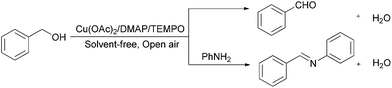
Practical organic solvent-free Cu(OAc)2/DMAP/TEMPO-catalyzed aldehyde and imine formation from alcohols under air atmosphere†
Mingyu Guana,
Chao Wanga,
Jingyu Zhang*b and
Yingsheng Zhao*a
aKey Laboratory of Organic Synthesis of Jiangsu Province College of Chemistry, Chemical Engineering and Materials Science Soochow University, Suzhou 215123, P. R. China. E-mail: yszhao@suda.edu.cn
bCollege of Physics, Optoelectronics and Energy & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215006, P. R. China. E-mail: jyzhang@suda.edu.cn
First published on 17th September 2014
Abstract
A highly efficient and practical organic solvent-free Cu(OAc)2/DMAP/TEMPO catalyst system for the selective aerobic oxidation of benzylic and allylic alcohols to aldehydes and phenones under an ambient air atmosphere was reported. A wide range of functional groups such as phenolic hydroxyl, amino, and methylthio are compatible with the catalyst system. The organic solvent-free aerobic oxidative imine synthesis from benzyl alcohol and amines was also achieved via the newly developed Cu(OAc)2/DMAP/TEMPO catalyst. 100 g-scale reactions for aldehyde and imine formation were achieved with over 90% yield using 0.5 mol% catalyst loading in 36 hours, presenting a potential valuable protocol for both economical and environmental considerations.
Introduction
Carbonyl compounds are significant intermediates used in the perfume, drug, dye, pharmaceutical and agrochemical industries. Thus, the development of highly selective catalytic oxidation of alcohols to their corresponding aldehydes or ketones is one of the most fundamental and challenging transformations for organic chemical synthesis.1 A variety of classical reagents such as manganese,2 chromium oxides,3 Swern reagents4 and Dess–Martin reagents5 have been developed for this reaction. However, these oxidation processes, stoichiometric hazardous or toxic oxidizing agents and large amount organic solvents were used. In the points of economic and environmental view, a catalytic method with oxygen as oxidant is quite attractive and important, which only gave water as byproduct. Accordingly, aerobic oxidation of alcohols to corresponding aldehydes or ketones with transition metal-free6 or transition metals7 catalyst system has been well developed over the past decades. Among the reports, the combination of copper salts and TEMPO (2,2,6,6-tetramehyl-1-piperidinyloxy) is one of the best catalytic system for this transformation.8 The first example was discovered by Semmelhack and co-workers in 1984, which was showed to be effective for the oxidation of benzylic and allylic alcohols in DMF.8a A similar catalyst system with 2,2′-bipyridine as a ligand and tBuOK as a base was disclosed by Sheldon and co-workers.9 Recently, Stahl and co-workers reported (bpy)Cu(OTf)/NMI (N-methylimidazole)/TEMPO combination which could selective aerobic of a broad range alcohols, including allylic, benzylic, and aliphatic derivatives, to the corresponding aldehydes in acetonitrile.10 Several nitrogen containing ligands such as fluoroalkyl substituted 2,2′-bipyridine,11 L-proline,12 and diaziridinone,13 N-heterocyclic carbenes (NHCs)14 and 1,10-phenanthroline (phen)15 were well studied. Despite the advantages of these reactions, the use of large amount of solvent or expensive ligands has limited their application in synthetic chemistry. Due to the importance of this transformation in organic chemical synthesis, to further develop environmentally benign and easy scale-up oxidation reactions are extremely important. Herein, we report a high practical organic solvent-free Cu(OAc)2/DMAP (4-dimethylaminopyridine)/TEMPO catalyst