Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: study of entrapment properties and in situ generation of gel–gold nanoparticle hybrid material†‡
Braja Gopal Bag* and
Rakhi Majumdar
Department of Chemistry and Chemical Technology, Vidyasagar University, Midnapore 72102, West Bengal, India. E-mail: braja@mail.vidyasagar.ac.in
First published on 14th October 2014
Abstract
A natural pentacyclic triterpenoid arjunolic acid self-assembled hierarchically in aqueous solvents yielding vesicular structures of nano to micrometer diameters affording gels. This self-assembly has been utilized for the entrapment and controlled release of anticancer drugs such as doxorubicin at physiological pH. A gel–gold nanoparticle hybrid material has also been prepared by in situ generated gold nanoparticles at room temperature.
1. Introduction
Spontaneous self-assembly of molecules in liquids yielding supramolecular structures of nano- to micro-meter dimensions such as vesicles, fibers, spheres, tubules, etc. has become an area of intense research in recent years for an improved understanding of different supramolecular architectures and because of their many potential and realized technological applications.1–4 Vesicular self-assemblies in aqueous medium are of special significance due to their range of applications in the areas of controlled-release drug delivery systems, medical implants, tissue engineering, etc.5,6 Additionally, the development of gel–nanoparticle composite materials consisting of supramolecular gels and metal nanoparticles has drawn special attention in recent years because of their applications in biomedicine, organic–inorganic advanced materials, etc.7 A literature study reveals that there are several examples of low molecular weight gelators (LMWGs), often obtained by multistep chemical synthesis, that are capable of gelling aqueous solvent mixtures.3,8 But, vesicular self-assemblies of molecules from renewable resources in aqueous solvent mixtures are rare.9Plant metabolites having diversified molecular frameworks and functional groups offer newer opportunities for the study of their self-assembly because they are available in renewable supply without extensive synthetic effort.10–12 Triterpenoids, the 30-carbon subset of the major plant secondary metabolite terpenoids, are attractive candidates because of their rather rigid structures, with lengths exceeding one nm.13–15 The nano-sized building blocks with several centres of chirality complimented by several hydroxyl and carboxyl groups at different positions and orientations make them significant for the study of their self-assemblies even without derivatizations.16–19 The structure of the triterpenoid framework, the number and the kind of functional groups and their spatial dispositions may have profound effect on the self-assembly property of a triterpenoid yielding different morphologies.16 Hence, to investigate the structure property relationships during self-assembly of the triterpenoids with minute structural variation and functional group dispositions, it occurred to us that arjunolic acid will be a suitable molecule for such investigations. Arjunolic acid 1 is a nano-sized, 6-6-6-6-6 pentacyclic triterpenoid having three hydroxyl and one carboxyl group at opposite ends of the rigid triterpenoid backbone. The compound is extractable from the heavy wood of Terminalia arjuna (T. arjuna) as the free acid20,21 having medicinal importance.22 Herein we report the self assembly properties of arjunolic acid 1 in different liquids (Fig. 1). The triterpenic acid preferentially formed spherical self-assemblies in aqueous solvents at low concentrations which were shown to be vesicular in nature by electron and atomic force microscopy and dye entrapment studies.4,5 Various fluorophores such as 5,6 carboxyfluorescein (CF), rhodamine B and the anticancer drug doxorubicin could be entrapped inside the vesicular self-assemblies in aqueous solvent mixtures and controlled release of the entrapped drug molecules carried out at physiological pH indicated their usefulness as drug delivery vehicle. We have also demonstrated that the bark extract of T. arjuna rich in antioxidants having medicinal significance as a cardiac tonic,23,24 forms a gel with arjunolic acid in alcohol–water mixture. By utilizing this phenomenon, a gel–gold nanoparticle hybrid material could be synthesized at room temperature by in situ generated gold nanoparticles under very mild conditions for possible futuristic applications in the fields of nanobiotechnology, biosensors, biomedicine, etc.7
2. Results and discussion
2.1 Study of self-assembly properties
Arjunolic acid 1 was extracted from the heavy wood powder of T. arjuna and purified by a chemical route, developed in our laboratory, as a white crystalline solid.20 As anticipated, the compound having a rigid lypophilic backbone with three hydroxyl and one carboxyl groups at the opposite ends, was poorly soluble in most of the common organic liquids except in polar solvents like DMSO, DMF, acetonitrile, etc. Interestingly, in aqueous ethanol, DMSO and DMF, compound 1 showed a tendency to self-assemble yielding spherical self-assemblies and gels at certain concentrations of 1 and solvent compositions (Table 1 and TS1–TS4, ESI‡). On treatment of a dilute ethanol solution of arjunolic acid (0.15 mL, 0.5% w/v) contained in a vial (capacity 4 mL, 1 cm id) with water (0.2 mL), turbidity appeared almost instantly. Interestingly, the clear solution obtained by heating the turbid mixture, transformed into a transparent soft solid-like material when allowed to cool at room temperature within ten minutes. The material did not flow by turning the vial upside down (Fig. 1) and the supramolecular gel3 thus obtained transformed thermo-reversibly into a clear solution at 50 °C. Even on lowering the concentration of arjunolic acid, excellent gels were obtained and the gel to sol transition temperature Tgel was 34 °C at its minimum gelator concentration MGC (2 mM) rendering it as an excellent gelator of ethanol–water mixture at a ratio of many well known alcoholic drinks25 (Fig. S1b, ESI‡). With increasing concentration of the solute, the Tgels increased when tested with the same solvent mixture. Strong gels were also obtained in aqueous DMSO and DMF. With increasing ratio of water, the gels became stronger in DMSO–water as evident from the increase in Tgel values at the same concentration of 1 (Tables 1 and TS3, ESI‡). The positive free energy change (ΔG° values) during gel to sol transition calculated from the Tgel vs. concentration plots (Table TS5 and Fig. S1c, ESI‡) indicated the stability of the gels.21,26| Serial no | Medium (v/v) | Statea | MGC | Tgelb |
