer (FRET) bioprobes.
Fluorescent Resonance Energy Transfer (FRET) is a mechanism of energy transfer between two chromophores otherwise than by radiation. Donor in the excited state can transfer excitation energy to acceptor being situated at a distance of less than 10 nm. The main effect of this transfer is the decreasing of the donor light emission and increasing of the acceptor light emission.
FRET is a widely used mechanism employed in the bioassays, predominantly due to its high sensitivity to the distance separating the donor from the acceptor (proportional to r6). This property has been extensively applied to study antigen–antibody interaction and protein real-time folding in vivo.3,4
Materials used in the FRET techniques can be divided into several different classes: organic materials (the “traditional” organic dyes (fluorophores), quencher molecules and polymers); inorganic materials (metal chelators, metal and semiconductor nanocrystals); biological fluorophores (fluorescent proteins and amino acids, and biological compounds that exhibit bioluminescence under enzymatic catalysis). These materials can function either as donors, acceptors, or both, depending on the experimental conditions.5
There are numerous reports of FRET effect between nanoparticles and organic dyes on their surface. Relatively nontoxicity of ZnO makes it a good replacement to II–VI semiconductors based on cadmium ions.6 Surface of colloidal ZnO nanoparticles can be successfully modified by various kind of dye molecules comprising catechol groups acting as the FRET acceptor.7 FRET has been also observed in the complex of ZnO nanoparticles (acting as donor) with oxazine 1 (acceptors) ex vivo.8 Energy transfer from the defect state of ZnO nanoparticles to Alexa Fluor 594 cadaverine has been studied using photoluminescence and time resolved measurements.9 However, there are no reports of ZnO FRET complexes being internalized in living cells and aimed to monitor changes in organisms in vivo. In the present study we focused on the formation of the complex molecules of ZnO/MgO core/shell nanoparticles coated with carboxymethyl-β-cyclodextrin (CMCD) (as a donor) with the Nile Red (as an acceptor). The synthesized complex was introduced into HeLa cells, where its luminescence was examined, to the best of our knowledge, for the first time.
Results and discussion
Fluorescence resonance energy transfer between ZnO/MgO core/shell nanoparticles and Nile Red
Due to the great need for early, fast and inexpensive cancer detection test we design sensors based on fluorescence resonance energy transfer between ZnO/MgO/CMCD nanoparticles (donor) and Nile Red (acceptor).We investigated the FRET effect between ZnO/MgO core/shell nanoparticles coated with carboxymethyl-β-cyclodextrin and Nile Red, an organic dye built into the cavities of β-cyclodextrin using a procedure similar to Rakshit S. et al.10 Preparation of ZnO/MgO and ZnO/MgO/CMCD nanoparticles and their properties are described in the ESI.† We examined the stability of the nanoparticles in physiologically relevant phosphate buffers for various incubation time and concentration of phosphate ions (Fig. S9 in ESI†). This is very important effect for use the nanoparticles in biology system. Rakshit S. et al.10 have claimed stability of the nanoparticles. Our results revealed that in solutions with low concentrations of phosphate ions (2 mM and 5 mM), the ZnO/MgO nanoparticles degraded until reached the equilibrium between the nanoparticles and the phosphate ions. At higher concentrations (10 mM, 25 mM), the nanoparticles decomposed almost completely after two hours. Hence we concluded that the ZnO/MgO nanoparticles could be used in buffers of 2 mM and 5 mM phosphate concentration (physiological concentration) (Fig. S9†).
The Nile Red was selected because its photoluminescence is temperature dependent, therefore, it is sensitive to the external environment. In addition, the absorption spectrum of the Nile Red overlaps with the luminescence spectrum of the ZnO/MgO crystals which derives from the surface defects (Fig. 1). The Nile Red also forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with β-cyclodextrin.10
1 complex with β-cyclodextrin.10
The FRET process is observed when energy from the donor excited state is transferred to the acceptor molecule by a dipole–dipole interaction: this process is more efficient when there is a substantial overlap between the emission spectrum of the donor with the absorption spectrum of the acceptor.11 Fig. 1 confirms that there is a sufficient emission coverage between the luminescent band of ZnO/MgO nanoparticles and the absorption band of Nile Red dyes.