system for selective aerobic oxidation of the alcohols to corresponding aldehydes or ketones at room temperature with air as oxidant. Different types of imines can also be synthesized from alcohols and amines in excellent yields under air with the new developed catalyst system. Both the formation of aldehydes and imines from alcohols could be easily scaled up and reduced the catalyst loading to 0.5 mol% (Scheme 1).Inspired by the ligands effect in copper/TEMPO catalyst system for oxidation of alcohols to aldehydes and ketones,8–15 we hypothesized that more efficient alcohol oxidation might be achieved in solvent-free conditions under ambient air atmosphere at room temperature by using a powerful ligand. At the outset of our study, we attempted to synthesize benzyl aldehyde from benzyl alcohol via aerobic oxidation with 1 mol% Cu(OAc)2 and 1 mol% TEMPO under ambient air (open system) at room temperature for 24 hours without solvent. Only a low yield of benzyl aldehyde was observed by GC (Table 1, entry 1). The nitrogen containing ligands played an important role in the aerobic oxidation which had been reported by several groups.8–15
| Entry | Ligand | Conv.b (%) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: benzyl alcohol (1 mmol), Cu(OAc)2 (1 mol%), ligand (1 mol% or 2 mol%), TEMPO (1 mol%), 25 °C solvent-free, open air.b Determined by GC using tridecane as an internal standard.c 1 atm O2.d No Cu(OAc)2 was added. | |||
| 1 | — | 20 | 9 |
| 2 | L2 | 45 | 21 |
| 3 | L3 | 98 | 78 |
| 4 | L4 | 55 | 36 |
| 5 | L5 | 83 | 72 |
| 6 | L6 | 64 | 43 |
| 7 | L7 | 66 | 47 |
| 8 | L8 | 70 | 51 |
| 9 | L9 | 47 | 35 |
| 10 | L10 | 43 | 31 |
| 11 | L1 | 100 | 97 |
| 12c | L1 | 97 | 80 |
| 13d | L1 | 0 | 0 |
 |
|||
We first tested 2,2′-bipyridine under the general reaction condition without base. We were pleased to find the yield increased to 30%, along with 60% of benzyl alcohol recovered. The 1,10-phenanthroline, 1,4-diazabicyclo[2.2.2]octane (DABCO)16 and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)17 gave the yields no higher than 80% respectively (Table 1, entry 3–5). When pyridine derivatives18 were employed as the ligand respectively, around 50% yield of benzyl aldehyde was obtained, accompany with the conversion of alcohol around 60%. However, the L-proline had suppression effect compared to 2,2′-bipyridine. To our delight, the simple and easy accessible DMAP turned out to be the best ligand, and an exciting yield of 97% was obtained. However, only 80% yield was achieved when the reaction carried out under oxygen atmosphere. We also tested other copper salts, such as CuCl, CuBr, CuI, CuCl2, CuBr2, all of them gave similar yields as Cu(OAc)2 (ESI†). Controlling experiments confirmed that no reaction happened without using copper, implicating the crucial role of copper salts for the transformation (Table 1, entry 13).
Once we had identified the optimal conditions for the aerobic oxidation of alcohol to corresponding aldehydes and ketones, the scope and limitation of this reaction were next explored and representative data are shown in Table 2 and 3. To our delight, a remarkable broad substrates scope of benzylic alcohols bearing various functional groups were tolerated. Both electron-rich and electron-deficient alcohols gave the corresponding aldehydes in good to excellent yields (Table 2, entry 1–27). As the melting point of part starting materials were higher than 25 °C, the reactions were tested at a little higher temperature (Tables 2 and 3), and gave good to excellent yields respectively. Notably, 2-furanmethanol, 2-thiophenemethanol and cinnamyl alcohol were all transformed to the corresponding aldehydes in excellent yield at room temperature.
| Entry | Substrate | Product | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: alcohol (1 mmol), Cu(OAc)2 (1 mol%), DMAP (2 mol%), TEMPO (1 mol%), solvent-free, open air, 25 °C.b Isolated yield.c 80 °C.d 100 °C. | ||||
 |
 |
|||
| 1 | X = H | 1a | 24 | 97 |
| 2 | X = p-F | 1b | 24 | 86 |
| 3c | X = o-Cl | 1c | 18 | 76 |
| 4c | X = p-Cl | 1d | 18 | 72 |
| 5c | X = o-Br | 1e | 18 | 88 |
| 6c | X = p-Br | 1f | 18 | 85 |
| 7d | X = o-l | 1g | 18 | 97 |
| 8c | X = p-l | 1h | 18 | 99 |
| 9 | X = m-OMe | 1i | 36 | 80 |
| 10 | X = p-OMe | 1j | 36 | 99 |
| 11 | X = p-tBu | 1k | 36 | 91 |
| 12c | X = o-NO2 | 1l | 24 | 98 |
| 13c | X = m-NO2 | 1m | 18 | 98 |
| 14d | X = p-NO2 | 1n | 18 | 83 |
| 15c | X = p-Me | 1o | 18 | 87 |
| 16 | X = 2,6-F | 2a | 24 | 92 |
| 17c | X = 2-F-6-Cl | 2b | 18 | 95 |
| 18d | X = 3,4-Cl | 2c | 18 | 97 |
| 19 | X = 3,4-OMe | 2d | 36 | 82 |
| 20c | X = 5-Br-2-OMe | 2e | 18 | 97 |
| 21d |  |
 |
18 | 89 |
| 22 | 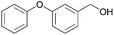 |
 |
24 | 99 |
| 23c |  |
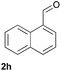 |
18 | 86 |
| 24 |  |
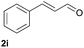 |
24 | 83 |
| 25 |  |
 |
24 | 93 |
| 26 | 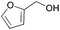 |
 |
36 | 87 |
| Entry | Alcohol | Product | Isolated yield (%) |
|---|---|---|---|
| a Reaction conditions: alcohol (1 mmol), Cu(OAc)2 (5 mol%), DMAP (10 mol%), TEMPO (5 mol%), solvent-free, open air.b Cu(OAc)2 (1 mol%), DMAP (2 mol%), TEMPO (1 mol%), 80 °C, 18 h.c 25 °C, 0.5 mL CH3CN, 24 h.d 25 °C, 0.5 mL CH3CN, 48 h.e 25 °C, 0.5 mL EtOH, 1 atm O2, 48 h.f 60 °C, 48 h.g Determined by 1H NMR analysis. | |||
| 1b |  |
 |
3a, 93 |
| 2c |  |
 |
3b, 94 |
| 3c |  |
 |
3c, 90 |
| 4d |  |
 |
3d, 89 |
| 5d | 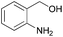 |
 |
3e, 77 |
| 6e |  |
 |
3f, 88 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 62 Z = 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38g 38g |
| 7f |  |
 |
3g, 72 |
| 8b | 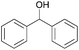 |
 |
3h, 99 |
Highly chemoselective oxidation of a hydroxyl group of molecules which contain an easily oxidized functional group such as phenolic hydroxyl, amino, and methylthio are challenging. With the new developed catalyst system, all these types of alcohols were oxidized to corresponding aldehydes with acetonitrile as solvent in 36 hours (Table 3). The over oxidized product wasn't observed in all the reactions respectively. The geraniol was also delivered the aldehyde in excellent yield at room temperature with ethanol as solvent under oxygen atmosphere. The secondary benzylic alcohols could also be transformed to ketones at a little higher temperature in good yields (Table 3, entry 7 and 8).
Imines derivatives are important versatile synthetic precursors in the synthesis of nitrogen heterocycles, fine chemicals, and pharmaceuticals.19,20 The new developed copper/DMAP/TEMPO catalyst system can also promoted the organic solvent-free imine synthesis from benzyl alcohols and aromatic or aliphatic amines in good excellent yields under air (open system) at 110 °C (Table 4).
| Entry | Alcohol | Aniline | Product | Isolated yield (%) |
|---|---|---|---|---|
| a Reaction conditions: alcohol (1 mmol), aniline (1.2 mmol) Cu(OAc)2 (1 mol%), DMAP (2 mol%), TEMPO (1 mol%), solvent-free, open air, 24 h.b Determined by GC using tridecane as an internal standard. | ||||
| 1 |  |
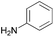 |
 |
4a, 95b |
| 2 |  |
 |
 |
4b, 81 |
| 3 |  |
 |
 |
4c, 89 |
| 4 |  |
 |
 |
4d, 84 |
| 5 |  |
 |
 |
4e, 66 |
| 6 |  |
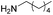 |
 |
4f, 69 |
| 7 |  |
 |
 |
4g, 73 |
Large-scale application of oxidation alcohols are constrained by safety concerns associated with the combination of oxidant, O2 and organic solvents, especially the frequent use of halogenated solvent.21 With the newly developed organic solvent-free and Cu(OAc)2/DMAP/TEMPO catalyst system, a 100 g-scale reactions for aldehyde and imine formation were achieved in higher than 90% yields in 36 hours, and the catalyst loading could even been decreased to 0.5 mol%, which highlights the power of this approach (Scheme 2).
Based on the primary study by our group and others' reports,8b,22 a possible mechanism for the aerobic copper/TEMPO was proposed (Scheme 3). The DMAP coordinated to copper(II) center to form a complex A which might similar to CuBr2 (2,2′-bipyridine)-TEMPO catalyst system which was shown in Scheme 3. The high efficiency imines formation might be attributed to the open system which water could be got rid of from the reaction mixture under 110 °C.
Conclusions
In conclusion, we have developed a highly efficient and practical organic solvent-free Cu(OAc)2/DMAP/TEMPO catalyst system for the selective oxidation of benzylic and allylic alcohols to aldehydes and phenones under ambient air atmosphere. A wide range of substrates which even contained phenolic hydroxyl, amino, and methylthio functional groups could be transformed into corresponding aldehydes with no trace of over oxidized carboxylic acids. The aerobic oxidative imine synthesis from benzyl alcohol and amines were achieved by employing the newly developed catalyst system. 100 g-scale reactions for aldehyde and imine formation were achieved in higher than 90% yield with low catalyst loading in 36 hours. To our best of knowledge, this is the most convenient way to synthesize these kinds of compounds in lab. The more detailed mechanism and synthetic applications of this new developed catalyst system are currently under investigation.Experimental
General
All the chemicals and solvents were obtained from commercial sources and used without further purification. Column chromatography purifications were performed using 300–400 mesh silica gel. Reactions were monitored by thin-layer chromatography (TLC) carried out on 0.2 mm Huanghai silica gel plates (HSGF-254) using UV light as visualizing agent. NMR spectra were recorded on Bruker DRX-400 instruments and calibrated using residual solvent peaks as internal reference.General procedures for synthesis of aldehydes
A mixture of alcohol (1 mmol, 1.0 eq.), Cu(OAc)2 (1.8 mg, 0.01 eq.), DMAP (2.4 mg, 0.02 eq.) and TEMPO (1.5 mg, 0.01 eq.) in a 15 mL glass tube (under air atmosphere) was heated and stirred at 25 °C for 24 hours. The reaction mixture was cooled to rt, and concentrated in vacuo. The resulting residue was purified by column chromatography on silica gel to give the aldehyde.Benzaldehyde(1a): 1H NMR (400 MHz, CDCl3) δ = 10.01 (s, 1H), 7.90–7.84 (m, 2H), 7.65–7.59 (m, 1H), 7.52 (dd, J = 7.9, 7.1 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ = 192.49, 136.47, 134.55, 129.82, 129.08.
General procedures for synthesis of imines
A mixture of alcohol (1 mmol, 1.0 eq.), amine (1.2 mmol, 1.2 eq.), Cu(OAc)2 (1.8 mg, 0.01 eq.), DMAP (2.4 mg, 0.02 eq.) and TEMPO (1.5 mg, 0.01 eq.) in a 15 mL glass tube (under air atmosphere) was heated and stirred at 110 °C for 24 hours. The reaction mixture was cooled to rt, and concentrated in vacuo. The resulting residue was purified by column chromatography on silica gel to give the imine. The silica gel column was leached by eluent (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Et3N = 100
Et3N = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at first.
1) at first.
N-Benzylideneaniline (4a): 1H NMR (400 MHz, CDCl3) δ = 8.45 (s, 1H), 7.91 (dd, J = 6.5, 3.1 Hz, 2H), 7.51–7.44 (m, 3H), 7.40 (dd, J = 10.6, 4.9 Hz, 2H), 7.27–7.17 (m, 3H). 13C NMR (101 MHz, CDCl3) δ = 160.56, 152.20, 136.33, 131.51, 129.28, 128.94, 128.90, 126.07, 120.99.
Procedures for synthesis of benzaldehyde on mole-scale
A mixture of benzyl alcohol (1 mol, 1.0 eq.), Cu(OAc)2 (900 mg, 0.005 eq.), DMAP (1200 mg, 0.01 eq.) and TEMPO (750 mg, 0.005 eq.) in a 250 mL round-bottomed flask (under air atmosphere) was heated at 80 °C for 36 hours. After the reaction was finished, the reaction mixture was extracted with n-pentane (5 × 250 mL). The combined n-pentane phase was concentrated in vacuo. The yield was determined by GC using tridecane as an internal standard and the residue was purified by vacuum distillation to afford benzaldehyde (87% isolated yield).Procedures for synthesis of N-benzylideneaniline on mole-scale
A mixture of benzyl alcohol (1 mol, 1.0 eq.), aniline (1.2 mol, 1.2 eq.), Cu(OAc)2 (900 mg, 0.005 eq.), DMAP (1200 mg, 0.01 eq.) and TEMPO (750 mg, 0.005 eq.) in a 500 mL round-bottomed flask (under air atmosphere) was heated at 110 °C with a decanter for 36 hours before it was quenched by NH4Cl (250 mL, sat. aq.). The layers were separated and the aqueous layer was extracted with EtOAc (3 × 250 mL). The combined ethyl acetate phase was concentrated in vacuo. The yield was determined by GC using tridecane as an internal standard and the residue was purified by recrystallization (ethyl acetate/n-pentane, three times) to afford N-benzylideneaniline (80% isolated yield).Acknowledgements
This research was financially supported by the financial support from the Natural Science Foundation of Jiangsu Province of China (L210903913), and a start-up fund (Q410901212) from Soochow University for support of this work. The PAPD and Qing Lan Project are also gratefully acknowledged.Notes and references
- (a) R. A. Sheldon and J. K. Kochi, Metal-Catalysed Oxidations of Organic Compounds, Academic Press, New York, 1981 Search PubMed; (b) R. C. Larok, Comprehensive organic transformations, John Wiley & Sons, New York, 2nd edn, 1999 Search PubMed.
- (a) J. W. Ladbury and C. F. Cullis, Chem. Rev., 1958, 58, 403 CrossRef CAS; (b) A. J. Fatiadi, Synthesis, 1976, 65 CrossRef CAS; (c) R. J. K. Taylor, M. Reid, J. Foot and S. A. Raw, Acc. Chem. Res., 2005, 38, 851 CrossRef CAS PubMed.
- (a) E. J. Corey and J. W. Suggs, Tetrahedron Lett., 1975, 16, 2647 CrossRef; (b) R. J. K. Taylor, M. Reid, J. Foot and S. A. Raw, Acc. Chem. Res., 2005, 38, 851 CrossRef CAS PubMed.
- (a) K. E. Pfitzner and J. G. Moffatt, J. Am. Chem. Soc., 1963, 85, 3027 Search PubMed; (b) A. J. Mancuso, S. L. Huang and D. Swern, J. Org. Chem., 1978, 43, 2480 CrossRef CAS; (c) T. T. Tidwell, Synthesis, 1990, 857 CrossRef CAS.