|---|---|---|---|---|
| a G = gel, VS = viscous suspension. Concentrations are in % w/v.b Gel to sol transition temperatures (Tgel) are provided at minimum gelator concentration MGC. | ||||
| 1 | EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
G | 0.11 | 34 |
| 2 | DMSO–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
G | 7.1 | 67 |
| 3 | DMF–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
G | 7.1 | 35 |
| 4 | EG–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
VS | 2.5 | — |
2.2 Morphological characteristics of the self-assemblies
Morphologies of the self-assemblies were studied by optical microscopy, scanning electron microscopy (SEM), transmission electron microscopy (TEM), dynamic light scattering (DLS), X-ray diffraction studies, etc. Optical microscopy of self-assembled arjunolic acid in aqueous ethanol, DMSO, DMF, and acetonitrile–DMSO mixture indicated the appearance of spherical aggregates of 3–5 μm diameters (Fig. 2 and S2, ESI‡). Whereas discrete spherical objects were observed at lower concentrations of 1 (below MGC, Fig. 2a and c), densely packed assemblies of spherical objects were observed at a higher concentration (above MGC, Fig. 2b and d). DLS studies carried out with a dilute ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mixture of arjunolic acid (0.043% w/v) revealed the average size of the spherical objects as 135 nm with 4% of the spherical objects having diameter in the micrometer range (Fig. S3d, ESI‡). Nano to micro-sized self-assemblies of arjunolic acid were also observed by DLS studies in DMSO–water mixture. The DLS studies unravelled that the micro-sized spherical objects observed by optical microscopy were only a small fraction of the total self-assemblies of nano- to micro-meter dimensions due to size limitation of the technique used. To overcome this limitation, we used different electron microscopy and atomic force microscopy techniques to thoroughly investigate the morphologies of the self-assemblies. Indeed SEM studies carried out with the dried self-assemblies of 1 from different aqueous solvent mixtures indicated the formation of nano-sized spherical objects along with the micro-sized spherical objects (Fig. 3 and S4, ESI‡). The average diameter of the dried self-assemblies of 1 in ethanol–water at its MGC (0.11% w/v, 3
4) mixture of arjunolic acid (0.043% w/v) revealed the average size of the spherical objects as 135 nm with 4% of the spherical objects having diameter in the micrometer range (Fig. S3d, ESI‡). Nano to micro-sized self-assemblies of arjunolic acid were also observed by DLS studies in DMSO–water mixture. The DLS studies unravelled that the micro-sized spherical objects observed by optical microscopy were only a small fraction of the total self-assemblies of nano- to micro-meter dimensions due to size limitation of the technique used. To overcome this limitation, we used different electron microscopy and atomic force microscopy techniques to thoroughly investigate the morphologies of the self-assemblies. Indeed SEM studies carried out with the dried self-assemblies of 1 from different aqueous solvent mixtures indicated the formation of nano-sized spherical objects along with the micro-sized spherical objects (Fig. 3 and S4, ESI‡). The average diameter of the dried self-assemblies of 1 in ethanol–water at its MGC (0.11% w/v, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was 185 nm (calculated from 200 spherical objects). Porous nature of the spherical objects were also obvious form the SEM images (Fig. 3a and b). Similarly, nano- to micro-sized spherical objects were observed in the dried self-assemblies of 1 from DMSO–water and DMF–water mixtures (5
4) was 185 nm (calculated from 200 spherical objects). Porous nature of the spherical objects were also obvious form the SEM images (Fig. 3a and b). Similarly, nano- to micro-sized spherical objects were observed in the dried self-assemblies of 1 from DMSO–water and DMF–water mixtures (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 0.71% w/v). Formation of interconnected spherical aggregates from the densely packed spherical objects were observed in SEM especially at higher concentration of the solute (Fig. 3).
2, 0.71% w/v). Formation of interconnected spherical aggregates from the densely packed spherical objects were observed in SEM especially at higher concentration of the solute (Fig. 3).
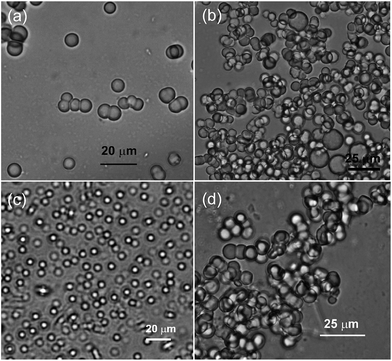 | ||
Fig. 2 Optical microscopy images of self-assembled arjunolic acid in (a and b) DMSO–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) at (a) 5.55% w/v, (b) 7.1% w/v; (c and d) DMF–water (5 4) at (a) 5.55% w/v, (b) 7.1% w/v; (c and d) DMF–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at (c) 0.10% w/v (d) at 6.25% w/v. 2) at (c) 0.10% w/v (d) at 6.25% w/v. | ||
Atomic force microscopy of the dried self-assemblies of 1 in DMF–water (1.0% w/v) also revealed the formation of soft-natured spherical objects of 40–50 nm diameters and heights of 6–8 nm diameters (Fig. S3a–c, ESI‡) which are normally observed for vesicular self-assemblies. The measured heights did not match with the radius of the spherical objects due to deformation by the AFM tip.4
Transmission electron microscopy of the dried self-assemblies of 1 in aqueous ethanol, DMF and DMSO revealed that the spherical objects were vesicular in nature (Fig. 4a, b and S5, ESI‡) having a sharp contrast between the centre and the distinct periphery. The vesicles were robust enough to retain their spherical shapes under the condition of TEM experiments. With the membrane thickness of 2.7 nm and length of arjunolic acid being 1.35 nm (Fig. S1a, ESI‡), a bilayer membrane structure can be proposed (Fig. 4c).21 The bilayer membrane structure is also supported by low angle X-ray diffraction studies carried out with a gel sample of 1 in DMF–water (7.1% w/v, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) that revealed a peak at 3.82° corresponding to a d-spacing of 2.68 nm (Fig. S6b, ESI‡).
