Fabrication of magnetic amino-functionalized nanoparticles for S-arylation of heterocyclic thiols†
Yanhong Liuabc,
Lincheng Zhou*a,
Xinping Hui*a,
Zhenwen Donga,
Hao Zhua,
Yanming Shaoa and
Yanfeng Lia
aCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China. E-mail: zhoulc@lzu.edu.cn; huixp@lzu.edu.cn
bKey Laboratory of Petroleum Resources Research, Institute of Geology and Geophysics, Chinese Academy of Sciences, Lanzhou 730000, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 19th September 2014
Abstract
A series of uniformed mono-disperse magnetic nanoligands (MNLs) (CoFe2O4–NH2 (MNL A), Fe3O4@Si(CH2)3NH2 (MNL B), Fe3O4@Si(CH2)3NHC(O) (CH2)2PEI (MNL C) and Fe3O4@Si(CH2)3NHC(O)PEI (MNL D)) were obtained by loading two ligands, an aminosilane coupling agent and PEI-600, onto magnetic nanoparticles prepared using a solvothermal method. The catalytic applications of the synthesized MNLs were explored for the cross-coupling reaction of heterocyclic thiols with aromatic iodides. The reactions were carried out in the presence of CuI (5 mol%), MNL (10 mol% N) and K2CO3 (1.3 eq.) in DMF at 120 °C. A variety of heterocyclic sulfides were afforded in good to excellent yields (up to 98%) when MNL B was used. The magnetic, crystal, organic matter structure and morphology of MNL B exhibited no obvious changes after five consecutive cycles. XPS characterization of MNL B revealed the combination of a small amount of Cu0 nanoparticles, but this had no significant effect on catalytic performance.
Introduction
Methods for the formation of C–S bonds are important tools in synthetic chemistry, but are comparatively less well understood than C–N or C–O bond formation.1 Couplings can be achieved under harsh reaction conditions, such as high reaction temperature combined with the use of toxic and high boiling polar solvents like quinoline or HMPA. Alternatively, sulfides can be prepared by reducing aryl sulfones or aryl sulfoxides using strong reducing agents such as LiAlH4 and DIBAL-H.2 To overcome these difficulties, considerable attention has been focused on the development of catalytic systems for C–S cross-coupling.3 Organosulfur chemistry has also benefitted significantly from progress associated with catalysis mediated by transition metals, including Pd,4 Ni,5 Cu,6,7 Co,8 Fe,9 In,10 La,11 Au12 and Bi.13 The strong coordination properties of organosulfur compounds are responsible for deactivation of metal catalysts.Nanoscale catalysts offer several advantages over traditional methodologies, including an increase in exposed active surface area for selective binding of substrates, which makes this a more effective process.14 Recently, nanocatalysts have been used to form C–S bonds between aryl halides and thiols. The use of CuO nanoparticles for the catalysis of C–S cross-coupling was first reported in 2007.15 These reactions are effective at 80 °C in DMSO in the presence of KOH under a nitrogen atmosphere without ligands, and CuO is found to be inferior to CuO nanoparticles as catalysts of the C–S coupling reaction. Additionally, Schwab16 et al. reported excellent product yields under ligand-free conditions by combining nanotechnology and ionic liquids to facilitate C–S cross-coupling using CuO nanopowder as a catalyst in [bmmim]+BF4−.