- (a) D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 48, 4155 CrossRef CAS; (b) M. Frigerio and M. Santagostino, Tetrahedron Lett., 1994, 35, 8019 CrossRef CAS; (c) Y. Jiang, W. Chen and W. Lu, Tetrahedron, 2013, 69, 3669 CrossRef CAS.
- (a) S. Wertz and A. Studer, Green Chem., 2013, 15, 3116 RSC; (b) M. C. López, G. Royal, C. Philouze, P. Y. Chavant and V. Blandin, Eur. J. Org. Chem., 2014, 4884 CrossRef; (c) R. Prebil, G. Stavber and S. Stavber, Eur. J. Org. Chem., 2014, 395 CrossRef CAS.
- (a) K. Yamaguchi, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2000, 122, 7144 CrossRef CAS; (b) R. A. Sheldon, I. W. C. E. Arends, G. J. T. Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774 CrossRef CAS PubMed; (c) T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037 CrossRef CAS PubMed; (d) S. S. Stahl, Angew. Chem., Int. Ed., 2004, 43, 3400 CrossRef CAS PubMed; (e) D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS PubMed; (f) M. Turner, V. B. Golovko, O. P. H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M. S. Tikhov, B. F. G. Johnson and R. M. Lambert, Nature, 2008, 454, 981 CrossRef CAS PubMed; (g) M. Uyanik and K. Ishihara, Chem. Commun., 2009, 2086 RSC; (h) S. Gowrisankar, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2011, 50, 5139 CrossRef CAS PubMed; (i) C. Parmeggiani and F. Cardona, Green Chem., 2012, 14, 547 RSC; (j) S. Sarina, H. Zhu, E. Jaatinen, Q. Xiao, H. Liu, J. Jia, C. Chen and J. Zhao, J. Am. Chem. Soc., 2013, 135, 5793 CrossRef CAS PubMed; (k) Y. Sasano, S. Nagasawa, M. Yamazaki, M. Shibuya, J. Park and Y. Iwabuchi, Angew. Chem., Int. Ed., 2014, 53, 3236 CrossRef CAS PubMed.
- (a) M. F. Semmelhack, C. R. Schmidt, D. A. Cortes and C. S. Chou, J. Am. Chem. Soc., 1984, 106, 3374 CrossRef CAS; (b) R. Ben-Daniel, P. Alsters and R. Neumann, J. Org. Chem., 2001, 66, 8650 CrossRef CAS PubMed; (c) I. A. Ansari and R. Gree, Org. Lett., 2002, 4, 1507 CrossRef CAS PubMed; (d) N. Jiang and A. J. Ragauskas, Org. Lett., 2005, 7, 3689 CrossRef CAS PubMed; (e) M. Shibuya, M. Tomizawa, I. Suzuki and Y. Iwabuchi, J. Am. Chem. Soc., 2006, 128, 8412 CrossRef CAS PubMed; (f) A. E. Wendlandt, A. M. Suess and S. S. Stahl, Angew. Chem., Int. Ed., 2011, 50, 11062 CrossRef CAS PubMed.
- (a) P. Gamez, I. W. C. E. Arends, J. Reedijk and R. A. Sheldon, Chem. Commun., 2003, 2414 RSC; (b) P. Gamez, I. W. C. E. Arends, R. A. Sheldon and J. Reedijk, Adv. Synth. Catal., 2004, 346, 805 CrossRef CAS.
- J. M. Hoover and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 16901 CrossRef CAS PubMed.
- (a) B. Betzemeier, M. Cavazzini, S. Quici and P. Knochel, Tetrahedron Lett., 2000, 41, 4343 CrossRef CAS; (b) G. Ragagnin, B. Betzemeier, S. Quici and P. Knochel, Tetrahedron, 2002, 58, 3985 CrossRef CAS.
- G. Zhang, X. Han, Y. Luan, Y. Wang, X. Wen and C. Ding, Chem. Commun., 2013, 49, 7908 RSC.
- B. Z. Ying, G. Zhu and Y. Shi, Org. Lett., 2012, 15, 992 Search PubMed.
- (a) J. Olguin, H. Muller-Bunz and M. Albrecht, Chem. Commun., 2014, 50, 3488 RSC; (b) X. Liu, Q. Xia, Y. Zhang, C. Chen and W. Chen, J. Org. Chem., 2013, 78, 8531 CrossRef CAS PubMed; (c) D. R. Jensen, M. J. Schultz, J. A. Mueller and M. S. Sigman, Angew. Chem., Int. Ed., 2003, 42, 3810 CrossRef CAS PubMed.
- (a) I. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown and C. J. Urch, Science, 1996, 274, 2044 CrossRef; (b) I. E. Marko, A. Gautier, R. Dumeunier, K. Doda, F. Philippart, S. M. Brown and C. J. Urch, Angew. Chem., Int. Ed., 2004, 43, 1588 CrossRef CAS PubMed.
- (a) A. J. Ragauskas, Org. Lett., 2005, 7, 3689 CrossRef PubMed; (b) S. Mannam, S. K. Alamsetti and G. Sekar, Adv. Synth. Catal., 2007, 349, 2253 CrossRef CAS.
- Y. Matano and H. Nomura, Angew. Chem., Int. Ed., 2002, 41, 3028 CrossRef CAS.
- (a) T. O. T. Nishimura, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 6750 CrossRef CAS PubMed; (b) B. A. S. Steinhoff and S. S. Stahl, Org. Lett., 2002, 4, 4179 CrossRef CAS PubMed.
- (a) S. F. Martin, Pure Appl. Chem., 2009, 81, 195 CrossRef CAS PubMed; (b) L. Shi, H. M. Ge, S. H. Tan, H. Q. Li, Y. C. Song, H. L. Zhu and R. X. Tan, Eur. J. Med. Chem., 2007, 42, 558 CrossRef CAS PubMed; (c) Z. Y. Liu, Y. M. Wang, Z. R. Li, J. D. Jiang and W. Boykin, Bioorg. Med. Chem. Lett., 2009, 19, 5661 CrossRef CAS PubMed.
- (a) R. W. Layer, Chem. Rev., 1963, 63, 489 CrossRef CAS; (b) J. K. Whitesell, Acc. Chem. Res., 1985, 18, 280 CrossRef CAS; (c) J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984 CrossRef CAS PubMed; (d) W. Tang and X. Zhang, Chem. Rev., 2003, 103, 3029 CrossRef CAS PubMed; (e) H. Groeger, Chem. Rev., 2003, 103, 2795 CrossRef CAS PubMed; (f) A. Cordova, Acc. Chem. Res., 2004, 37, 102 CrossRef CAS PubMed; (g) S. J. Connon, Angew. Chem., Int. Ed., 2006, 45, 3909 CrossRef CAS PubMed; (h) K. I. Yamada and K. Tomioka, Chem. Rev., 2008, 108, 2874 CrossRef CAS PubMed; (i) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (j) J. Wang, X. Liu and X. Feng, Chem. Rev., 2011, 111, 6947 CrossRef CAS PubMed.
- (a) A. Dijksman, A. Marino-González, A. M. I. Payeras, I. W. C. E. Arends and R. A. Sheldon, J. Am. Chem. Soc., 2001, 123, 6826 CrossRef CAS PubMed; (b) M. Shibuya, Y. Osada, Y. Sasano, M. Tomizawa and Y. Iwabuchi, J. Am. Chem. Soc., 2011, 133, 6497 CrossRef CAS PubMed.
- (a) A. J. Ragauskas, Org. Lett., 2005, 7, 3689 CrossRef PubMed; (b) P. J. Figiel, M. Leskelä and T. Repo, Adv. Synth. Catal., 2007, 349, 1173 CrossRef CAS; (c) M. Uyanik and K. Ishihara, Chem. Commun., 2009, 2086 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09851j |
| This journal is © The Royal Society of Chemistry 2014 |