2 v/v) that revealed a peak at 3.82° corresponding to a d-spacing of 2.68 nm (Fig. S6b, ESI‡).
Observation of discrete vesicular self-assemblies at a lower concentration and densely packed vesicular self-assemblies at a higher concentration of 1 by optical microscopy in a liquid (native state) and identical observation by SEM in the xero-gels of 1 both at lower and higher concentrations (discussed previously) prompted us to propose a mechanism for the formation of gel via vesicular self-assemblies (Fig. 4c). The viscosity of the solutions increased with increasing concentration of 1 due to immobilization of the liquid by the interconnected and densely packed vesicles yielding a gel above its MGC. The observation that the alkyl chained esters of 1 having three free hydroxyl groups at one end of the triterpenoid yield gels in different liquids via the formation of fibrillar networks21b and some ketals of 1 having the free carboxyl group on the other end of the triterpenoid yield gels via the formation of bilayer vesicles in different liquids21a indicate the importance of the free carboxyl group in the formation of bilayered vesicular structures both in the ketals as well as the free acid. Formation of gels via vesicular self-assemblies, though not very common, has been reported by us previously on a monohydroxy triterpenoid and by others on synthetic supramolecular systems.4,9,27,28 The ‘C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O’ stretching frequency appearing at 1708 cm−1 for the powder sample of arjunolic acid shifted to 1696, 1696, 1693 cm−1 in the xero-gel samples obtained from DMSO–water (5
O’ stretching frequency appearing at 1708 cm−1 for the powder sample of arjunolic acid shifted to 1696, 1696, 1693 cm−1 in the xero-gel samples obtained from DMSO–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), ethanol–water (3
3), ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) and DMF–water (5
4) and DMF–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) respectively showing a shift of 12–15 cm−1 compared to that in the powder (Fig. S7, ESI‡). This observation indicated that the carboxyl groups were in a highly H-bonded environment in the xero-gels. The ‘O–H’ peak in the FTIR of the powder sample of 1 became broader and shifted to lower frequency in all the xero-gel samples indicating that the ‘O–H’ groups were also highly H-bonded in the xero-gel samples.
2) respectively showing a shift of 12–15 cm−1 compared to that in the powder (Fig. S7, ESI‡). This observation indicated that the carboxyl groups were in a highly H-bonded environment in the xero-gels. The ‘O–H’ peak in the FTIR of the powder sample of 1 became broader and shifted to lower frequency in all the xero-gel samples indicating that the ‘O–H’ groups were also highly H-bonded in the xero-gel samples.
2.3 Study of entrapment of fluorophores including anticancer drug doxorubicin
To examine whether the vesicular self-assemblies of arjunolic acid are capable of entrapping various guest molecules inside,29,30 entrapment studies were carried out with a cationic fluorophore rhodamine B and an anionic fluorophore CF. Interestingly, both the fluorophores were entrapped inside the vesicular self-assemblies of compound 1 (Fig. 5). For example, a hot solution of arjunolic acid (4.38 mM) in ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mixture containing rhodamine B (2.5 × 10−3 mM) was cooled at room temperature and their epifluorescence was examined. Observation of reddish fluorescence from the spherical objects indicated the entrapment of the fluorophore inside the vesicles (Fig. S8, ESI‡). In DMSO–water mixture also the entrapment of rhodamine B inside the vesicular self-assemblies of arjunolic acid was observed (Fig. 5a–c). Similarly, the anionic fluorophore CF (0.25 mM) was entrapped inside the vesicular self-assemblies of arjunolic acid (63.9 mM) in DMSO–water as observed by greenish fluorescence from the vesicular self-assemblies (Fig. 5d–f) under epifluorescence microscopy. To verify the entrapment of fluorophores inside the vesicular self-assemblies, we treated the rhodamine B (3.57 × 10−3 mM) entrapped spherical self-assemblies of arjunolic acid (73.1 mM) with a small amount of triton X-100 (1.1 × 10−3 mM). Lysis of the spherical self-assemblies confirmed their vesicular nature (Fig. S9, ESI‡).31
4) mixture containing rhodamine B (2.5 × 10−3 mM) was cooled at room temperature and their epifluorescence was examined. Observation of reddish fluorescence from the spherical objects indicated the entrapment of the fluorophore inside the vesicles (Fig. S8, ESI‡). In DMSO–water mixture also the entrapment of rhodamine B inside the vesicular self-assemblies of arjunolic acid was observed (Fig. 5a–c). Similarly, the anionic fluorophore CF (0.25 mM) was entrapped inside the vesicular self-assemblies of arjunolic acid (63.9 mM) in DMSO–water as observed by greenish fluorescence from the vesicular self-assemblies (Fig. 5d–f) under epifluorescence microscopy. To verify the entrapment of fluorophores inside the vesicular self-assemblies, we treated the rhodamine B (3.57 × 10−3 mM) entrapped spherical self-assemblies of arjunolic acid (73.1 mM) with a small amount of triton X-100 (1.1 × 10−3 mM). Lysis of the spherical self-assemblies confirmed their vesicular nature (Fig. S9, ESI‡).31
There has been an increasing research interest in recent years in the utilization of vesicles as drug delivery vehicle.5a,32 Inspired by the entrapment abilities of the vesicular self-assemblies of 1, we examined their entrapment abilities with the well known anticancer drug doxorubicin. A hot solution of 1 (1.29 mM) in DMSO–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) containing doxorubicin (4.04 × 10−3 mM) was cooled at room temperature and the sample was examined under epifluorescence microscopy. Reddish fluorescence observed from the vesicular self-assemblies indicated the entrapment of the chemotherapeutic drug (Fig. 5g–i). Fluorescence quenching due to entrapment of doxorubicin inside the vesicles and partial release of the entrapped drug molecules by sonication also confirmed its entrapment inside the vesicles (Fig. S10, ESI‡).