Although these results are promising, the small size of nanoparticles often makes their separation and recycling difficult. In order to circumvent such problems, super-paramagnetic nanocatalysts with high surface area,17 whose flocculation and dispersion can be controlled reversibly by application of a magnetic field, have been employed as a heterogeneous catalyst for C–S cross-coupling reactions. CuFe2O4 nanopowder has been used to catalyze aryl–sulfur bond formation between aryl halides and thiols/disulfides.18 Also, a magnetically recoverable heterogeneous Cu catalyst (nano-FeDOPACu) has been used to synthesize diarylsulfides via a one-pot multicomponent reaction under microwave irradiation.19 Hence, the development of an alternative, inexpensive, air-resistant, and recyclable ligand, for the formation of the C–S bond, is highly desirable. In previous studies, the small molecular ligand 1,10-phenanthroline, had proven to be efficient in a copper(I)-catalyzed C–S cross-coupling reaction.20 Similarly, we report in this study the cross-coupling of heterocyclic thiols with aromatic iodides, catalyzed by CuI and magnetic nanoligands (MNLs) modified by the addition of an aminosilane coupling agent aminopropyl-triethoxysilane (APTES) and polyethylene imine-600 (PEI-600) (Fig. S1†). Additionally, we report a detailed characterization (i.e., magnetic properties, crystal structure, organic structure and morphology) of the nanocomposite, before and after use.
Results and discussion
Characterization of magnetic nanoligands
Fe3O4 MNPs were chosen as catalyst supports based on their high magnetic susceptibility and their capacity for surface functionalization. The preparation of MNLs is presented in Scheme 1. The nitrogen content values for MNLs A, B, C and D were 0.64, 2.71, 2.28, 2.0 mmol g−1, respectively. The smaller size of the Fe3O4 NPs (8 nm) resulted in a larger surface area with higher activity, and the nitrogen content of MNL B may reach as much as 3.8 wt%, which is higher than the previously reported value of 0.61%.21Transmission electron microscope (TEM) was used to characterize the morphology and crystal structure. CoFe2O4 particles are spherical, nearly monodisperse, and have average diameters of approximately 23 ± 3 nm (Fig. 1a and b). TEM images of Fe3O4 MNPs prepared via the thermal decomposition of Fe(acac)3 in benzyl ether and oleylamine, clearly indicate that the 8 nm iron oxide MNPs are rounded and monodisperse (Fig. 1c and d). Additionally, we observed no significant changes in the crystalline structure, size, or morphology of the Fe3O4 MNPs after modification (Fig. 1e and f).
In the XRD pattern of MNLs A, B, C and D (Fig. S2†), the half-peak widths of the ligands remained unchanged, and the NP sizes were consistent with the sizes observed before surface modification. The field dependent magnetization curves of the ligands at room temperature (Fig. S3†) illustrate the importance of the dispersion state of the magnetic nanoparticles. The saturation magnetization (Ms) value for Fe3O4 nanoparticles was 48.9 emu g−1, and Ms value for MNLs A, B, C and D were found to be 70.6 emu g−1, 33.5 emu g−1, 16.3 emu g−1, and 45.3 emu g−1, respectively. The decreasing Ms values were found to correspond with the addition of surface functionalization, although these species could still be separated magnetically. In the IR spectra (Fig. S4†) of the nanoparticles, the appearance of characteristic bands confirmed the existence of the magnetic core and provided evidence of surface amine-functionalization. Also, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks for MNLs C and D were observed at 1711 cm−1 and 1558 cm−1, respectively. It may be that the amino group makes the electron cloud of the C
O peaks for MNLs C and D were observed at 1711 cm−1 and 1558 cm−1, respectively. It may be that the amino group makes the electron cloud of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond move to the oxygen atom, resulting in a decrease in the bond force constant.
O double bond move to the oxygen atom, resulting in a decrease in the bond force constant.
