The high capacity and excellent rate capability of Ti-doped Li2MnSiO4 as a cathode material for Li-ion batteries
Min Wang,
Meng Yang*,
Liqun Ma* and
Xiaodong Shen
College of Materials Science and Engineering, Nanjing Tech University, Nanjing 210009, China. E-mail: maliqun@njtech.edu.cn; yangmengyy@njtech.edu.cn; Fax: +86 25 83240205; Tel: +86 25 83587240
First published on 13th November 2014
Abstract
Ti-doped Li2Mn1−xTixSiO4 as cathode materials for Li-ion batteries was successfully synthesized by a facile sol–gel method. The addition of Ti to the precursors changed the particle sizes and specific surface areas of Li2MnSiO4. The galvanostatic charge–discharge measurements showed that Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1, 0.2) electrodes delivered high charge capacity of 298, 286, 248 mA h g−1 and discharge capacity of 203, 211, 171 mA h g−1 in the first cycle, much higher than that of undoped Li2MnSiO4 (52 mA h g−1 for charging and 26 mA h g−1 for discharging). Moreover, the Ti-doped samples exhibited good cycling stability and superior rate capability, as compared to that of pristine sample. Even at a high rate (2 C), the Ti-doped Li2Mn1−xTixSiO4 still maintained high discharge capacities. The remarkable enhancement of battery performance in terms of capacity and rate capability for doped Li2Mn1−xTixSiO4 was primarily attributed to the decrease of charge transfer resistance and the improvement of the Li+ diffusion coefficient.
Introduction
Recently, Li2MnSiO4 as a cathode material for Li-ion batteries has attracted remarkable attention since it was first reported by R. Dominko et al.1 in 2007. This material is expected to be the candidate cathode material for next-generation rechargeable Li-ion batteries due to its high energy density, power density, high thermal stability, abundant resources, and environmentally friendly etc.2–5 Most importantly, the transition metal manganese ion can easily achieve the transformation of Mn2+/Mn3+ and Mn3+/Mn4+ to carry out extraction of two Li+, resulting high theoretical capacity of 330 mA h g−1.1,3 However, low intrinsic electronic conductivity and slow Li+ mobility which cause excessive polarization during charge–discharge process, lead to low capacity and inferior electrochemical performance of this material.6,7 Although carbon coating can improve electronic conductivity and reduce particle size which can shorten the pathway of Li+ migration, Li2MnSiO4 still has large irreversible capacity loss and unsatisfactory cycling stability so that not sufficient for practical battery applications.8,9 Thus, other feasible methods to enhance its battery performance should be found in urgent. Recently, few studies on ion doping of Li2MnSiO4 material have been reported. Zhang et al.10 prepared Cr-doped Li2MnSiO4 with a citric acid-assisted sol–gel method. The obtained Li2Mn0.94Cr0.06SiO4 showed the best cycling performance and could deliver the discharge capacity of 306 mA h g−1 in the fifth cycle. Choi et al.11 synthesized various ions (Al3+, Ga3+, Mg2+) doped Li2MnSiO4 via the conventional sol–gel method. The sample doped with Ga3+ showed high charge–discharge capacity and initial efficiency with well-dispersed nanoparticle formation. Hence, doping with metal ions might be a new strategy to improve the electrochemical performance of Li2MnSiO4 material.Herein, we chose Ti element as doping cation for following reasons: (1) according to defect chemistry, aliovalent ion doping can induce defects (vacancies or interstitials) in the lattice by charge compensation so as to enhance the materials' conductivity.11–14 (2) this element is inactive in the potential range of 1.5–4.8 V, so the lattice shrinking of Li2MnSiO4 during charging is minimized by the support of unchangeable radius of Ti4+.10,15
In this paper, we successfully synthesized pristine Li2MnSiO4 and Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) materials by a facile sol–gel method. The effects of Ti-doped Li2MnSiO4 materials in terms of structure, morphology and electrochemical properties have been investigated by X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), inductively coupled plasma-atomic emission spectrometer (ICP-AES), Brunauer–Emmett–Teller (BET), galvanostatic charge–discharge and electrochemical impedance spectra (EIS) in details.