3) containing doxorubicin (4.04 × 10−3 mM) was cooled at room temperature and the sample was examined under epifluorescence microscopy. Reddish fluorescence observed from the vesicular self-assemblies indicated the entrapment of the chemotherapeutic drug (Fig. 5g–i). Fluorescence quenching due to entrapment of doxorubicin inside the vesicles and partial release of the entrapped drug molecules by sonication also confirmed its entrapment inside the vesicles (Fig. S10, ESI‡).
2.4 Release study of the entrapped anticancer drug doxorubicin at physiological pH
Study of the release of the entrapped drug molecules to buffer solutions at physiological pH is an integral part of drug entrapment studies for the prospective use of the self-assemblies as drug delivery vehicle.5 To examine this, the anticancer drug doxorubicin (0.31 mM) loaded gels of arjunolic acid (4.26 mM) in ethanol–water were covered with 1 mL of PBS buffer (10 mmol, pH 7.2 and 6.6) and their release were monitored by UV-visible spectroscopy at various time intervals (Fig. S11, ESI‡). Indeed, slow release of the loaded doxorubicin drug from the gel to the buffer solutions was observed making it useful as a prospective drug delivery vehicle.2.5 In situ generation of gel–gold nanoparticle hybrid material
The development of gel–gold nanoparticle hybrid material is an emerging area of research due to its wide range of applications in the areas of nanobiotechnology, biomedicine, biosensors, etc.7 Many of such applications require the synthesis and stabilization of gold nanoparticles (AuNPs) from non-toxic, biomolecules (via the reduction of Au(III) to Au(0)) under very mild reaction conditions. The bark extract of T. arjuna (BETA), a well known cardiac tonic is rich in polyphenolic compounds including various types of antioxidants,24 and the polyphenolic compounds can be utilized for the very mild synthesis and in situ stabilization of gold nanoparticles.33 Hence, it occurred to us that it can be utilized for the synthesis of a gel–gold nanoparticle hybrid material. Initial investigations on whether a gel can be prepared from a mixture of 1 and BETA, an ethanol solution of 1 (0.5% w/v) was mixed with the aqueous BETA (0.006% w/v), maintaining the ratio of alcohol to water as (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Indeed, a transparent gel was obtained within 30 min. Inspired by this observation, a dilute ethanol solution of arjunolic acid (0.15 mL, 0.5% w/v) contained in a vial was treated with an aqueous solution of BETA (0.2 mL, 0.006% w/v) and Au(III) solution (0.008 mL, 10.42 mM) at room temperature. Appearance of reddish violet color within 5–10 minutes indicated the formation of gold nanoparticles. Interestingly, a reddish violet colored hybrid-gel containing gold-nanoparticles was formed within 1 hour at room temperature (Fig. 6a). A characteristic Surface Plasmon Resonance (SPR) band at 533 nm confirmed the formation of the AuNPs (Fig. 6b). The gel–gold nanoparticle hybrid material was stable at room temperature for several months under sealed condition and the Tgel values increased with increasing concentration of the solutes. Moreover, in the case of the gel–nanoparticle hybrid material, the Tgel values were higher compared to the composite gel containing BETA (0.006% w/v) as well as the alcohol–water gel (Fig. S1c, ESI‡). This is perhaps due to the extra stability provided by the stabilized gold nanoparticles in the hybrid material.34 Densely packed and interconnected spherical objects were observed by optical microscopy and SEM (Fig. S2e, f and S4b, c, ESI‡). HRTEM images of samples prepared by drop-casting of a diluted sample of the hybrid material (1.1 mM) showed the presence of gold nanoparticles along with vesicular self-assemblies (Fig. 6d–h). The wide angle X-ray diffraction of a xero-gel sample from the gel–gold nanoparticle hybrid material showed the presence of crystalline gold nanoparticles with characteristic reflections of the planes (111), (200), (220) and (311) at 2θ = 44.69°, 52.11°, 76.84° and 93.17° respectively (Fig. 6c). The data for the gold nanoparticle are in agreement with the reported standards JCPDS file no. 04-0784. Additionally, the diffraction peaks for the xero-gel sample of 1 obtained in aqueous ethanol (Fig. S6a, ESI‡) were also present in the xero-gel of the hybrid material. Selected area electron diffraction (SAED) image (Fig. 6i) and energy disperse X-ray (EDX) spectra (Fig. 6j) also confirmed the formation of AuNPs.