ZnO/MgO nanoparticles covered with CMCD were dissolved in water. Then the nanoparticles were titrated with various concentrations of Nile Red dissolved in acetone by adding the dye dissolved in the same volume. The variations of the ZnO/MgO/CMCD luminescence were monitored for several Nile Red concentrations (Fig. 2A). Excitation wavelength at 356 nm, corresponding to ZnO/MgO nanocrystals absorption band, was selected.
With the increasing acceptor to donor ratio, the visible emission (510–580 nm) of ZnO/MgO nanoparticles is effectively quenched while Nile Red emission measured at 650 nm is increased. The spectra clearly indicate the presence of the FRET process between ZnO/MgO nanoparticles and Nile Red. The presence of the isosbestic point in Fig. 2A is consistent with relatively simple two component system in thermodynamic equilibrium. This indicates that the filling of the CMCD cavities anchored to the nanoparticles by Nile Red molecules is an independent process. At higher Nile Red concentrations, when the Nile Red to CMCD cavities ratio is very large, the ZnO/MgO/CMCD/Nile Red complexes precipitate from the aqueous solution. This may be due to hydrophilic surface of the nanocrystals being covered by Nile Red. This experiment confirmed a previous observations of the FRET process between the ZnO/MgO/CMCD and Nile Red performed by Rakshit S. et al.10
Further evidence supporting that the 650 nm (Nile Red) emission, in the sample of ZnO/MgO/CMCD/Nile Red, comes from the FRET is summarized in Fig. 2B. In the absence of CMCD on the surface of the nanoparticles, no luminescence increase originating from the acceptor (Nile Red) was observed, only the donor quenching is apparent.
Our observations show that changing the excitation wavelength from 355 nm to the multiphoton excitation (705 nm) does not change the luminescence wavelength originating from the ZnO defects (about 545 nm) (the results are in Fig. 11 in ESI†). It is of practical significance because a departure from the UV excitation may reduce the tissue damage and improve the quality of live cells imaging.
We made additional measurements of luminescence decay of the donor, in the presence of various acceptor concentrations which are shown in the ESI.† The luminescence decay time of the visible light from ZnO/MgO/CMCD (donor) and the donor in the presence of Nile Red resulting from 705 nm multiphoton excitation was measured, to complement the stationary fluorescence measurements to prove the FRET processes between nanoparticles and dye.
Effect of temperature on the fluorescence resonance energy transfer between ZnO/MgO nanoparticles and Nile Red
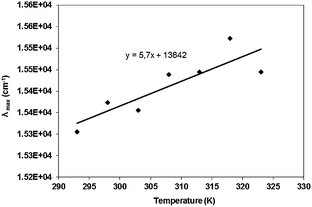
To investigate sensing properties of the complex, we performed the FRET determinations in various temperatures. The effect of temperature on the Nile Red wavelength maximum intensity for the ZnO/MgO/CMCD/Nile Red complex was measured from 293 to 323 K (Fig. 3). In this temperature range, changes of the wavelength of the maximum luminescence intensity of Nile Red in the ZnO/MgO/CMCD/Nile Red complex are linear with a thermochromic shift of 5.7 ± 1.5 cm−1 K−1. The thermochromic shift values for Nile Red in nonpolar solvents vary from 0.9 to 4 cm−1 K−1.12 The thermochromic shift value obtained in aqueous solution in the FRET system is larger than for non-polar solvents and larger than the value in an aqueous solution obtained by other investigators (3 cm−1 K−1).10 Nile Red thermochromism observed in the ZnO/MgO/CMCD/Nile Red complex can be used to determine temperature changes in the aqueous solutions.In order to verify the effect of temperature on the luminescence intensity, the maximum intensity wavelength and the reversibility of these changes were determined. The control measurements for the solutions containing ZnO/MgO/CMCD, ZnO/MgO and Nile Red were also performed. From the results in Fig. 4A, the differences between the intensity of the luminescence at 20 °C and 45 °C were calculated. The results are summarized in Fig. 4B.