Experimental
Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) powders were prepared by a facile sol–gel method. Analytical grade lithium acetate dihydrate, manganese acetate tetrahydrate, ethyl silicate and tetrabuty titanate were employed as raw materials. First, stoichimetric amounts of lithium acetate dihydrate and manganese acetate tetrahydrate were dissolved in ethanol stirring for half an hour. Then, ethyl silicate in ethanol solution and tetrabuty titanate in ethanol solution was slowly added to above mixed solutions in order. The solutions were maintained at 80 °C for 24 hours under vigorously stirring and then dried in oven at 80 °C overnight. Subsequently, the obtained xerogel were fine ground by ball-milling and then calcined at 650 °C for 10 hours with a flow of argon. For comparison, undoped Li2MnSiO4 powder was prepared by identical process without adding tetrabuty titanate as the doping agent.X-ray powder diffraction (XRD) was collected by diffractmeter (SmartLab, Japan) with Cu Kα (λ = 1.5406 Å) radiation. The scanning angle 2θ was ranging from 10 to 80° at a step of 0.02°. The morphology and particle size distribution were observed by field emission scanning electron microscopy (FE-SEM, HITACHIS-4800, Japan). N2 adsorption–desorption isotherms were measured by F-Sorb 2400 analyzer. The specific surface areas were calculated by using Brunauer–Emmett–Teller (BET) method. Chemical composition of all the samples were determined by inductively coupled plasma-atomic emission spectrometer (ICP-AES).
The electrochemical characterization of the materials was measured using a typical CR2032 coin cell. The working electrodes were prepared by mixing 80 wt% active materials, 10 wt% carbon black with 10 wt% polyvinylidene fluoride (PVDF) via using 1-methyl-2-pyrrolidone as a solvent. Then, the resulting slurries were uniformly pasted on Al foils and dried in vacuum at 100 °C overnight. Circular electrodes were cut-off from the sheets with a diameter of 12 mm and the active materials (Li2Mn1−xTixSiO4) weights were maintained in the range of 1.9–2.1 mg cm−2. The coin cells were assembled in a glove box filled with high pure argon (less than 1 ppm of water and oxygen) using Li metal as the anodes, celgard2400 film as the separator and 1 M LiPF6 dissolved in EC and DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) as the electrolyte. Meanwhile, galvanostatic charge–discharge measurements on (Land, China) were performed in the voltage range of 1.5–4.8 V (vs. Li+/Li) under the current density of 10 mA g−1 at 30 °C. Rate capability of electrodes was examined at various current densities: 0.03 C, 0.1 C, 0.5 C, 1 C, 2 C (1 C = 330 mA g−1). Electrochemical impedance spectra (EIS) were recorded by electrochemical workstation (CHI660D, China) in a frequency range from 10 mHz to 100 kHz with amplitude of 5 mV.
1 w/w) as the electrolyte. Meanwhile, galvanostatic charge–discharge measurements on (Land, China) were performed in the voltage range of 1.5–4.8 V (vs. Li+/Li) under the current density of 10 mA g−1 at 30 °C. Rate capability of electrodes was examined at various current densities: 0.03 C, 0.1 C, 0.5 C, 1 C, 2 C (1 C = 330 mA g−1). Electrochemical impedance spectra (EIS) were recorded by electrochemical workstation (CHI660D, China) in a frequency range from 10 mHz to 100 kHz with amplitude of 5 mV.
