Low cost and large-area fabrication of self-cleaning coating on polymeric surface based on electroless-plating-like solution deposition approach†
Liangzhuan Wu,
Yuan Yu and
Jinfang Zhi*
Key Laboratory of Photochemical Conversion and Optoelectronic Materials, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: zhi-mail@mail.ipc.ac.cn
First published on 2nd January 2015
Abstract
A novel low-cost controllable solution-based coating process for realization of self-cleaning coating on large-area flexible polymeric substrates based on an improved Electroless-Plating-Like Solution Deposition (EPLSD) approach was developed. In a typical coating procedure, a layer of aniline was first adsorbed onto the surface of a flexible polymeric substrate by dip-coating; and as the pretreated flexible substrate was then immersed into an nanosol including a Peroxo-Titanium-Complex (PTC) modified silica (P–Si), after the thermal treatment at 80 °C for 30 min, the flexible polymeric substrate coated by P–Si composite thin films could be obtained. The as-prepared TiO2–SiO2 thin film was characterized by TEM, SEM, XRD, and FTIR analysis, and its self-cleaning characteristics were also evaluated by the contact angle and the methyl blue degradation test.
I. Introduction
Semiconducting metal oxides are an important class of functional materials promising a wide range of new technological applications. In particular, when they are prepared into thin films, the utilized efficiency and applicability of these materials can be significantly improved. For instance, titanium oxide (TiO2) films which are coated on various substrates, such as glasses, polymers, and silicon wafers, have many important applications in catalytic decomposition of organic pollutants, anti-bacterial activity, and self-cleaning activity, as well as solar cells, etc.1–6Among these applications, combining the photo-induced hydrophilicity and photocatalytic properties of TiO2 film, which make use of sunlight and natural rainfall to keep surfaces self-cleaning, is one of the most attractive and promising fields, as it can save significant cost for maintenance in industry and daily life.7 However, the hydrophilic surface of a simple TiO2 film will slowly become hydrophobic again without UV light irradiation, resulting in a deterioration or failure of its self-cleaning performance. Thus, a self-cleaning surface containing SiO2 and TiO2 is widely adopted,8–10 as the SiO2 component can preserve a lasting hydrophilicity without the UV light irradiation.
On the other hand, considering the various substrates that have been tried for supporting TiO2 photocatalysts, although polymer substrate seems to be very promising due to its several advantages such as flexible, low-cost, and ease of availability, most of the as reported TiO2 films were usually coated on the inorganic substrates, such as glasses, tile, etc. Because, concerning the generally adopted preparing methods for fabrication TiO2 self-cleaning coating, such as sol–gel method, chemical vapor deposition (CVD), and hydrothermal methods, etc.,11–14 a high temperature sintering process was generally inevitable, as the sintering process can benefit not only a high efficient photocatalytic activity which were generated only in well crystallized anatase TiO2 phases, but also a strong adhesion between the coating layer and the substrate due to a chemical bond formation between the TiO2 and the inorganic substrate during the high temperature process. Thus the low thermal stability/tolerance of polymeric substrate precludes the use of the above mentioned physical and chemical methods that need high sintering temperature for adherence of titania on the polymer surface. This calls for development of novel, low temperature and economical methods for fabrication of polymer supported titania photocatalysts.