4). Indeed, a transparent gel was obtained within 30 min. Inspired by this observation, a dilute ethanol solution of arjunolic acid (0.15 mL, 0.5% w/v) contained in a vial was treated with an aqueous solution of BETA (0.2 mL, 0.006% w/v) and Au(III) solution (0.008 mL, 10.42 mM) at room temperature. Appearance of reddish violet color within 5–10 minutes indicated the formation of gold nanoparticles. Interestingly, a reddish violet colored hybrid-gel containing gold-nanoparticles was formed within 1 hour at room temperature (Fig. 6a). A characteristic Surface Plasmon Resonance (SPR) band at 533 nm confirmed the formation of the AuNPs (Fig. 6b). The gel–gold nanoparticle hybrid material was stable at room temperature for several months under sealed condition and the Tgel values increased with increasing concentration of the solutes. Moreover, in the case of the gel–nanoparticle hybrid material, the Tgel values were higher compared to the composite gel containing BETA (0.006% w/v) as well as the alcohol–water gel (Fig. S1c, ESI‡). This is perhaps due to the extra stability provided by the stabilized gold nanoparticles in the hybrid material.34 Densely packed and interconnected spherical objects were observed by optical microscopy and SEM (Fig. S2e, f and S4b, c, ESI‡). HRTEM images of samples prepared by drop-casting of a diluted sample of the hybrid material (1.1 mM) showed the presence of gold nanoparticles along with vesicular self-assemblies (Fig. 6d–h). The wide angle X-ray diffraction of a xero-gel sample from the gel–gold nanoparticle hybrid material showed the presence of crystalline gold nanoparticles with characteristic reflections of the planes (111), (200), (220) and (311) at 2θ = 44.69°, 52.11°, 76.84° and 93.17° respectively (Fig. 6c). The data for the gold nanoparticle are in agreement with the reported standards JCPDS file no. 04-0784. Additionally, the diffraction peaks for the xero-gel sample of 1 obtained in aqueous ethanol (Fig. S6a, ESI‡) were also present in the xero-gel of the hybrid material. Selected area electron diffraction (SAED) image (Fig. 6i) and energy disperse X-ray (EDX) spectra (Fig. 6j) also confirmed the formation of AuNPs.
3. Conclusions
In conclusion, we have reported the formation of vesicular self-assemblies of a natural triterpenoid arjunolic acid in aqueous media yielding supramolecular gels in most of the liquids studied. According to our knowledge, this is the first report of the vesicular self-assemblies of a trihydroxy triterpenic acid without any synthetic modification. The vesicular self-assemblies have been utilized for the entrapment and controlled release of different fluorophores including anticancer drug doxorubicin (at physiological pH), demonstrating its usefulness as a prospective drug delivery vehicle. Interestingly, arjunolic acid formed gel in alcohol–water mixture at a ratio of many well known alcoholic drinks. Formation of a composite gel of an ethanol solution of arjunolic acid with the aqueous bark extract of T. arjuna (rich in antioxidants) has also been demonstrated by us. By utilizing this phenomenon, preparation of a gel–gold nanoparticle hybrid material has been demonstrated via green synthesis of gold-nanoparticles providing opportunities for the development of organic–inorganic advanced materials, biosensors, etc. some of which are ongoing in our laboratory and will be reported in due course.4. Experimental
4.1 Materials and methods
Arjunolic acid 1 was extracted from the heavy wood of T. arjuna and purified by following a method developed in our laboratory.20 HAuCl4 was purchased from SRL (Sisco Research Laboratory) and used without further purification. Synthesis of gold nanoparticles utilizing the bark extract of T. arjuna and HAuCl4 has been carried out by following a procedure reported by us previously.33 The gel to sol transition temperatures Tgel were recorded by gradual heating of a gel sample contained in a vial (capacity 4 mL, 1 cm i.d.) until the material started to flow when observed by tilting the vial. All the commercial grade solvents were purified by distillation before use.4.2 Characterization
TEM images of samples prepared by drop-casting over Formvar coated Cu-grid were recorded in JEOL JEM-2100 instrument. Dried self-assemblies of the samples on glass plates were coated in a sputter coater with gold for 120 s and then recorded in a Zeiss FESEM. Optical microscopy was carried out using a Nikon Ecliplse LV100POL instrument with fluorescence attachment. X-ray diffraction (XRD) patterns of the stabilized AuNPs were recorded in Panalytical X'pert Pro diffractometer with Co-Kα radiation (λ = 1.789 Å). UV-Visible spectra were recorded in Shimadzu 1601 spectrophotometer. FTIR spectra of samples were recorded in Perkin Elmer FTIR Spectrum-II model using KBr pellet.4.3 Study of self-assembly properties
Arjunolic acid (5 mg) was solubilised in ethanol (1 mL) by heating with continuous stirring and the clear solution (0.5% w/v) thus obtained was allowed to cool at room temperature. Aliquots of 0.025, 0.050, 0.075, 0.100, 0.125, 0.150, 0.175, 0.200 mL were added to eight vials containing 0.2 mL of water in each of them at room temperature. All the mixtures were heated over a hot plate for 2–3 min with magnetic stirring and the clear solutions thus obtained were kept at room temperature. Cloudiness appeared in all the vials within 30 minutes. Self-assemblies were observed by optical microscopy. In 5th and 6th vials containing 0.19 and 0.21% w/v of 1 respectively in ethanol–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 and 6
8 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v respectively), formation of a soft solid-like material was observed. As the materials did not flow by turning the vials upside down, we called these as gels (Table TS1, ESI‡). Similar studies were performed in methanol–water system (Table TS2, ESI‡). In DMSO–water and DMF–water mixtures, the concentration of 1 was kept constant and the solvent ratio was varied (Tables TS3 and TS4, ESI‡). The increase in Tgel values were observed with increasing percentage of water in DMSO–water system.
8 v/v respectively), formation of a soft solid-like material was observed. As the materials did not flow by turning the vials upside down, we called these as gels (Table TS1, ESI‡). Similar studies were performed in methanol–water system (Table TS2, ESI‡). In DMSO–water and DMF–water mixtures, the concentration of 1 was kept constant and the solvent ratio was varied (Tables TS3 and TS4, ESI‡). The increase in Tgel values were observed with increasing percentage of water in DMSO–water system.
4.4 Lyses of entrapped vesicles with Triton-X
To verify the entrapment of fluorophores inside the vesicular self-assemblies, we treated the rhodamine B (3.57 × 10−3 mM) entrapped spherical self-assemblies of arjunolic acid (73.1 mM) with a small amount of triton X-100 (1.1 × 10−3 mM). The vesicles became larger in size within 20 min and appeared as black spot with a highly reddish fluorescent background. Disappearance of the vesicular self-assemblies was observed within a couple of hours (Fig. S9, ESI‡). Triton-X-100 is known to disrupt the vesicular structures by damaging the vesicular membranes.31 Our observation of the disruption of the spherical self-assemblies confirms their vesicular structures.4.5 Entrapment studies with doxorubicin
To examine whether the well known anticancer drug doxorubicin can be entrapped in the vesicular self-assemblies of arjunolic acid, we prepared a hot solution of 1 (1.29 mM) in DMSO–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) containing doxorubicin (4.04 × 10−3 mM) and cooled at room temperature. Entrapment of doxorubicin inside the vesicles were observed by epifluorescence microscopy (Fig. 5g–i). Reddish fluorescence was observed form the spherical self-assemblies of 1 confirmed the entrapment of doxorubicin. Similarly, entrapment studies with rhodamine-B and CF were also carried out (Fig. 5a–f).
3) containing doxorubicin (4.04 × 10−3 mM) and cooled at room temperature. Entrapment of doxorubicin inside the vesicles were observed by epifluorescence microscopy (Fig. 5g–i). Reddish fluorescence was observed form the spherical self-assemblies of 1 confirmed the entrapment of doxorubicin. Similarly, entrapment studies with rhodamine-B and CF were also carried out (Fig. 5a–f).
4.6 Preparation of doxorubicin loaded gel and release study of the entrapped drug molecules
Crystalline arjunolic acid (5 mg) contained in a vial was dissolved in ethanol (1 mL) by heating with magnetic stirring to prepare a stock solution (0.5% w/v). An aliquot of the freshly prepared ethanol solution of arjunolic acid (0.15 mL) contained in a vial was mixed with distilled water (0.17 mL) and an aqueous solution of doxorubicin hydrochloride (0.03 mL, 3.68 mM) maintaining the alcohol–water ratio as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The mixture was heated over a hot plate with stirring for 1 min and then the dark orange colored solution was allowed to cool at room temperature. No gravitational flow of the material observed by turning the vial upside down indicated the formation of a gel. The gel was stable for several weeks at room temperature under sealed condition. For the release study of gel entrapped doxorubicin to PBS buffer at pH 6.6 and 7.2, two sets of the above drug loaded gels contained in vials were covered with 1 mL each of PBS buffers at pH 6.6 and 7.2. The buffer solutions (0.8 mL) were removed carefully and UV-visible spectra were recorded at certain time intervals. After each spectroscopic measurement, the buffer solutions were returned to the respective vials. Release studies carried out for 18–19 hours indicated slow release of the entrapped doxorubicin to the buffer solutions (Fig. S11, ESI‡). Whereas 51% of the loaded drug was released at pH 6.6 buffer in 19 h at room temperature, 63% of the loaded drug was released at pH 7.2. The color of the gel faded with time during the course of our release studies.
4. The mixture was heated over a hot plate with stirring for 1 min and then the dark orange colored solution was allowed to cool at room temperature. No gravitational flow of the material observed by turning the vial upside down indicated the formation of a gel. The gel was stable for several weeks at room temperature under sealed condition. For the release study of gel entrapped doxorubicin to PBS buffer at pH 6.6 and 7.2, two sets of the above drug loaded gels contained in vials were covered with 1 mL each of PBS buffers at pH 6.6 and 7.2. The buffer solutions (0.8 mL) were removed carefully and UV-visible spectra were recorded at certain time intervals. After each spectroscopic measurement, the buffer solutions were returned to the respective vials. Release studies carried out for 18–19 hours indicated slow release of the entrapped doxorubicin to the buffer solutions (Fig. S11, ESI‡). Whereas 51% of the loaded drug was released at pH 6.6 buffer in 19 h at room temperature, 63% of the loaded drug was released at pH 7.2. The color of the gel faded with time during the course of our release studies.
4.7 Preparation of a composite gel of arjunolic acid and T. arjuna bark extract
An aliquot of freshly prepared ethanol solution of arjunolic acid (0.5% w/v) contained in a vial (0.30 mL) was mixed with an aqueous bark extract of T. arjuna (0.4 mL, 0.006% w/v) maintaining the alcohol–water ratio as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The turbid mixture thus obtained was heated over a hot plate with magnetic stirring to obtain a clear solution and it was allowed to cool at room temperature. A transparent gel was obtained within 30 min as observed by turning the vial upside down.
4. The turbid mixture thus obtained was heated over a hot plate with magnetic stirring to obtain a clear solution and it was allowed to cool at room temperature. A transparent gel was obtained within 30 min as observed by turning the vial upside down.
4.8 Preparation of gel–gold nanoparticle hybrid material
A turbid mixture of freshly prepared ethanol solution of arjunolic acid (0.5% w/v, 0.30 mL) and the bark extract of T. arjuna (0.4 mL) contained in a vial was heated over a hot plate with magnetic stirring to obtain a clear solution. The solution was allowed to cool at room temperature for 2 min, an aliquot of Au(III) solution (0.016 mL, 10.42 mM) was added and the mixture was stirred at room temperature for 30 seconds. The resulting solution was kept at room temperature. Appearance of pinkish-red color appeared within 5 min indicating the formation of stabilized gold nanoparticles. A transparent reddish violet colored hybrid-gel containing gold-nanoparticles was formed within 1 hour at room temperature (Fig. 6a) as observed by turning the vial upside down.Acknowledgements
We thank CSIR (02(0068)/12/EMR-II), UGC-SAP and DST-FIST New Delhi, for financial support. RM thanks the UGC, New Delhi, for a research fellowship.Notes and references
- (a) A. Sorrenti, O. Illa and R. M. Ortuno, Chem. Soc. Rev., 2013, 42, 8200 RSC; (b) C. A. E. Hauser and S. Zhang, Nature, 2010, 468, 516 CrossRef CAS PubMed; (c) N. Amdursky, M. Molotskii, E. Gazit and G. Rosenman, J. Am. Chem. Soc., 2010, 132, 15632 CrossRef CAS PubMed.