Catalytic studies
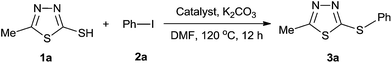
Experiments were performed to identify the appropriate conditions for the coupling reaction of 5-methyl-1,3,4-thiadiazol-2-thiol (1a) with iodobenzene (2a) (1.2 equiv.) using K2CO3 (1.3 equiv.) as a base and DMF as a solvent at 120 °C. The desired product 3a was obtained at 41% yield when 5 mol% CuI was used (Table 1, entry 1), and 5% yield in the presence of 10 mol% MNL A (Table 1, entry 2). The reaction gave 83% yield catalyzed by 5 mol% CuI and 10 mol% MNL A (Table 1, entry 3).Of the four ligands, MNL B was the most effective, due to its ability to form a more active catalytic centre with CuI (Table 2, entries 1–4). MNL A, obtained though one-step synthesis, exhibited the lowest nitrogen content. In comparison to the aliphatic amine of MNL B, the nitrogen content of MNLs C and D included amides, and these ligands showed weak coordination with CuI. So, the catalytic activity of MNLs C and D was relatively low. After investigating the reaction in different solvents (Table 2, entries 5–7), we found that the reaction catalyzed by MNL B failed to yield the desired product in THF or toluene. However, a moderate yield was obtained for MNL B in DMSO. In addition, for various bases (e.g., Cs2CO3, NaOAc, KOH and Na3PO4), the product 3a was obtained in 75–92% yield (Table 2, entries 8–11). Thus, the optimized reaction conditions included CuI (5 mol%), K2CO3 (1.3 equiv.) and MNL B (10 mol%) in DMF at 120 °C for 12 h.
| Entry | MNL | Base | Solvent | Temp. (°C) | Yieldb(%) |
|---|---|---|---|---|---|
a Reaction conditions: MNL/CuI/base/5-methyl-1,3,4-thiadiazol-2-thiol/iodobenzene = 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 (mmol); reaction time: 12 h.b Isolated yield. 1.2 (mmol); reaction time: 12 h.b Isolated yield. |
|||||
| 1 | A | K2CO3 | DMF | 120 | 83 |
| 2 | B | K2CO3 | DMF | 120 | 94 |
| 3 | C | K2CO3 | DMF | 120 | 87 |
| 4 | D | K2CO3 | DMF | 120 | 79 |
| 5 | B | K2CO3 | Toluene | 110 | NR |
| 6 | B | K2CO3 | THF | 66 | NR |
| 7 | B | K2CO3 | DMSO | 120 | 77 |
| 8 | B | Cs2CO3 | DMF | 120 | 75 |
| 9 | B | NaOAc | DMF | 120 | 92 |
| 10 | B | KOH | DMF | 120 | 89 |
| 11 | B | Na3PO4 | DMF | 120 | 84 |
Using the optimized reaction conditions, we extended the scope of this catalytic protocol to the cross-coupling reactions of aryl iodides with heterocyclic thiols (Table 3). The coupling of 5-methyl-1,3,4-thiadiazol-2-thiol with aryl iodides having electron-donating groups resulted in a slightly low yields (Table 3, entries 2–3), however, high yields were obtained in the presence of electron-withdrawing groups (Table 3, entries 4 and 5). For 5-aryl-1,3,4-thiadiazol-2-thiol, both electron-donating and electron-withdrawing groups led to high yields (Table 3, entries 6–12). When 4,5-diphenyl-4H-1,2,4-triazol-3-thiol, 4,6-di-methylpyrimidine-2-thiol and 5-(o-tolyl)-1,3,4-oxadiazol-2-thiol were treated with iodobenzene, the desired products were afforded at 94, 91 and 88% yield, respectively (Table 3, entries 13–15). Furthermore, thiophenols were investigated for this reaction. As expected, the coupling reactions of thiophenols with 5-methyl-1,3,4-thiadiazol-2-thiol proceeded in good yields (Table 3, entries 16–18). The results demonstrated that the catalytic activity of magnetic nano-ligands was slightly lower than that resulting from the 1,10-phenanthroline catalyst,20 although these novel catalysts exhibited good reusability.