Alternative approaches for embedding of titania on the polymer surface without thermal sintering, such as binding,15 polymerization the organic monomer in the presence of TiO2 nanoparticles,16 and thermal bonding (hot pressing)17 have then been explored. But a following loss catalytic activity, which was attributed to the partial embedment of TiO2 particles on the polymer surface, clearly suggested a need in improvement of the deposition method. Recently, our group developed an electroless-plating-like solution deposition (EPLSD) approach for the fabrication of flexible metal oxide film,18 i.e., crystallized metal oxide (such as TiO2, V2O5 and MoO3) can be attached on the surface of PET film adhesively by a redox reaction between d0 configured peroxo metal complex (Ti(IV), V(V), Mo(VI)) with the conductive (electroactive/conjugated) polymer monomer (EDOT, pyrrole, and aniline). In this EPLSD process, the formed metal oxide films showed highly crystalline phase, all processes were performed under 80 °C conditions using large-area polymer substrates, and the procedure is simple and cost-effective. Moreover, conductive (electroactive/conjugated) polymer (PAni) formed during the EPLSD procedure, characterized with an extended π-conjugated electron system, can obtain a synergic effect with inorganic semiconductor metal oxides like TiO2,18 i.e., PAni causes rapid charge separation, slow charge recombination and thus an enhanced photocatalytic activity of the prepared TiO2/PAni photocatalysts. Therefore, EPLSD may suitable for the use in the polymer substrate coating of self-cleaning by enhanced self-cleaning ability instead of a catalytic activity loss due to the partial embedment of TiO2.
However, the as described EPLSD process was limited only early transition metal series with d0 configuration, such as Ti, V, Mo, etc. While for the silica, which is necessary for keeping the good self-cleaning effect with TiO2 in the coating, as described in above, was not involved in the candidates for EPLSD procedure, i.e., Si element is not an early transition metal. This hindered the application of EPLSD procedure on fabrication of the TiO2–SiO2 composite self-cleaning coating on polymeric surface. To overcome this limitation, in the present report, we developed an improved EPLSD process which can be used to non-d0 configured nanoparticles to fabricated an oxides composited film. This improved process includes modifying the silica nanoparticles with the Peroxo-Titanium-Complex (PTC) aqueous solution, then the PTC coated silica (P–Si) solution instead of peroxo metal complex solution was applied for the EPLSD approach, and a corresponding TiO2–SiO2 composite flexible thin film was successfully coated onto the PET substrate.
II. Experimental section
Materials
All chemical reagents used in present experiments were analytical grade and purchased from Beijing Chem. Co. Ltd. The silica nano-sol (SiO2) was purchased from Beijing Institute of Aeronautical Materials. A PET film as a flexible substrate was purchased from Tianjin Shiqi Co. Ltd.A typical experiment process for the film preparation can be described in Scheme 1.
 | ||
| Scheme 1 Scheme of electroless-plating-like solution deposition (EPLSD) process for fabricating the TiO2–SiO2 self-cleaning polymeric film. | ||
The coating solution for the EPLSD procedure was prepared as following: firstly, TiCl4 was reacted with ammonia to form titanic acid precipitates, the precipitate was dissolved into 30 wt% H2O2 solution, and then the solution were diluted to [Ti] = 0.125 mol L−1, and a detailed description for the preparation of peroxo-titanium-complex (PTC) solution can be found in the ref. 18. Secondly, the as prepared PTC aqueous solution was added to the as-purchased silica nanosol, the molar ratios of silica/Ti were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Finally, the mixtures were stirred and kept on boiling for ca. 4 h, and a final coating solution, named as PTC modified silica (P–Si) solution, was thus achieved.
1. Finally, the mixtures were stirred and kept on boiling for ca. 4 h, and a final coating solution, named as PTC modified silica (P–Si) solution, was thus achieved.
As for the pretreatment of the PET substrate, the swelling process of PET was carried out in a 2 L beaker, 20 × 40 cm PET film (10.98 g) was immersed in the 1.5 L mixed organic solvent of aniline and EtOH in the above flask for a 2 h to reach the plateau value of 8 wt% of aniline, and the swelling film was then taken out and performed a drying procedure in air for 10 min, allowing nearly 0.87 g aniline monomer was absorbed onto the PET surface. The swelling kinetics of PET film in aniline was discussed in ESI Fig. S1.†
As for the EPLSD process, as was illustrated in Scheme 1, it was performed as following: the P–Si coating solutions was firstly heated and remain at a stable temperature of 80 °C, and then the as-pretreated PET substrates were immersed into P–Si solution to fulfill coating; the reaction time can be varied from several minute to hours depending on requirement.