Changes in the luminescence intensity of Nile Red (acceptor) in the FRET complex are approximately twice larger than the changes in the luminescence intensity of ZnO/MgO (donor) in the FRET. The effect of temperature on the intensity of the ZnO/MgO luminescence in water (Fig. 4A) possibly results from an increased probability of a non-radial processes at higher temperature which decrease the luminescence intensity. The lowering of green luminescence intensity with temperature raising was investigated in detail for ZnO microrods13 and thin films.14 Cited authors observed a similar magnitude of changes to our observations. The lowering in intensity of green luminescence can be explain by the temperature dependent changes of lattice vibrations of ZnO which are strongly coupled to electronic transitions at defects.15
The effects of temperature on the position of the maximum intensity and the reversibility of the process were compared. The luminescence maximum for Nile Red in the FRET complex (Fig. 5A) is sensitive to temperature and this is a reversible process, unlike the position of the maximum for the donor intensity in water (Fig. 5B). Therefore, only the FRET complex is a potential nanosensor of temperature changes. Aqueous solutions of ZnO/MgO/CMCD and ZnO/MgO nanoparticles do not exhibit these properties.
The effects of temperature on the FRET efficiency between the donor (ZnO/MgO/CMCD) and acceptor (Nile Red) in the ZnO/MgO/CMCD/Nile Red complex were studied (Fig. 6). Determination of the FRET efficiency from the measurements of stationary luminescence and decay times is described in the ESI.†
 | ||
| Fig. 6 Changes in FRET efficiency in the ZnO/MgO/CMCD/Nile Red system (Nile Red background luminescence was subtracted). The efficiency was determined from the eqn (3) for each temperature (ESI†). | ||
It was observed that the FRET efficiency is reduced by approximately 30% with increasing temperature and the process is a reversible. The FRET efficiency is reduced with consecutive changes of temperature.
In this part of our work, the effect of temperature on the intensity and the wavelength of the maximum luminescence of Nile Red and ZnO/MgO/CMCD in ZnO/MgO/CMCD/Nile Red complex studies compared with ZnO/MgO/CMCD and ZnO/MgO in water studies confirmed the sensitivity of the FRET complex towards temperature. Reversible changes of FRET efficiency as a function of the periodic changes of temperature were observed.
We observed temperature dependent changes in the maximum luminescence wavelength intensity of Nile Red in the ZnO/MgO/CMCD/Nile Red complex with a thermochromic shift of 5.7 ± 1.5 cm−1 K−1. Nile Red thermochromism observed in the ZnO/MgO/CMCD/Nile Red complex can be used as a potential nanosensor in aqueous solutions after appropriate calibration.
Fluorescence resonance energy transfer between ZnO/MgO/CMCD and Nile Red inside HeLa cells
In order to investigate the biosensory properties of ZnO/MgO/CMCD/Nile Red complexes in living organisms, the FRET complexes were transfected into the HeLa cells (Fig. 7). Procedure of introducing and results of control experiments are described in ESI.†The Nile Red luminescence was observed (Fig. 7B) when excited with a 355 nm laser at 0.3 mW of power. The luminescence intensity increased for the intracellularly localized FRET complexes compared to the donor (ZnO/MgO/CMCD) luminescence or the acceptor (Nile Red) luminescence under the same experimental conditions (ESI†). Neither photodamage nor biodegradation of the FRET complexes inside HeLa cells were observed.
In addition to causing significant cellular damage, UV light is responsible for a high cellular autofluorescence. Therefore, the experiments were repeated using a femtosecond multiphoton excitation at 705 nm. The results are shown in Fig. 8. Our previous results were confirmed. The appropriate control experiments are shown in the ESI.† It can be concluded that we were able to introduce FRET complexes into the HeLa cells without damaging them. We were also able to observe the FRET complexes with multiphoton excitation (λ = 705 nm) of the donor.