Recovery and reusability of MNL B
The reusability of MNL B was also examined (Table 4). MNL B was magnetically separated from the mixture after completion of the reaction, washed with deionized (DI) water, ethanol, ethyl acetate by sonication, and dried at 40 °C in vacuum for direct use in additional catalytic reactions. The results showed that MNL B could be reused at least five times without major loss of activity.To further study the changes of MNL B activity, the morphology and structures of both the freshly synthesized MNL B and MNL B after the 5th run were evaluated. The powder X-ray diffraction analysis, the typical hysteresis loops at room temperature (300 K) and peaks in the IR spectra (Fig. S5†) indicated that the material exhibited good stability under the described reaction conditions. The TEM analysis revealed degradation of both the morphology and the dispersity of the MNL B after the initial successful catalytic cycles (Fig. S6†). We could conclude that the small change resulting from coordination interaction between the ligand and copper during the reaction did not significantly alter catalytic performance.
The XPS technique had been employed to characterize the surface chemical composition of nanoligands. The XPS spectra of freshly synthesized MNL B and MNL B after the 5th run (Fig. S7†) indicated no obvious differences. The high-resolution spectra of Fe 2p, C 1s, N 1s, Si 2p, O 1s and Cu 2p were showed in Fig. S8†. The N 1s signal of freshly synthesized MNL B was observed at 398.3 eV which corresponding to the binding energy (Eb) of aliphatic amine (N–C, N–H) (Fig. S8c†). After the 5th run, this value increased approximately 1.3 eV due to the coordination interaction between copper and the nitrogen atom. The peaks for Cu 2p 1/2 (951.7 eV) and Cu 2p 3/2 (932.4 eV) (Fig. S8f†) were close to the standard data of Cu0, which was produced by reduction of Cu(I). The existence of Cu 2p was consisted with an increase of N 1s binding energy, which was consisted with the formation of Cu0 nanoparticles. This was slightly reduced the catalytic performance of the material as the nitrogen ligands, even though the Cu0 nanoparticles could catalyze the C–S bond formation.19
Conclusions
Four uniformed monodisperse magnetic nano-ligands CoFe2O4–NH2 (MNL A), Fe3O4@Si(CH2)3NH2 (MNL B), Fe3O4@Si(CH2)3NHC(O) (CH2)2PEI (MNL C), Fe3O4@Si(CH2)3NHC(O)PEI (MNL D) were obtained through simple procedures and used as ligands with CuI for C–S bond coupling reactions. MNL B was found to be tolerant to the cross-coupling reaction of heterocyclic thiols with aryl iodides having different functional groups, and a variety of heterocyclic sulfides were obtained in good to excellent yields (up to 98%). The magnetic nature of these ligands allowed for easy and rapid separation of the heterogeneous ligands from the reaction mixture. MNL B continued to exhibit good catalytic activity after five consecutive cycles.Experimental section
General procedure for copper-catalyzed cross-coupling reaction of heterocyclic thiols 1 with aryl iodides 2
Aryl iodides 2 (0.24 mmol) were added to a solution of CuI (0.01 mmol, 1.9 mg), a magnetic nanoligand (0.02 mmol N, 9 mg), heterocyclic thiols 1 (0.2 mmol), and K2CO3 (0.26 mmol, 90 mg) in DMF (1.0 mL). The reaction mixture was heated at 120 °C for 12 h under a nitrogen atmosphere. After cooling to room temperature, ethyl acetate (4 mL) and H2O (4 mL) were added, and the organic phase was separated. The aqueous phase was extracted with ethyl acetate (3 × 4 mL). The combined organic phases were washed with saturated brine and dried over anhydrous Na2SO4. The organic solvent was then concentrated in vacuum to yield the crude products, which was purified by silica gel column chromatography using petroleum ether/ethyl acetate.Recyclability experiments
Recycling reactions were performed using MNL B. After completion of the reaction, MNL B was recovered magnetically and the reaction solution was decanted. MNL B was washed with DI water (2 × 5 mL), ethanol (2 × 5 mL), ethyl acetate (2 × 5 mL), dried at 40 °C under vacuum and reused for the next run. Recycle reactions were performed as described above.Acknowledgements
We are grateful for the financial support of the National Natural Science Foundation of China (21172099 and 21372106).Notes and references
- T. Kondo and T. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed.