Results and discussion
The typical powder X-ray diffraction (XRD) patterns of as-prepared pristine Li2MnSiO4 and Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) powders are shown in Fig. 1. It can be seen that all the samples are well indexed to orthorhombic structure with Pmn21 space group, which is in accord with previous reports.16,17 However, with the increasing of Ti content, some diffraction peaks identified as Mn2TiO4 and Li2SiO3 impurities are observed in the case of x = 0.2, which are also reported by other researchers.18–21 But neither of these impurities is considered for quantitative analysis, since they are electrochemically inactive in the voltage window of 1.5–4.8 V.10 In order to clarify the actual sites of dopants, Rietveld refinement is adopted. The refinements results are presented in Fig. 2. We examine all the possible ways in which dopants occupy in the crystal lattice and the best fit results are obtained when Ti occupied Mn site. The reasonable values of Rp and Rwp can support this conclusion. The calculated lattice parameters of all these materials are listed in Table 1. Noting, doping Ti element induces the slight volume shrinkage of Li2MnSiO4, which might be ascribed to the smaller ionic radius of Ti4+ (0.68 Å)22 than that of Mn2+ (0.80 Å), but the values of b-axis are a little enlargement. According to previous reports,23,24 layers of SiO4 and MnO4 tetrahedral lying on the ac-plane link along the b-axis by LiO4 tetrahedra, so enlargement of b values can expand the Li+ diffusion channel to facilitate Li+ mobility so as to enhance the electrochemical activity. The results of ICP-AES element analysis are demonstrated in Table 2, where the amount of element Mn is normalized to stoichiometry. As is seen, the actual compositions of all as-prepared powders are generally in line with nominal compositions. | ||
| Fig. 1 XRD patterns of as-prepared pristine Li2MnSiO4 and doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) powders. | ||
| Samples | a/Å | b/Å | c/Å | V/Å3 |
|---|---|---|---|---|
| Li2MnSiO4 | 6.3077 | 5.3884 | 4.9753 | 169.1025 |
| Li2Mn0.94Ti0.06SiO4 | 6.3185 | 5.3908 | 4.9708 | 169.3142 |
| Li2Mn0.9Ti0.1SiO4 | 6.3128 | 5.3913 | 4.9676 | 169.0683 |
| Li2Mn0.8Ti0.2SiO4 | 6.2943 | 5.4117 | 4.9457 | 168.4647 |
| Samples | Li | Mn | Ti |
|---|---|---|---|
| Li2MnSiO4 | 1.91 | 1.0 | — |
| Li2Mn0.94Ti0.06SiO4 | 1.93 | 0.94 | 0.064 |
| Li2Mn0.9Ti0.1SiO4 | 2.03 | 0.9 | 0.101 |
| Li2Mn0.8Ti0.2SiO4 | 2.06 | 0.8 | 0.198 |
FE-SEM images display the morphology of pristine and Ti-doped samples, as shown in Fig. 3a–d. We can see that pristine Li2MnSiO4 powders exhibit severe agglomeration by nanosized primary particles (∼50 nm), while Ti-doped samples represent alleviative agglomeration between particles and the mean particle sizes are approximately 30 nm, much less than that of pristine sample. Moreover, the BET specific surface areas of Li2Mn1−xTixSiO4 are calculated to be 11.919 (x = 0), 44.376 (x = 0.06), 40.6 (x = 0.1) and 35.197 (x = 0.2) m2 g−1, respectively. Obviously, doping Ti element can decrease the particle sizes, thus dramatically increasing the specific surface areas. The results can shorten the pathway of lithium-ion transfer and enable good contact between particles and the electrolyte so as to benefit the electrochemical reaction.
 | ||
| Fig. 3 FE-SEM images of as-prepared Li2Mn1−xTixSiO4 powders: (a) x = 0; (b) x = 0.06; (c) x = 0.1; (d) x = 0.2. | ||