ZnO/MgO/CMCD/Nile Red complex was excited by a two photon excitation (705 nm). The excited ZnO/MgO transfers energy to Nile Red, which emits at 600–690 nm. The FRET mechanism is identical as in the case of the UV excitation.
The effect of the cellular localization (extracellular vs. cytosol) of the nanoparticles on the FRET emission spectra was examined. The tests were performed for two different concentrations of Nile Red in the FRET complex and for two different excitation wavelengths: 355 nm and 705 nm (Fig. 9).
The luminescence originating from the donor (ZnO/MgO/CMCD nanoparticles) is visible only in the case of multi-photon excitation. In the case of UV excitation it coincides with the autofluorescence of the cells. Although the presence of ZnO nanoparticles in the cellular cytosol was observed previously,16 no luminescence spectrum has been published previously by others. We reported this luminescence inside HeLa cells recently.17
Spectral displacement of the Nile Red luminescence was observed in the ZnO/MgO/CMCD/Nile Red complex and Nile Red localized either outside or inside the cells. This effect is more evident for the 355 nm excitation. This may be due to less efficient multiphoton ZnO excitation compared to the UV excitation and significant contribution of Nile Red alone to the multiphoton autofluorescence.
Fig. 9 depict changes of the Nile Red luminescence depending on the complex location (inside/outside the cell). The results are summarized in Table S3† and in Fig. 10. The results were recorded for two acceptor concentrations: 3.2 μM and 16 μM and constant concentration of the donor: 20 mg ml−1. Values are reported as a mean ± SEM (standard error of the mean). The standard error was calculated by the procedure described in the ESI (Fig. S23).† The difference between the Nile Red luminescence (from the FRET probes) inside cells and outside cells is statistically significant (correlation coefficients > 0.9). For the Nile Red probes (without donor) the differences are not statistically significant due to the unmatched excitation wavelength for the Nile Red maximum absorption.
Maximum intensity of luminescence of Nile Red in the FRET complex inside the cells is shifted towards a higher wavelength compared to the complex present outside the cells for both 355 nm and 705 nm excitation. The statistically significant (correlation coefficients > 0.9) shift noted results from different environments outside vs. inside of the cells (pH, ionic strength, dielectric constant). The differences obtained for 705 nm excitation were not statistically significant, as a result of unmatched excitation wavelength, for the Nile Red maximum absorption. No acceptor molecules were found outside the cells for 3.2 mM Nile Red concentration.
We obtained the evidence of presence of FRET complexes inside cells from the ratio of the Nile Red luminescence intensity to the intensity of luminescence at 450 nm wavelength (autofluorescence of cells) measurements – ESI.† Nile Red luminescence to autofluorescence ratios are much higher in the case of FRET complexes inside and outside of the cells compared with the acceptor when the excitation wavelength was 355 nm. In the case of 705 nm excitation, there were no changes in the ratio of the Nile Red luminescence intensity to the autofluorescence. This is probably due to lower efficiency of multi-photon excitation compare to the high energy single photon donor excitation.
Finally, we performed the acceptor quenching measurements at 705 nm excitation to test the nature of the energy transfer. The results confirmed the observed donor–acceptor mode of the energy transfer (Fig. 11).
Note an increase in luminescence donor (green curve) resulted from photobleaching of the acceptor (a decrease of the luminescence intensity – red curve). At time 0 s, energy transfer occurs from ZnO/MgO/CMCD nanoparticles to Nile Red (excitation wavelength: 705 nm). Therefore, a high luminescence intensity of Nile Red and a low intensity of ZnO nanoparticles were observed. Followed by a high power 561 nm irradiation, corresponding to the maximum absorption of the Nile Red, the acceptor within the cells underwent photobleaching, as manifested by reduction of the Nile Red luminescence intensity, shown in Fig. 11 at about 15 s. As a result, ZnO nanoparticles were unable to transfer energy, thus an increase in the visible luminescence of the donor was observed. We noted a slow increase of the acceptor luminescence intensity and the decrease of the donor luminescence intensity resulted from a diffusion of unbleached nanoparticles present in the other cellular locations. Comparison with the results of the control samples are shown in Table inside Fig. 11.
Summary
For early, fast and inexpensive cancer detection test we designed sensors based on fluorescence resonance energy transfer between ZnO/MgO/CMCD nanoparticles (donor) and Nile Red (acceptor). We observed temperature dependent changes in the maximum luminescence wavelength intensity of Nile Red in the ZnO/MgO/CMCD/Nile Red complex with a thermochromic shift of 5.7 ± 1.5 cm−1 K−1. Nile Red thermochromism observed in the ZnO/MgO/CMCD/Nile Red complex can be used as a potential nanosensor in aqueous solutions after appropriate calibration.The ZnO/MgO/CMCD/Nile Red complexes were introduced into HeLa cells. Maximum luminescence shift, under 355 nm excitation, of Nile Red was observed in the FRET complexes sensing the localization of complexes, either inside or outside of the cells. The change of the observed emission was caused by differences in the probes' local environment (different pH, dielectric constant, ion content). The FRET complexes were sensitive to temperature and to the local environmental changes.
In order to minimize the strong autofluorescence and light scattering present during 355 nm excitation, a 705 nm femtosecond laser was applied to excite the FRET complexes. Acceptor photobleaching measurements confirmed the nature of the energy transfer. Finally, the environmentally sensitive shift of the Nile Red luminescence was confirmed following the 705 nm multiphoton excitation.
The properties of the synthesized nanosensing probe suggest that it may be useful for the further development of biofunctional specific biosensors.
Methods
Fluorescence resonance energy transfer measurements in aqueous solution
When we measured FRET between ZnO/MgO/CMCD nanoparticles and Nile Red, the concentration of the donor (ZnO/MgO/CMCD nanoparticles) was constant, while the acceptor concentration (Nile Red) was varied similarly to the previous publication.10 The methods of the ZnO/MgO nanoparticles synthesis and coating with CMCD as well as their properties are described in ESI.† Donor solution was prepared by dissolving 2 g of ZnO/MgO nanoparticles coated by CMCD in 100 ml of distilled water. Nile Red solutions were obtained at concentrations ranging from 0.005 to 1.5 mM in acetone. For the FRET measurements, 0.1 ml of Nile Red solution at a given concentration was added to 3 ml of the donor ZnO/MgO/CMCD solution in quartz cuvette and a luminescence spectrum was monitored for every concentration of the organic dye. The excitation wavelength was 356 nm.The control experiment consisted of repeating exactly the same procedure but applied to uncoated ZnO/MgO nanoparticles. The initial solution was prepared by dissolving 6 mg of ZnO/MgO nanoparticles in 100 ml of distilled water. Nile Red solutions were obtained at concentrations ranging from 0.005 to 1.5 mM in acetone. For the FRET measurements, 0.1 ml of Nile Red solution of a given concentration was added to 3 ml of the ZnO/MgO nanoparticles solution in quartz cuvette. Luminescence spectra, at 356 nm excitation, were observed for various concentrations of the organic dye.
The luminescence spectra were recorded using a Fluorolog III Spectrophotometer. The instrument was equipped with a thermostatic cuvette holder. The appropriate solvents were used as reference samples.
Measurements of temperature effect on the fluorescence resonance energy transfer in aqueous solution
2 g of ZnO/MgO/CMCD nanoparticles (donor) were dissolved in 100 ml of distilled water. 3 ml of the solution was added to the cuvette and then 0.1 ml of Nile Red solution at a concentration of 0.6 mM was added. The solution was brought to the desired temperature and the FRET luminescence spectrum was measured. Measurements were carried out separately for each temperature point (excitation wavelength 356 nm).For the measurements of the FRET reversibility in function of temperature, 0.25 g of ZnO/MgO/CMCD was dissolved in 12.5 ml of distilled water. Later, 0.1 ml of Nile Red solution at a concentration of 0.6 mM was added to 3 ml of a nanoparticles solution in a quartz cuvette. The FRET luminescence spectra were measured in varying temperature from 20 °C to 45 °C. The cycles were repeated several times. The same experiment was repeated with ZnO/MgO nanoparticles, ZnO/MgO/CMCD nanoparticles and Nile Red.
The luminescence spectra were monitored and recorded using a Fluorolog III Spectrophotometer, as above.
Introducing of ZnO/MgO/CMCD/Nile Red into the HeLa cells
A well-studied cervical cancer derived HeLa cells line was used in the study. Human cells were routinely cultured with DMEM (Dulbecco's Modified Eagle Medium) containing 10% fetal calf serum (FCS), 100 units per ml penicillin and 100 μg ml−1 streptomycin. Cell cultures of cells were kept at 37 °C in a humidified atmosphere containing 5% CO2. Liposomal vesicle transfection agent (Lipofectamine 2000) was applied to introduce nanoparticles into the cells. Cells were cultured in 6 well plate dishes (6 × 10 cm2) at a density of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well.
000 cells per well.
The transfection protocol was developed based on information provided by the manufacturer (ESI†).
Cells were imaged using a Zeiss 710 NLO confocal microscope equipped with an infrared femtosecond laser (Coherent, Chameleon), and a Carl Zeiss LSM 780 confocal microscope equipped with a an continuously ultraviolet laser (355 nm).
Throughout the imaging experiments of the ZnO/MgO/CMCD/Nile Red nanoparticles inside HeLa cells, two channels were observed: the first at the 488 nm wavelength excitation (total laser power of 30 mW), and detection ranging from 520 to 560 nm; and the second at the 355 nm wavelength excitation (total laser power of 20 mW) and detection in range from 560 to 700 nm.
The luminescence spectra of ZnO/MgO/CMCD/Nile Red complexes, depending on their location in or around of HeLa cells, were observed using confocal microscope with UV excitation and a confocal microscope with NIR excitation using the lambda mode scan. The procedure includes creation of a composite image from the confocal images collected from a number of simultaneously emitted wavelength, and then generation, for the selected point on the image, of the luminescent spectra by applying the software provided by the manufacturer.
The FRET effect inside the HeLa cells was observed at the excitation wavelength of 705 nm, by the photobleaching of the acceptor. The experiment involves laser irradiation of a selected location over several minutes. The irradiation wavelength corresponds to the absorption maximum of the acceptor (excitation at 561 nm with a 10% of laser power for Nile Red). Following the irradiation, the emission intensity was recorded in the donor channel (ZnO/MgO/CMCD nanoparticles) and acceptor channel (Nile Red). The acceptor channel was recorded at an excitation wavelength of 561 nm and in the range of emission: 629–650 nm (2 mW laser power). The donor channel emission was recorded at the excitation wavelength of 705 nm (30 mW laser power) in the range of 530–560 nm.
Conflict of interest
The authors declare no competing financial interest.Acknowledgements
We acknowledge G. Wilczyński (Nencki Institute of Experimental Biology, Polish Academy of Science) for enabling us access to the confocal microscope. The research was partially supported by the European Union within European Regional Development Fund, through the grant Innovative Economy (POIG.01.01.02-00-008/08), the grants of Polish National Science Center NN 302 663 940, NN UMO-2013/08/A/ST3/00297 and DEC-2012/07/B/ST5/02080. This work has been done in the NanoFun laboratories co-financed by the European Regional Development Fund within the Innovation Economy Operational Programme, the Project no. POIG.02.02.00-00-025/09/.References
- Y. Wang and L. Chen, Nanomedicine, 2011, 7, 385 CrossRef CAS PubMed
.
- O. Zohar, M. Ikeda, H. Shinagawa, H. Inoue, H. Nakamura, D. Elbaum, D. L. Alkon and T. Yoshioka, Biophys. J., 1998, 74, 82 CrossRef CAS
.
- U. Schobel, H. J. Egelhaaf, A. Brecht, D. Oelkrug and G. Gauglitz, Bioconjugate Chem., 1999, 10, 1107 CrossRef CAS PubMed
.
- M. P. Lillo, B. K. Szpikowska, M. T. Mas, J. D. Sutin and J. M. Beechem, Biochemistry, 1997, 36, 11273 CrossRef CAS PubMed
.
- K. E. Sapsford, L. Berti and I. L. Medintz, Angew. Chem., Int. Ed., 2006, 45, 4562 CrossRef CAS PubMed
.
- S. Gulia and R. Kakkar, Adv. Mater. Lett., 2013, 4(12), 876 CAS
.
- R. Marczak, F. Werner, J.-F. Gnichwitz, A. Hirsch, D. M. Guldi and W. Peukert, J. Phys. Chem. C, 2009, 113, 4669 CAS
.
- A. Makhal, S. Sarkar, T. Bora, S. Baruah, J. Dutta, A. K. Raychaudhuri and S. K. Pal, Nanotechnology, 2010, 21, 265703 CrossRef PubMed
.
- G. A. Beane, A. J. Morfa, A. M. Funston and P. Mulvaney, J. Phys. Chem. C, 2012, 116, 3305 CAS
.
- S. Rakshit and S. Vasudevan, ACS Nano, 2008, 2(7), 1473 CrossRef CAS PubMed
.
- J.-F. Baussard, Synthesis of New Ionic Functional Polymers by Free Radical Polymerization via the RAFT Process, Dissertation, Catholic University of Louvain, 2004, ch. 2
.
- M. G. Christina, W. B. Williams and J. B. Foresman, J. Fluoresc., 1998, 8, 395 CrossRef
.
- F. H. Su, Y. F. Liu, W. Chen, W. J. Wang, K. Ding, G. H. Li, A. G. Joly and D. E. McCready, J. Appl. Phys., 2006, 100, 013107 CrossRef PubMed
.
- X. Meng, Z. Shi, X. Chen, X. Zeng and Z. Fu, J. Appl. Phys., 2010, 107, 023501 CrossRef PubMed
.
- S. L. Shi, G. Q. Li, S. J. Xu, Y. Zhao and G. H. Chen, J. Phys. Chem. B, 2006, 110, 10475 CrossRef CAS PubMed
.
- H. M. Xiong, Y. Xu, Q. G. Ren and Y. Y. Xia, J. Am. Chem. Soc., 2008, 130, 7522 CrossRef CAS PubMed
.
- B. Sikora, K. Fronc, I. Kamińska, K. Koper, P. Stępień and D. Elbaum, J. Phys.: Condens. Matter, 2013, 25, 194104 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and properties of ZnO/MgO nanoparticles. Structural properties of ZnO/MgO nanoparticles. Absorption and emission properties of ZnO/MgO nanoparticles and their aggregation and stability. Coating of ZnO/MgO nanoparticles by carboxymethyl-β-cyclodextrin (CMCD). FRET between ZnO/MgO nanoparticles and Nile Red. FRET between ZnO/MgO nanoparticles and Nile Red, measurements of the luminescence decay time. FRET efficiency – stationary measurements. FRET efficiency – decay time measured. Introducing of ZnO/MgO/CMCD/Nile Red into the HeLa cells. Negative controls: ZnO/MgO/CMCD (no acceptor) and Nile Red (no donor) inside the cells. Fluorescence resonance energy transfer inside HeLa cells – 705 excitation wavelength. Spectral displacement of the Nile Red luminescence localized either outside or inside the cells. The statistical analysis of the experimental results. Evidence of fluorescence resonance energy transfer inside HeLa cells. See DOI: 10.1039/c4ra13011a |
| ‡ The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. B. Sikora carried out the synthesis of ZnO/MgO/CMCD nanoparticles, FRET measurements, confocal microscope measurements and designed and analysis experiments with assistance from K. Fronc, I. Kamińska and D. Elbaum. B. Sikora with D. Elbaum wrote this paper. K. Koper and P. Stępień carried out the experiments with HeLa cells. M. Chwastyk contributed to the statistical analysis. W. Paszkowicz carried out the XRD measurements. T. Wojciechowski carried out the SEM and EDX measurements. K. Sobczak carried out TEM measurements. |
| This journal is © The Royal Society of Chemistry 2015 |