Characterization
X-ray diffraction (XRD) experiments were performed with an XD-2 diffractometer (Purkinje General Instrument Co. Ltd.), using monochromatic Cu Kα radiation at 36 kV and 30 mA. Fourier transform infrared (FT-IR) spectra were obtained by using an Excalibur 3100 spectrometer with a resolution of 4 cm−1. Measurements were performed in the transmission mode in spectroscopic grade KBr pellets for all the powders. Transmission electron microscopy (TEM) studies were performed using Philips CM200/FEG field emission-gun transmission electron microscope, operating at 200 kV. The surface composition and elemental chemical state of the samples were examined by X-ray photoelectron spectroscopy (XPS) using a Model Axis Ultra (Kratos Analytical Ltd.) apparatus. The surface morphology of the sample was investigated by a scanning electron microscopy (Hitachi Ultra-High-Resolution S-4300).III. Results and discussion
Morphology of the as-purchased silica and the P–Si composite solution were characterized by TEM observations (Fig. 1). Fig. 1a revealed that the as-purchased silica nanosol was an aqueous solution of SiO2 nanoparticles of with the size of about 4–6 nm in diameters, and the insert part of the digital photo indicated the solution was homogeneous and milky transparent. The amorphous nature of the silica nanoparticles can be identified by the HRTEM image of Fig. 1b. Fig. 1c and d are the TEM images of the samples taken from the P–Si composite solution, which was prepared as described in the Experimental section. Compared with the original silica sol, after reaction with PTC, the latter P–Si solution shows a significant color variation, i.e., from milky to yellow, but remained as a homogeneous and transparent aqueous sol. This P–Si sol can be kept in a sealed case for several months without precipitation. Furthermore, as was observed in the Fig. 1c, this yellow transparent solution was proved to be a solution of nanoparticles with the size of about 8–10 nm in diameters. Different from the amorphous nature of the original silica nanoparticles, a new crystalline phase, as was shown in Fig. 1d (marked with the inset white arrow lines), can be clearly distinguished, verified the existence of crystallized nanoparticles attached on the silica spheres, which was characterized with a distinct lattice spacings of 0.35 nm correspond to the (101) planes spacings of the anatase phase TiO2 (JCPDS: no. 02-0406). These results suggested the P–Si solution contains an anatase crystallized TiO2, moreover, it might be a nanocomposite of SiO2/TiO2, concerning on an increased of dimension in particle sizes, i.e., from 4–6 nm to 8–10 nm, compared with the original silica nanoparticles. In order to further identify the structure of the as prepared nanocomposite SiO2/TiO2, extensive TEM observations were made on samples collected from the P–Si composite solution (see ESI Fig. S3†), and a random disordered structure of the as prepared nanocomposite SiO2/TiO2 can be characterized. | ||
| Fig. 1 TEM images of the (a) and (b) are as-purchased silica sol, and (c) and (d) are P–Si composite solution, the insert photos are the corresponding digital photos of the samples. | ||
As a part of efforts to obtain a better understanding of the formation of P–Si nanocomposite, a serious of analysis were performed on the silica and P–Si solution. The zeta potential analysis was shown in Fig. 2a, the mixture of a positively charged silica nanosols (2.7 mV) and negatively charged PTC aqueous solution (−7.9 mV) would result in a negatively charged solution characterized with a relatively low zeta potential of −2.1 mV, and this implies the modification of PTC on silica nanoparticles may attribute to the electrostatic adsorption between the opposite charging of the PTC precursor and silica nanoparticle. Fig. 2b shows the corresponding XRD patterns of the silica and the P–Si sample. Compared with the typical amorphous result of the silica sample, all the diffraction peaks of the PTC modified samples are the anatase crystalline phase of titania (JCPDS no. 02-0406), confirming that the P–Si obtain the component of TiO2.