Additionally, the electrochemical performance of Ti-doped samples shows remarkable improvement, especially for capacity. For comparison, the charge–discharge curves of doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) and pristine Li2MnSiO4 electrodes are presented in Fig. 4, which were tested in a voltage range of 1.5–4.8 V under a current density of 10 mA g−1 at 30 °C. It is clearly seen that pristine Li2MnSiO4 deliver extremely low capacity of 52 mA h g−1 for charging and 26 mA h g−1 for discharging in the first cycle, corresponding to 0.16 Li+ and 0.08 Li+ per formula unit, respectively. The poor electrochemical activity may be attributed to its low intrinsic electric conductivity (<10−14 S cm−1 at RT).1 However, in the case of Ti-doped Li2Mn1−xTixSiO4, all three samples demonstrate dramatically increased capacities. For x = 0.06, 0.1 and 0.2, the capacities are 298, 286, 248 mA h g−1 for first charging (corresponding to 1.81, 1.73 and 1.50 Li+ per formula unit) and 203, 211, 171 mA h g−1 for first discharging (corresponding to 1.23, 1.28 and 1.04 Li+ per formula unit), respectively. Although the capacities decrease during prolonged cycle, they are still greatly improved, as compared to that of pristine Li2MnSiO4 electrodes. Among these Ti-doped samples, Li2Mn0.8Ti0.2SiO4 shows the best cycling stability, as shown in Fig. 5. From 20th to 50th cycles, the capacity maintains around 100 mA h g−1 and it has a relatively high capacity retention rate of 60% after 50 cycles. As far as we know, this should be the best result of Li2MnSiO4 electrodes without additional carbon coating so far.25–27 The reasonable explanations of achieved high capacity and structural stability for doped Li2Mn1−xTixSiO4 electrodes are ascribed to (i) according to the XRD results, for Ti-doped samples, the enlargement of Li+ diffusion channel is beneficial to facilitate Li+ mobility; (ii) decreased particle size and increased specific surface area can shorten the Li+ migration pathway and enable good contact between particles and electrolyte so as to benefit the electrochemical reaction; (iii) Li2MnSiO4 is a typical n-type semiconductor, its electronic conduction takes place by electron hopping between high-valence (Mn4+ or Mn3+) and low-valence (Mn3+ or Mn2+) cations, Ti doping can increase the amount of Mn3+ and probably Mn4+, thus increase its electric conductivity; (iv) the lattice shrinking of Li2MnSiO4 during charging is minimized by the support of unchangeable radius of Ti4+ to stabilize the structure.
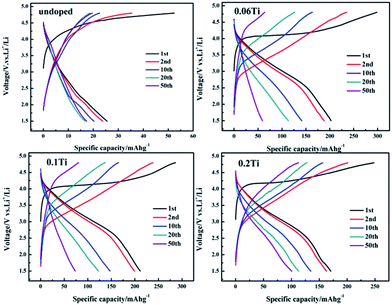 | ||
| Fig. 4 Charge–discharge curves of Li2Mn1−xTixSiO4 (x = 0, 0.06, 0.1 and 0.2) electrodes in different cycles. | ||
 | ||
| Fig. 5 Cycling performance of Li2Mn1−xTixSiO4 (x = 0, 0.06, 0.1 and 0.2) electrodes in the voltage range of 1.5–4.8 V under the current density of 10 mA g−1 at 30 °C. | ||
Fig. 6 demonstrates the rate capabilities of pristine Li2MnSiO4 and Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) samples. The charge–discharge current densities are various from 0.03 C to 2 C (1 C = 330 mA g−1). It is surprising to note that Ti-doped Li2Mn1−xTixSiO4 samples exhibit superior rate capability, as shown in the Fig. 6. At 1 C-rate, the discharge capacities of Ti-doped samples in the second cycle are 114, 120, 76 mA h g−1 for 0.06Ti, 0.1Ti, 0.2Ti, respectively. Even at higher rate (2 C), the Ti-doped samples still keep the discharge capacity of around 70 mA h g−1, whereas the undoped sample almost loses the ability to discharge. The excellent rate capabilities of Ti-doped samples should be ascribed to the enhancement of electronic conductivity and migration rate of Li ions, which can be further confirmed by the results of electrochemical impedance spectra in the following section.