- (a) E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098 RSC; (b) H.-B. Yao, H.-Y. Fang, X.-H. Wang and S.-H. Yu, Chem. Soc. Rev., 2011, 40, 3764 RSC; (c) M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Marzán, ACS Nano, 2010, 4, 3591 CrossRef CAS PubMed.
- (a) E. Carretti, M. Bonini, L. Dei, B. H. Berrie, L. V. Angelova, P. Baglioni and R. G. Weiss, Acc. Chem. Res., 2010, 43, 751 CrossRef CAS PubMed; (b) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed; (c) M. Suzuki and K. Hanabusa, Chem. Soc. Rev., 2009, 38, 967 RSC; (d) M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489 CrossRef CAS PubMed; (e) R. G. Weiss and P. Terech, in Molecular Gels: Materials with Self-Assembled Fibrillar Networks, Springer, Dordrecht, 2006 Search PubMed.
- (a) A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644 CrossRef CAS PubMed; (b) A. Ajayaghosh, R. Varghese, S. Mahesh and V. K. Praveen, Angew. Chem., 2006, 118, 7893 (Angew. Chem., Int. Ed., 2006, 45, 7729) CrossRef; (c) T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401 CrossRef CAS PubMed; (d) A. Ajayaghosh, R. Varghese, S. Mahesh and V. K. Praveen, Angew. Chem., 2006, 118, 3339 (Angew. Chem., Int. Ed., 2006, 45, 3261) CrossRef.
- (a) P. Moitra, K. Kumar, P. Kondaiah and S. Bhattacharya, Angew. Chem., Int. Ed., 2014, 53, 1113 CrossRef CAS PubMed; (b) S. K. Misra, P. Kondaiah, S. Bhattacharya and C. N. R. Rao, Small, 2012, 8, 131 CrossRef CAS PubMed; (c) S. K. Misra, P. Moitra, B. S. Chhikara, P. Kondaiah and S. Bhattacharya, J. Mater. Chem., 2012, 22, 7985 RSC.
- (a) A. Friggeri, B. L. Feringa and J. van Esch, J. Controlled Release, 2004, 97, 241 CrossRef CAS PubMed; (b) Z. Yang, G. Liang, L. Wang and B. Xu, J. Am. Chem. Soc., 2006, 128, 3038 CrossRef CAS PubMed; (c) Z. Yang, H. W. Gu, D. G. Fu, P. Gao, K. J. K. Lam and B. Xu, Adv. Mater., 2004, 16, 1440 CrossRef CAS; (d) Z. Yang and B. Xu, Chem. Commun., 2004, 2424 RSC; (e) K. J. C. van Bommel, M. C. A. Stuart, B. L. Feringa and J. van Esch, Org. Biomol. Chem., 2005, 3, 2917 RSC.
- (a) D. Das, T. Kar and P. K. Das, Soft Matter, 2012, 8, 2348 RSC; (b) P. Koley and A. Pramanik, Adv. Funct. Mater., 2011, 21, 4126 CrossRef CAS.
- (a) S. Dutta, T. Kar, D. Mandal and P. K. Das, Langmuir, 2013, 29, 316 CrossRef CAS PubMed; (b) P. K. Vemula, J. Li and G. John, J. Am. Chem. Soc., 2006, 128, 8932 CrossRef CAS PubMed; (c) P. K. Vemula and G. John, Chem. Commun., 2006, 2218 RSC; (d) N. Sreenivasachary and J.-M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 5938 CrossRef CAS PubMed.
- (a) B. G. Bag and K. Paul, Asian J. Org. Chem., 2012, 1, 150 CrossRef CAS; (b) M. Delample, F. Jérôme, J. Barrault and J.-P. Douliez, Green Chem., 2011, 13, 64 RSC; (c) B. Novales, L. Navailles, M. Axelos, F. Nallet and J.-P. Douliez, Langmuir, 2008, 24, 62 CrossRef CAS PubMed.
- (a) P. K. Vemula and G. John, Acc. Chem. Res., 2008, 41, 769 CrossRef CAS PubMed; (b) G. John, B. V. Shankar, S. R. Jadhav and P. K. Vemula, Langmuir, 2010, 26, 17843 CrossRef CAS PubMed; (c) P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed.
- S. Grassi, E. Carretti, L. Dei, C. W. Branham, B. Kahr and R. G. Weiss, New J. Chem., 2011, 35, 445 RSC.
- (a) N. Baccile, F. Babonneau, J. Jestin, G. Pehau-Arnaudet and I. Van Bogaert, ACS Nano, 2012, 6, 4763 CrossRef CAS PubMed; (b) N. Baccile, N. Nassif, L. Malfatti, I. N. A. Van Bogaert, W. Soetaert, G. Pehau-Arnaudet and F. Babonneau, Green Chem., 2010, 12, 1564 RSC; (c) S. Zhou, C. Xu, J. Wang, W. Gao, R. Khverdiyeva, V. Shah and R. Gross, Langmuir, 2004, 20, 7926 CrossRef CAS PubMed.
- (a) B. G. Bag, C. Garai, R. Majumdar and M. Laguerre, Struct. Chem., 2012, 23, 393 CrossRef CAS; (b) R. Xu, G. C. Fazio and S. P. T. Matsuda, Phytochemistry, 2004, 65, 261 CrossRef CAS PubMed.