 | ||
| Fig. 2 (a) Zeta potential graph the original silica, peroxo-titanium-complex and the P–Si nanosols, and (b) XRD pattern of the original silica and the P–Si samples. | ||
The samples were further investigated by FT-IR spectra and the results were shown in ESI S4,† not only the Ti–O–Ti stretching vibration of TiO2 (462 cm−1 and 815 cm−1) and the Si–O–Si stretching vibration of SiO2 (1096 cm−1), but also a stretching vibration Ti–O–Si (958 cm−1) can be clearly characterized, and this result revealed the existence of a chemical bond between TiO2 and SiO2 nanoparticle. Concerning on the fact the TiO2 precursor, i.e. PTC, was absorbed on the silica surface, we may suggesting that TiO2 were mostly nucleated on silica surface during the crystallization process and result in a nanocomposite of TiO2/SiO2 connected by a chemical bond of Ti–O–Si.
In order to clarify the long term stability of the as prepared P–Si nano sol, the thermal treatment time of the P–Si solution was varied from 0.5 h to 8 h. The products of the as prepared P–Si samples were characterized by electron microscopy and the result was shown in Fig. 3. As was shown in Fig. 3a, at the beginning of the thermal treatment of the P–Si solution, some nanoparticles with the size of about 4–6 nm in diameters surrounded by the amorphous materials can be identified, and this may attribute to the adsorption of PTC onto the surface of the silica due to the electrostatic adsorption between the opposite charging of the PTC precursor and silica nanoparticle. As the thermal treating time of the P–Si solution increased to 4 h, as was shown in Fig. 3b, monodispersed nanocomposite SiO2/TiO2 can be observed. Meanwhile, the color of the as obtained nanosols changed from transparent orange to transparent yellow (see the insert digital photo of Fig. 3a and b), which can be attribute to the partly decomposition of PTC since it was the exclusive precursor of TiO2. Finally, accompanied with the completely decomposition of PTC, identified by the disappearing of PTC unique color (see the insert digital photo of Fig. 3c), agglomeration of the nanocomposite SiO2/TiO2 can be characterized in Fig. 3c, which means the destruction of the long term stability and precipitation can also be observed in the as achieved P–Si solution.
The result discussed above suggested that the PTC groups absorbed on the nanoparticles surface may accounted for the long term stability of the as prepared P–Si nanosol. Actually, Ragai19 had also mentioned the long term stability of peroxidized titanium solution for more than one year. According to Schwarzenbach's work,20,21 the condensation reaction of the PTC may result in a polynuclear product, and it is thus reasonable to conclude that the polynuclear PTC absorbing onto the surface of the nanocomposite SiO2/TiO2 may responsible for the long term stability of P–Si solution due to a steric stabilization.
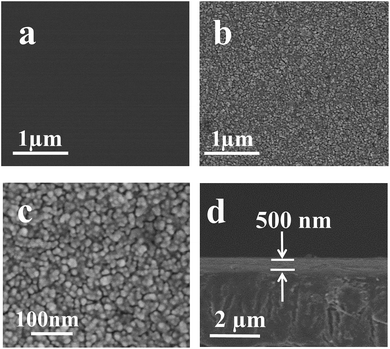
Fig. 4 were the SEM characterization result of the original and P–Si coated PET films, As shown in Fig. 4, compared to a blank and smooth surface of the original PET film, the surfaces of PET films treated by P–Si were covered with a layer of fine and closed nanoparticles (Fig. 4b and c), and this layer thickness is about 500 nm (Fig. 4d). The EDS (see ESI S5†) spectra analysis suggested that the composition of these nanoparticles which formed on the surface of the PET films mainly contains Ti, Si. C and O elements, and an atomic ratio of C (44.69%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti (2.96%)
Ti (2.96%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si (2.22%) can be identified. A saddle-shaped distribution of the element Ti and Si can be observed (see ESI S6†), suggesting that the element Ti and Si were mainly centralized on the surface of the PET film.
Si (2.22%) can be identified. A saddle-shaped distribution of the element Ti and Si can be observed (see ESI S6†), suggesting that the element Ti and Si were mainly centralized on the surface of the PET film.
We also scraped off the samples from the PET substrate with a knife and then dispersed the as-obtained sample in water to analysis its component. As shown in the Fig. 5a, an amorphous aggregation can be observed from the TEM image, a further magnificated image (Fig. 5b), some crystalline nanoparticles surrounded by the amorphous materials with the size of about 4–6 nm in diameters can be identified. The lattice planes shown in the Fig. 4b agree well with distances of (101) lattice planes of anatase TiO2 (Fig. 5b) (JCPDS no. 02-0406). Thus, this indicated that the improved EPLSD process can truly be applied to deposit the as prepared P–Si nanoparticles onto the PET surface. As to the amorphous component coming from the scraped off coating samples as observed in Fig. 5, as was discussed in our previous report,18,22 it might be the polymerization product of the aniline monomer during the EPLSD procedure.
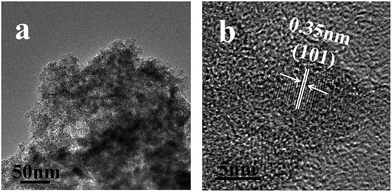 | ||
| Fig. 5 TEM images of the samples that were scraped off from the PET substrate with a knife, the PET substrate was coated with P–Si sol based on EPLSD procedure. | ||
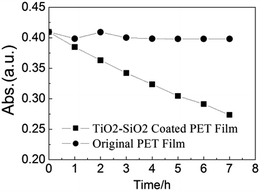 | ||
| Fig. 6 Photocatalytic degradation curves of aqueous MB irradiated under UV light (365 nm) of (a) blank PET film and (b) TiO2–SiO2 coated PET film. | ||
Based on the above discussion, we can preliminarily interpret the mechanism of the improved the EPLSD process which can realize the TiO2–SiO2 nanocomposite coating on the polymeric PET substrate surface. As was shown in Scheme 2, firstly, by refluxing the mixture of the negatively charged PTC precursor with the positively charged silica nanoparticles solution, the surface of silica nanoparticle can be modified with TiO2 nanocrystals, and a P–Si solution of TiO2–SiO2 nanocomposite particles was thus obtained; secondly, by dipping the PET film which was pretreated with aniline into the P–Si solution, triggered by the O–O group absorbed on the P–Si nanoparticles surface, which was identified by the feature yellow color in aqueous solution and a O–O stretching vibration at 890 cm−1 of the P–Si nanoparticles (see ESI S4†), a radical polymerization reaction of the organic aniline which absorbed onto the PET substrated begins; finally, as was described in a typical EPLSD procedure,18 the in situ produced polymer could be regarded as a self-binder reagent, and this process makes the TiO2–SiO2 nanocomposite attached on the surface of PET firmly.
 | ||
| Scheme 2 Mechanism of the improved electroless-plating-like solution deposition (EPLSD) process for large area TiO2–SiO2 self-cleaning PET film coating. | ||
The effectiveness of TiO2–SiO2 as a hydrophilic coating on treated PET film obtained was investigated by contact angle measurements. Table 1 depicts the durability test result of water static contact angles with or without UV light irradiation for untreated and treated samples. The decrease of the water contact angle in the treated surfaces (from 82.7° to 18.7°) indicates that sufficient surface hydrophilicity was achieved. Furthermore, UV light irradiation can enhances the decrease of the water contact angle (from 18.7° to 9.7°), and which are considered as the photo induced hydrophilicity of the TiO2. What more, a long time durability test result revealed that the as achieved hydrophilicity of the as prepared PET film can be retained more than one year.
The photocatalytic activities of the as-prepared TiO2–SiO2 film were evaluated by methylene blue degradation in an aqueous solution. In a typical experiment, the films slides (20 mm × 40 mm) were first dipped in 5 × 10−5 M methylene blue for the saturated adsorption process. The adsorption equilibrium testing film was then put into a watch glass containing 20 mL of 1 × 10−5 M methylene blue solution and exposed to the 365 nm UV light for 480 min. The result of methylene blue degradation tests (Fig. 6) revealed that the TiO2–SiO2 coated PET film demonstrated an obvious photocatalytic activity (33.21% methylene blue concentration decrease) compared with a photocatalytic inactivity bare PET film.
IV. Conclusions
By surface modification of SiO2 nanoparticles with the d0 configured peroxo metal complex precursor, self-cleaning coating of TiO2–SiO2 on large-area PET film via EPLSD procedure was successfully prepared. A durable hydrophilicity and color fading test result revealed the as prepared polymeric film surface obtained a desired self-cleaning ability. The as reported novel low temperature and economical solution-based procedure will representing not only a promising step toward polymer substrate self-cleaning, but also a widen application scope of the EPLSD from d0 configured metal oxide nanoparticles to non-d0 configured nanoparticles.Acknowledgements
This research was supported by the Beijing Natural Science Foundation (no. 2120002) and the International Science & Technology cooperation program of China (no. 2013DFG 50150).References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1–21 CrossRef CAS.
- D. A. Tryk, A. Fujishima and K. Honda, Electrochim. Acta, 2000, 45, 2363–2376 CrossRef CAS.
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- I. K. Konstantinou and T. A. Albanis, Appl. Catal., B, 2004, 49, 1–14 CrossRef CAS PubMed.
- A. Millis and S. Le Hunte, J. Photochem. Photobiol., A, 1997, 108, 1–35 CrossRef.
- R. Wang, K. Hashimoto and A. Fujishima, et al., Nature, 1997, 338, 431–432 CrossRef PubMed.
- B. Xi, L. K. Verma and J. Li, et al., ACS Appl. Mater. Interfaces, 2012, 4, 1093–1102 CAS.
- K. Qi, X. Chen and Y. Liu, et al., J. Mater. Chem., 2007, 17, 3504–3508 RSC.
- L. Pinho and J. Mosquera, J. Phys. Chem. C, 2011, 115, 22851–22862 CAS.
- K. O. Awitor, A. Rivaton and J. L. Gardette, Thin Solid Films, 2008, 516, 2286–2291 CrossRef CAS PubMed.
- M. Okuya, K. Nakade and S. Kaneko, Sol. Energy Mater. Sol. Cells, 2002, 70, 425–435 CrossRef CAS.
- Y. B. Ding, C. Z. Yang, L. H. Zhu and J. D. Zhang, J. Hazard. Mater., 2010, 175, 96–103 CrossRef CAS PubMed.
- A. Kafizas, C. W. Dunnill and I. P. Parkin, J. Mater. Chem., 2010, 20, 8336–8349 RSC.
- L. Song, R. Qiu, Y. Mo, D. Zhang and H. Wei, Catal. Commun., 2007, 8, 429–433 CrossRef CAS PubMed.
- X. Li, D. Wang, G. Cheng, Q. Luo and Y. Wang, Appl. Catal., B, 2008, 81, 267–273 CrossRef CAS PubMed.
- S. Naskar, S. A. Pillay and M. Chanda, J. Photochem. Photobiol., A, 1998, 113, 257–264 CrossRef CAS.
- L. Z. Wu, Y. Yu and J. F. Zhi, J. Mater. Chem. C, 2014, 2, 2262–2271 Search PubMed.
- J. Ragai, Nature, 1987, 325, 703–705 CrossRef CAS.
- G. Schwarzenbach, J. Muehlebach and K. Mueller, Inorg. Chem., 1970, 9, 2381–2390 CrossRef CAS.
- D. Schwarzenbach, Inorg. Chem., 1970, 9, 2391–2397 CrossRef CAS.
- Y. Z. Li, Y. Yu, L. Z. Wu and J. F. Zhi, Appl. Surf. Sci., 2013, 273, 135–143 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10513c |
| This journal is © The Royal Society of Chemistry 2015 |