- (a) T. Yamamoto and Y. Sekine, Can. J. Chem., 1984, 62, 1544 CrossRef CAS; (b) J. Lindley, Tetrahedron, 1984, 40, 1433 CrossRef CAS; (c) R. J. S. Hickman, B. J. Christie, R. W. Guy and T. White, Aust. J. Chem., 1985, 38, 899 CrossRef CAS; (d) A. V. Bierbeek and M. Gingras, Tetrahedron Lett., 1998, 39, 6283 CrossRef.
- (a) H. J. Cristau, B. Chabaud, A. Chene and H. Christol, Synthesis, 1981, 11, 892 CrossRef; (b) K. Takagi, Chem. Lett., 1987, 16, 2221 CrossRef; (c) G. Mann, D. Baranano, J. F. Hartwig, A. L. Rheingold and I. A. Guzei, J. Am. Chem. Soc., 1998, 120, 9205 CrossRef CAS; (d) C. Millois and P. Diaz, Org. Lett., 2000, 2, 1705 CrossRef CAS PubMed; (e) P. S. Herradura, K. A. Pendola and R. K. Guy, Org. Lett., 2000, 2, 2019 CrossRef CAS PubMed; (f) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2002, 4, 4309 CrossRef CAS PubMed; (g) F. Y. Kwongand and S. L. Buchwald, Org. Lett., 2002, 4, 3517 CrossRef; (h) C. G. Bates, P. Saejueng, M. Q. Doherty and D. Venkataraman, Org. Lett., 2004, 6, 5005 CrossRef CAS PubMed; (i) M. Carril, R. SanMartin, E. Domínguez and I. Tellitu, Chem.–Eur. J., 2007, 13, 5100 CrossRef CAS PubMed; (j) E. Sperotto, G. P. M. van Klink, J. G. de Vries and G. van Koten, J. Org. Chem., 2008, 73, 5625 CrossRef CAS PubMed; (k) Y. Jiang, Y. Qin, S. Xie, X. Zhang, J. Dong and D. Ma, Org. Lett., 2009, 11, 5250 CrossRef CAS PubMed; (l) S. Bhadra, B. Sreedhar and B. Ranu, Adv. Synth. Catal., 2009, 351, 2369 CrossRef CAS; (m) C. K. Chen, Y. W. Chen, C. H. Lin, H. P. Lin and C. F. Lee, Chem. Commun., 2010, 46, 282 RSC; (n) M. Gohain, C. Marais and B. C. B. Bezuidenhoudt, Tetrahedron Lett., 2012, 53, 1048 CrossRef CAS; (o) Z. Q. Ni, S. C. Wang, H. Mao and Y. J. Pan, Tetrahedron Lett., 2012, 53, 3907 CrossRef CAS; (p) P. Mishra, H. K. Maurya, B. Kumar, V. K. Tandon and V. J. Ram, Tetrahedron Lett., 2012, 53, 1056 CrossRef CAS; (q) C. F. Lee, Y. C. Liu and S. S. Badsara, Chem.–Asian J., 2014, 9, 706 CrossRef CAS PubMed; (r) M. M. Sontakke, A. A. Jichkar, M. G. Dhonde, C. S. Bhaskar and B. N. Berad, Synth. Commun., 2014, 44, 340 CrossRef CAS.