 | ||
| Fig. 6 Charge–discharge curves of Li2Mn1−xTixSiO4 (x = 0, 0.06, 0.1 and 0.2) electrodes with various current densities at 30 °C (1 C = 330 mA g−1). | ||
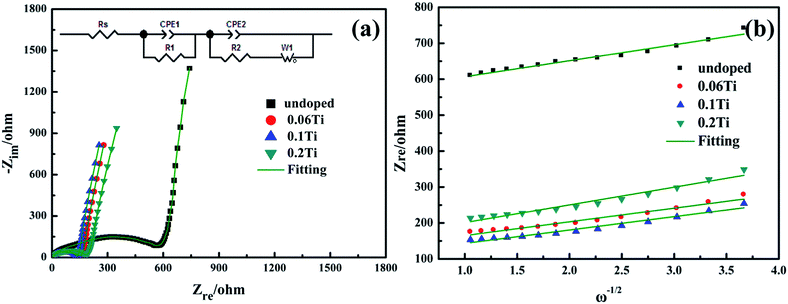
Electrochemical impedance spectra (EIS) are employed to characterize the kinetic properties of pristine Li2MnSiO4 and Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) samples. Fig. 7a demonstrates the Nyquist plots of the impedance spectra for all samples, and the equivalent circuit mode in the inset of Fig. 7a is used for fitting the impedance data. All spectrums are consisted of a depressed small semicircle in the high-frequency region, a large semicircle in the middle-frequency and a slightly inclined line in the low-frequency region. The semicircles represent the solution resistances (Rs), film Resistances (R1) and charge transfer resistance (R2), respectively. The inclined lines are attributed to the Li ions diffusion in the electrode bulks, which are called Warburg diffusion (Zw).28–31 The obtained fitting parameters are displayed in Table 3. It is seen that almost all of the resistances of Ti-doped Li2Mn1−xTixSiO4 are smaller than that of undoped Li2MnSiO4, especially charge transfer resistance (R2). The values of charge transfer resistance are 81.14, 68.3, 88.65 Ω for 0.06Ti, 0.1Ti, 0.2Ti samples, respectively, much smaller than that of undoped sample (433.6 Ω). The lower R2 of Ti-doped samples indicates that Ti doping facilitates the charge transfer at the interface, leading to high capacity and superior rate capability as observed. Besides, the diffusion coefficients of Li+ in the electrodes can be calculated based on the following equation:
| DLi+ = (R2T2)/(2A2n4F4C2σw2) | (1) |
| Zre ∝ σwω−1/2 | (2) |
| Rs/ohm | R1/ohm | R2/ohm | DLi+/cm2 s−1 | |
|---|---|---|---|---|
| Li2MnSiO4 | 1.923 | 100.2 | 433.6 | 2.3 × 10−15 |
| Li2Mn0.94Ti0.06SiO4 | 1.727 | 55.6 | 81.14 | 3.2 × 10−15 |
| Li2Mn0.9Ti0.1SiO4 | 3.82 | 57.69 | 68.3 | 3.3 × 10−15 |
| Li2Mn0.8Ti0.2SiO4 | 1.733 | 81.14 | 88.65 | 1.9 × 10−15 |
Conclusions
In summary, we successfully prepared pristine Li2MnSiO4 and Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) powders by a facile sol–gel method. XRD measurements confirm the structures of all the as-prepared powders are in agreement with orthorhombic Pmn21 space group. FE-SEM images and BET specific surface areas demonstrate that Ti-doped Li2Mn1−xTixSiO4 (x = 0.06, 0.1 and 0.2) samples possess smaller particle sizes, better monodispersion and larger specific surface areas, as compared to those of pristine Li2MnSiO4. Ti-doped Li2Mn1−xTixSiO4 electrodes exhibit dramatically increased capacity of 298, 286, 248 mA h g−1 for first charging and 203, 211, 171 mA h g−1 for first discharging, respectively, much higher than that of pristine sample. Among these Ti-doped materials, Li2Mn0.8Ti0.2SiO4 shows the best cycling performance, maintaining the capacity around 100 mA h g−1 after 50 cycles. Moreover, Ti-doped samples also present superior rate capability. At 1 C rate, they have the discharge capacities of 114, 120, 76 mA h g−1 for 0.06Ti, 0.1Ti, 0.2Ti samples, respectively. Even at higher rate (2 C), the Ti-doped samples still keep the discharge capacity of around 70 mA h g−1. The remarkable enhancement of battery performance in terms of capacity and rate capability for Ti-doped Li2Mn1−xTixSiO4 is primarily attributed to the decrease of charge transfer resistance and the improvement of Li+ mobility coefficient. Nevertheless, we don't clearly understand the mechanism of defects formation about aliovalent doping at present, the results indeed demonstrate a new strategy for conductivity enhancement and the possible structural stabilization which may lead to excellent electrochemical properties of Li2MnSiO4 cathode materials.Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (2012M521064) and the Natural Science Foundation of Jiangsu Province (BK20140936). The support from the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD) was also acknowledged.Notes and references
- R. Dominko, M. Bele and A. Kokalj, J. Power Sources, 2007, 174, 457 CrossRef CAS PubMed.
- R. J. Gummow, N. Sharma and V. K. Peterson, J. Solid State Chem., 2012, 188, 32 CrossRef CAS PubMed.
- K. Karthikeyan, V. Aravindan and S. B. Lee, J. Power Sources, 2010, 195, 3761 CrossRef CAS PubMed.