- (a) A. Eschenmoser, L. Ruzicka, O. Jeger and D. Arigoni, Helv. Chim. Acta, 1955, 38, 1890 CrossRef CAS; (b) A. Eschenmoser and D. Arigoni, Helv. Chim. Acta, 2005, 88, 3011 CrossRef CAS.
- (a) D. J. Reinert, G. Balliano and G. E. Schulz, Chem. Biol., 2004, 11, 121 CrossRef CAS PubMed; (b) T. Hoshino, S.-i. Nakano, T. Kondo, T. Sato and A. Miyoshi, Org. Biomol. Chem., 2004, 2, 1456 RSC.
- (a) B. G. Bag and R. Majumdar, RSC Adv., 2012, 2, 8623 RSC; (b) B. G. Bag and S. S. Dash, Nanoscale, 2011, 3, 4564 RSC.
- (a) L. Smentek and B. A. Hess Jr, J. Am. Chem. Soc., 2010, 132, 17111 CrossRef CAS PubMed; (b) B. A. Hess Jr and L. Smentek, Org. Lett., 2004, 6, 1717 CrossRef PubMed.
- (a) K. U. Wendt, G. E. Schulz, E. J. Corey and D. R. Liu, Angew. Chem., Int. Ed., 2000, 39, 2812 CrossRef CAS; (b) K. U. Wendt, Angew. Chem., Int. Ed., 2005, 44, 3966 CrossRef CAS PubMed.
- (a) S. Lodeiro, Q. Xiong, W. K. Wilson, M. D. Kolesnikova, C. S. Onak and S. P. T. Matsuda, J. Am. Chem. Soc., 2007, 129, 11213 CrossRef CAS PubMed; (b) H. Mitsuguchi, Y. Seshime, I. Fujii, M. Shibuya, Y. Ebizuka and T. Kushiro, J. Am. Chem. Soc., 2009, 131, 6402 CrossRef CAS PubMed.
- B. G. Bag, P. P. Dey, S. K. Dinda, W. S. Sheldrick and I. M. Oppel, Beilstein J. Org. Chem., 2008, 4, 24 Search PubMed.
- (a) B. G. Bag, R. Majumdar, S. K. Dinda, P. P. Dey, G. C. Maity, V. Ajay Mallia and R. G. Weiss, Langmuir, 2013, 29, 1766 CrossRef CAS PubMed; (b) B. G. Bag, S. K. Dinda, P. P. Dey, V. A. Mallia and R. G. Weiss, Langmuir, 2009, 25, 8663 CrossRef CAS PubMed; (c) B. G. Bag and S. K. Dinda, Pure Appl. Chem., 2007, 79, 2031 CrossRef CAS.
- (a) J. Ghosh and P. C. Sil, Biochimie, 2013, 95, 1098 CrossRef CAS PubMed; (b) T. Hemalath, S. Pulavendran, C. Balachandran, B. M. Manohar and R. Puvanakrishnan, Indian J. Exp. Biol., 2010, 48, 238 Search PubMed.
- P. D. Lokhande, S. C. Jagdale and A. R. Chabukswar, Indian Journal of Traditional Knowledge, 2006, 5, 420 Search PubMed.
- S. Jain, P. P. Yadav, V. Gill, N. Vasuda and N. Singla, Phytochem. Rev., 2009, 8, 491 CrossRef CAS.
- Many of the alcoholic drinks contain 42–43% alcohol. Arjunolic acid forms gel with alcohol–water mixture containing 42–43% alcohol making it as a versatile gelator of such alcoholic drinks. Indeed a branded whisky sample could be gelled using an alcoholic solution of arjunolic acid (0.14% w/v, Fig. S1b, ESI‡).
- D. Rizkov, J. Gun, O. Lev, R. Sicsic and A. Melman, Langmuir, 2005, 21, 12130 CrossRef CAS PubMed.
- N. S. Saleesh Kumar, S. Varghese, G. Narayan and S. Das, Angew. Chem., Int. Ed., 2006, 45, 6317–6321 CrossRef PubMed.
- T. Rehm, V. Stepanenko, X. Zhang, F. Wulrthner, F. Gro1hn, K. Klein and C. Schmuck, Org. Lett., 2008, 10, 1469 CrossRef CAS PubMed.
- Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542 CrossRef CAS PubMed.
- D.-S. Guo, K. Wang, Y. Wang and Y. Liu, J. Am. Chem. Soc., 2012, 134, 10244 CrossRef CAS PubMed.
- (a) S. Bhattacharya and J. Biswas, Langmuir, 2011, 27, 1581 CrossRef CAS PubMed; (b) C. Guo, S. Liu, C. Jiang, W. Li and Z. Dai, Langmuir, 2009, 25, 13114 CrossRef CAS PubMed.
- B. Tian, X. Tao, T. Ren, Y. Weng, X. Lin, Y. Zhang and X. Tang, J. Mater. Chem., 2012, 22, 17404 RSC.
- R. Majumdar and B. G. Bag, Int. J. Res. Chem. Environ., 2012, 2, 338 CAS.
- P. K. Vemula, U. Aslam, V. Ajay Mallia and G. John, Chem. Mater., 2007, 19, 138 CrossRef CAS.
Footnotes |
| † This paper is dedicated to Professor Guenter von Kiedrowski on his 61st birthday. |
| ‡ Electronic supplementary Information (ESI) available: Energy minimized structure, Tgel profile, AFM, additional SEM, TEM, OM images, XRD, FTIR, thermodynamic calculations. See DOI: 10.1039/c4ra08710k |
| This journal is © The Royal Society of Chemistry 2014 |