- (a) M. J. Dickens, J. P. Gilday, T. J. Mowlem and D. A. Widdowson, Tetrahedron, 1991, 47, 8621 CrossRef CAS; (b) T. Ishiyama, M. Mori, A. Suzuki and N. Miyaura, J. Organomet. Chem., 1996, 525, 225 CrossRef CAS; (c) N. Zheng, J. C. McWilliams, F. J. Fleitz, J. D. Armstrong and R. P. Volante, J. Org. Chem., 1998, 63, 9606 CrossRef CAS; (d) U. Schopfer and A. Schlapbach, Tetrahedron, 2001, 57, 3069 CrossRef CAS; (e) G. Y. Li, G. Zheng and A. F. Noonan, J. Org. Chem., 2001, 66, 8677 CrossRef CAS PubMed; (f) G. Y. Li, Angew. Chem., Int. Ed., 2001, 40, 1513 CrossRef CAS; (g) M. Murata and S. L. Buchwald, Tetrahedron, 2004, 60, 7397 CrossRef CAS; (h) T. Itoh and T. Mase, Org. Lett., 2004, 6, 4587 CrossRef CAS PubMed; (i) C. Mispelaere-Canivet, J. F. Spindler, S. Perrio and P. Beslin, Tetrahedron, 2005, 61, 5253 CrossRef CAS; (j) M. A. Fernández-Rodríguez, Q. Shen and J. F. Hartwig, Chem.–Eur. J., 2006, 12, 7782 CrossRef PubMed; (k) M. A. Fernández-Rodríguez, Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 2180 CrossRef PubMed; (l) R. S. Barbiéri, C. R. Bellato, A. K. C. Dias and A. C. Massabni, Catal. Lett., 2006, 109, 171 CrossRef; (m) Z. Jiang, J. She and X. Lin, Adv. Synth. Catal., 2009, 351, 2558 CrossRef CAS; (n) C. F. Fu, Y. H. Liu, S. M. Peng and S. T. Liu, Tetrahedron, 2010, 66, 2119 CrossRef CAS; (o) Y. Hirai and Y. Uozumi, Chem. Lett., 2011, 40, 934 CrossRef CAS; (p) N. Iranpoor, H. Firouzabadi and A. Rostami, Appl. Organomet. Chem., 2013, 27, 501 CrossRef CAS.
- (a) N. Taniguchi, J. Org. Chem., 2004, 69, 6904 CrossRef CAS PubMed; (b) Y. Zhang, K. N. Ngeow and J. Y. Ying, Org. Lett., 2007, 9, 3495 CrossRef CAS PubMed; (c) A. Saxena, A. Kumar and S. Mozumdar, Appl. Catal., A, 2007, 317, 210 CrossRef CAS; (d) S. Jammi, P. Barua, L. Rout, P. Saha and T. Punniyamurthy, Tetrahedron Lett., 2008, 49, 1484 CrossRef CAS; (e) Y. Q. Cao, Z. Zhang, Y. X. Guo and G. Q. Wu, Synth. Commun., 2008, 38, 1325 CrossRef CAS; (f) P. Guan, C. S. Cao, Y. Liu, Y. F. Li, P. He, Q. Chen, G. Liu and Y. H. Shi, Tetrahedron Lett., 2012, 53, 5987 CrossRef CAS; (g) K. Okamoto, J. B. Housekeeper and C. K. Luscombe, Appl. Organomet. Chem., 2013, 27, 639 CrossRef CAS.
- (a) C. Palomo, M. Oiarbide, R. Lopez and E. Gomez-Bengoa, Tetrahedron Lett., 2000, 41, 1283 CrossRef CAS; (b) C. G. Bates, R. K. Gujadhur and D. Venkataraman, Org. Lett., 2002, 4, 2803 CrossRef CAS PubMed; (c) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS PubMed; (d) Y. J. Wu and H. He, Synlett, 2003, 1789 CrossRef CAS; (e) K. Kauz, U. Schlotz and D. Ganzer, Synlett, 2003, 2428 Search PubMed; (f) W. Deng, Y. Zou, Y. F. Wang, L. Liu and Q. X. Guo, Synlett, 2004, 1254 CAS; (g) I. P. Beletskaya and A. V. Cheprakov, Coord. Chem. Rev., 2004, 248, 2337 CrossRef CAS; (h) G. Zeni, Tetrahedron Lett., 2005, 46, 2647 CrossRef CAS; (i) Y. J. Chen and H. H. Chen, Org. Lett., 2006, 8, 5609 CrossRef CAS PubMed; (j) D. Zhu, L. Xu, F. Wu and B. Wan, Tetrahedron Lett., 2006, 47, 5781 CrossRef CAS; (k) A. K. Verma, J. Singh and R. Chaudhary, Tetrahedron Lett., 2007, 48, 7199 CrossRef CAS; (l) X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863 CrossRef CAS PubMed; (m) B. C. Ranu, A. Saha and R. Jana, Adv. Synth. Catal., 2007, 349, 2690 CrossRef CAS; (n) H. Zhang, W. Cao and D. Ma, Synth. Commun., 2007, 37, 25 CrossRef CAS; (o) H.-J. Xu, X.-y. Zhao, Y. Fu and Y.-S. Feng, Synlett, 2008, 3063 CAS; (p) P. Buranaprasertsuk, J. W. W. Chang, W. Chavasiri and P. W. H. Chan, Tetrahedron Lett., 2008, 49, 2023 CrossRef CAS; (q) H. J. Xu, X. Y. Zhao, J. Deng, Y. Fuand and Y. S. Feng, Tetrahedron Lett., 2009, 50, 434 CrossRef CAS; (r) S. Murru, H. Ghosh, S. K. Sahoo and B. K. Patel, Org. Lett., 2009, 11, 4254 CrossRef CAS PubMed; (s) Y. Feng, X. Zhao, J. Wang, F. Zheng and H. Xu, Chin. J. Chem., 2009, 27, 2423 CrossRef CAS; (t) N. R. Jogdand, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 6092 CrossRef CAS; (u) Y. Feng, H. Wang, F. Sun, Y. Li, X. Fu and K. Jin, Tetrahedron, 2009, 65, 9737 CrossRef CAS; (v) D. J. C. Prasad, A. B. Naidu and G. Sekar, Tetrahedron Lett., 2009, 50, 1411 CrossRef CAS; (w) P.-F. Larsson, A. Correa, M. Carril, P.-O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691 CrossRef CAS PubMed; (x) S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971 CrossRef CAS PubMed; (y) N. Jung and S. Brase, J. Comb. Chem., 2009, 11, 47 CrossRef CAS PubMed; (z) X. Ku, H. Huang, H. L. Jiang and H. Liu, J. Comb. Chem., 2009, 11, 338 CrossRef PubMed.
- (a) J. She, Z. Jiang and Y. Wang, Tetrahedron Lett., 2009, 50, 593 CrossRef CAS; (b) T. Ramana, P. Saha, M. Das and T. Punniyamurthy, Org. Lett., 2010, 12, 84 CrossRef CAS PubMed; (c) M. S. Kabir, M. Lorenz, M. L. Van Linn, O. A. Namjoshi, S. Ara and J. M. Cook, J. Org. Chem., 2010, 75, 3626 CrossRef CAS PubMed; (d) Y.-S. Feng, Y.-Y. Li, L. Tang, W. Wu and H.-J. Xu, Tetrahedron Lett., 2010, 51, 2489 CrossRef CAS; (e) H.-L. Kao and C.-F. Lee, Org. Lett., 2011, 13, 5204 CrossRef CAS PubMed; (f) X. Y. Zhang, X. Y. Zhang and S. R. Guo, J. Sulfur Chem., 2011, 32, 23 CrossRef CAS; (g) Q. Z. Zhou, B. Zhang, T. Q. Du, H. N. Gu, H. J. Jiang and R. N. Chen, Tetrahedron, 2012, 68, 4233 CrossRef CAS; (h) K. Su, Y. T. Qiu, Y. W. Yao, D. Y. Zhang and S. Jiang, Synlett, 2012, 2853 CAS; (i) Y. S. Feng, H. X. Qi, W. C. Wang, Y. F. Liang and H. J. Xu, Tetrahedron Lett., 2012, 53, 2914 CrossRef CAS; (j) R. S. Xu, L. Yue and Y. J. Pan, Tetrahedron, 2012, 68, 5046 CrossRef CAS; (k) J. Mondal, N. Salam and A. Bhaumik, J. Nanosci. Nanotechnol., 2013, 13, 4883 CrossRef CAS PubMed; (l) A. Banerjee, S. K. Santra, S. K. Rout and B. K. Patel, Tetrahedron, 2013, 28, 9096 CrossRef; (m) S. G. Babu and R. Karvembu, Tetrahedron Lett., 2013, 54, 1677 CrossRef CAS; (n) B. Y. Bhong, A. V. Shelke and N. N. Karade, Tetrahedron Lett., 2013, 54, 739 CrossRef CAS.
- Y. C. Wong, T. T. Jayanth and C. H. Cheng, Org. Lett., 2006, 8, 5613 CrossRef CAS PubMed.
- (a) A. Correa, M. Carril and C. Bolm, Angew. Chem., Int. Ed., 2008, 47, 2880 CrossRef CAS PubMed; (b) A. Correa, O. G. Mancheño and C. Bolm, Chem. Soc. Rev., 2008, 37, 1108 RSC; (c) W. Y. Wu, J. C. Wang and F. Y. Tsai, Green Chem., 2009, 11, 326 RSC; (d) H. B. Wang, L. Wang, J. S. Shang, X. Li, H. Y. Wang, J. Gui and A. W. Lei, Chem. Commun., 2012, 48, 76 RSC.
- (a) V. P. Reddy, K. Swapna, A. V. Kumar and K. R. Rao, J. Org. Chem., 2009, 74, 3189 CrossRef CAS PubMed; (b) V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 1697 CrossRef CAS PubMed.
- S. N. Murthy, B. Madhav, V. P. Reddy and Y. V. D. Nageswar, Eur. J. Org. Chem., 2009, 5902 CrossRef CAS.
- (a) M. Jean, J. Renault, P. van de Weghe and N. Asao, Tetrahedron Lett., 2010, 51, 378 CrossRef CAS; (b) Y. M. Xi, B. L. Dong, E. J. McClain, Q. Y. Wang, T. L. Gregg, N. G. Akhmedov, J. L. Petersen and X. D. Shi, Angew. Chem., Int. Ed., 2014, 53, 4657 CrossRef CAS PubMed.
- P. Malik and D. Chakraborty, Appl. Organomet. Chem., 2012, 26, 557 CrossRef CAS.
- (a) V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC; (b) A. M. Balu, B. Baruwati, E. Serrano, J. Cot, J. Garcia-Martinez, R. S. Varma and R. Luque, Green Chem., 2011, 13, 2750 RSC; (c) R. B. Nasir Baig and R. S. Varma, Green Chem., 2012, 14, 625 RSC.
- L. Rout, T. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583 CrossRef CAS PubMed.
- R. S. Schwab, D. Singh, E. E. Alberto, P. Piquini, O. E. D. Rodrigues and A. L. Braga, Catal. Sci. Technol., 2011, 1, 569 CAS.
- (a) S. Shylesh, W. R. Thiel and V. Schünemann, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed; (b) C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412 CrossRef CAS; (c) V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed; (d) R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2013, 49, 752 RSC.
- (a) K. Swapna, S. N. Murthy, M. T. Jyothi and Y. V. D. Nageswar, Org. Biomol. Chem., 2011, 9, 5989 RSC; (b) N. Panda, A. K. Jena and S. Mohapatra, Appl. Catal., A, 2012, 433–434, 258 CrossRef CAS.
- R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2012, 48, 2582 RSC.
- L.-F. Niu, Y. Cai, C. Liang, X.-P. Hui and P.-F. Xu, Tetrahedron, 2011, 67, 2878 CrossRef CAS.
- Z. C. Xu, C. M. Shen, Y. L. Hou, H. J. Gao and S. H. Sun, Chem. Mater., 2009, 21, 1778 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08782h |
| This journal is © The Royal Society of Chemistry 2014 |