- M. S. Islam, R. Dominko and C. Masquelier, J. Mater. Chem., 2011, 21, 9811 RSC.
- M. K. Devaraju, T. Tomai and A. Unemoto, RSC Adv., 2013, 3, 608 RSC.
- Y. X. Li, Z. L. Gong and Y. Yang, J. Power Sources, 2007, 174, 528 CrossRef CAS PubMed.
- S. Zhang, Y. Li and G. J. Xu, J. Power Sources, 2012, 213, 10 CrossRef CAS PubMed.
- I. Belharouak, A. Abouimrane and K. Amine, J. Phys. Chem., 2009, 113, 20733 CAS.
- W. G. Liu, Y. H. Xu and R. Yang, J. Alloys Compd., 2009, 480, L1 CrossRef CAS PubMed.
- S. Zhang, Z. Lin and L. W. Ji, J. Mater. Chem., 2012, 22, 14661 RSC.
- S. H. Choi, S. J. Kim and Y. J. Yun, Mater. Lett., 2013, 105, 113 CrossRef CAS PubMed.
- N. Kuganathan and M. S. Islam, Chem. Mater., 2009, 21, 5196 CrossRef CAS.
- C. Deng, S. Zhang and Y. X. Wu, J. Electroanal. Chem., 2014, 719, 150 CrossRef CAS PubMed.
- C. Deng, S. Zhang and S. Y. Yang, J. Power Sources, 2011, 196, 386 CrossRef CAS PubMed.
- S. H. Wang, J. Yang and X. B. Wu, J. Power Sources, 2014, 245, 570 CrossRef CAS PubMed.
- D. M. Kempaiah, D. Rangappa and I. Honma, Chem. Commun., 2012, 48, 2698 RSC.
- I. B. ouak, A. Abouimrane and K. Amine, J. Phys. Chem. C, 2009, 113, 20733 Search PubMed.
- H. Y. Wang, T. L. Hou and D. Sun, J. Power Sources, 2014, 247, 497 CrossRef CAS PubMed.
- V. Aravindan, K. Karthikeyan and S. Amaresh, Ionics, 2011, 17, 3 CrossRef CAS.
- C. Deng, Y. H. Sun and S. Zhang, Int. J. Electrochem. Sci., 2012, 7, 4559 CAS.
- R. Y. Chen, R. Heinzmann and S. Mangold, J. Phys. Chem., 2011, 117, 884 Search PubMed.
- B. B. Tian, H. F. Xiang and L. Zhang, Electrochim. Acta, 2010, 5, 5453 CrossRef PubMed.
- L. Y. Bao, W. Gao and Y. F. Su, Chin. Sci. Bull., 2013, 58, 575 CrossRef CAS PubMed.
- M. E. Arroyo-deDompablo, R. Dominko and J. M. Gallardo-Amores, Chem. Mater., 2008, 20, 5574 CrossRef CAS.
- V. Aravindan, S. Ravi and W. S. Kim, J. Colloid Interface Sci., 2011, 355, 472 CrossRef CAS PubMed.
- V. Aravindan, K. Karthikeyan and S. Amaresh, Electrochem. Solid-State Lett., 2011, 14, A33 CrossRef CAS PubMed.
- Q. Q. Zhang, Q. C. Zhuang and S. D. Xu, Ionics, 2012, 18, 487 CrossRef CAS.
- C. L. Tan, H. J. Zhou and W. S. Li, J. Power Sources, 2008, 184, 408 CrossRef CAS PubMed.
- A. Bhaskar, M. Deepa and T. N. Rao, J. Electrochem. Soc., 2012, 159, A1954 CrossRef CAS PubMed.
- Z. Z. Feng, J. Yang and Y. N. Nuli, J. Power Sources, 2008, 184, 604 CrossRef CAS PubMed.
- C. Deng, S. Zhang and B. L. Fu, Mater. Chem. Phys., 2010, 120, 14 CrossRef CAS PubMed.
- M. Yang, X. Y. Zhao and Y. J. Bian, J. Mater. Chem., 2012, 22, 6200 RSC.
- S. K. Liu, J. Xu and D. Z. Li, J. Power Sources, 2013, 232, 258 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |