Distorted ring-banded spherulites in poly(L-lactic acid)/poly(ε-caprolactone) blends†
Febryan Harmansyaha,
Eamor M. Wooa,
Li-Ting Lee*b and
Huei-Ru Chienb
aDepartment of Chemical Engineering, National Cheng Kung University, Tainan, 701-01, Taiwan
bDepartment of Materials Science and Engineering, Feng Chia University, Taichung, 40724, Taiwan. E-mail: ltlee@fcu.edu.tw; Fax: +886 4 24510014; Tel: +886 4 24517250 ext. 5306
First published on 25th September 2014
Abstract
Unique distorted ring bands with wrinkles and concentric structures in blends of poly(L-lactic acid) (PLLA)/poly(ε-caprolactone) (PCL) were obtained by two-step isothermal crystallization and investigated by using polarized optical microscopy (POM) and atomic-force microscopy (AFM). Peculiar morphologies were revealed while the blends were crystallized by two-step crystallization with a fixed first crystallization temperature of 117 °C and different second crystallization temperatures. From the microscopy results, the morphology as the alternating ridges and valleys of wrinkle bands along with concentric ring bands can be resolved. The PLLA crystals could result in the structures of wrinkle bands and small concentric ring bands at the first crystallization temperature. Furthermore, formation of the alternating ridges and valleys of wrinkle bands could be suggested to be attributed to the different amounts of PCL inserted into the pre-formed crystals of PLLA while crystallizing the blends under a lower second crystallization temperature with fast crystallization of PCL. On the other hand at a higher second crystallization temperature, the wrinkle band changed to a branch-like band. PCL chains with high mobility could be sufficiently diffused and uniformly inserted into the pre-formed crystals of PLLA to generate more perfect crystals of PCL at the higher second crystallization temperature. The wrinkle bands with a significant ridge and valley structure vanished by changing the second crystallization to a higher one.
Introduction
Biodegradable polymers have attracted increasing interest owing to their potential application in fields such as biomedicine, agriculture, and packaging.1–8 Many researchers believe that biodegradable polymer materials will reduce the need for synthetic polymer production (thus reducing pollution) at a low cost. New environmentally friendly high-performance materials have been reported.9 Aliphatic polyester derivatives, such as D, L- and L-lactic acid, β-hydroxybutyrate, ethylene oxide, glycolic acid, and ε-caprolactone, are considered as biodegradable polymers.Poly(ε-caprolactone) (PCL) is another biodegradable polymer with a low melting point of around 60 °C and a glass transition temperature (Tg) of around −60 °C. The polymer is widely used as an additive for resins to improve their processing characteristics and end use properties (e.g., impact resistance). Polymer blends of PLLA and PCL have been extensively investigated, and PLLA/PCL is a typical immiscible blend of biocompatible polymers.10–14 In spite of the miscibility studies, crystallization investigations on the PLLA/PCL blends should not be ignored. Discussions and investigations on crystallization behaviors and morphologies would also provide useful information for future applications of the PLLA/PCL blends. Recently, many researchers have extensively investigated the preferential nucleation of crystals near the phase interface in immiscible polymer blends. In early reports, Bartczak et al.15 investigated the crystallization of isotactic polypropylene (iPP) in the presence of amorphous atactic polystyrene (aPS) and reported the preferential nucleation of iPP at the surface of the aPS phase by polarized optical microscopy (POM) observation. They suggested that the enhancement effect is caused by a decrease of the surface free energy in the formation of crystal nuclei due to the presence of the phase interface. Wenig et al.16 investigated the crystallization kinetics of isotactic polypropylene (iPP)/ethylene-propylene-diene terpolymer (EPDM) blends. The nuclei density of iPP increased by blending both crystalline and amorphous EDPMs. Avrami analysis of the isothermal crystallization revealed that the crystallization kinetics changed from a homogeneous nucleation system in neat iPP to a heterogeneous system in the iPP/EDPM blend, indicating that the nucleation of iPP was enhanced heterogeneously at the surface of dispersed EDPM domains. The enhanced nucleation induced by interfacial interaction in immiscible blends has also been found by Shao et al.17 for ultrahigh-molecular-weight polyethylene (UHMWPE)/iPP blends. Tsuburaya et al.18 reported that nucleation occurred simultaneously with liquid–liquid phase separation (LLPS) in polycarbonate (PC)/poly(ethylene oxide) (PEO) blend systems. Han et al.19–21 also investigated the relation between nucleation and LLPS in the poly(ethylene-co-butene) (PEB)/poly(ethylene-co-hexene) (PEH) blend system and claimed that the nuclei mainly resided near the interface of the phases, as determined from atomic-force microscopy (AFM) observations. The authors argued that the diffusion flux associated with the phase separation induced a specific orientation of polymer chains, thereby resulting in nucleation. Computational simulation of nucleation in a polymer blend was also investigated by Han et al.20 undergoing LLPS. The results indicated the generation of nuclei with high probability near the phase interface. Sakai et al.22 investigated PLLA/PCL blends with various compositions prepared via the solution casting method. They focused on the effect of chain mobility on nucleation behavior in the immiscible polymer blend. The authors suggested that the presence of PCL activates PLLA chain mobility. Dense nuclei were generated at lower temperature, probably even below Tg of PLLA. They explained that the limited miscibility of PLLA/PCL leads to the aggregation of PCL and depresses Tg locally and deeply at the interface of PCL domains, resulting in strong enhancement of PLLA nucleation.22
In this study, the crystallization behaviors and morphologies of PLLA/PCL blends were investigated. To obtain novel information for the crystallization behaviors of PLLA/PCL blends, “two-step crystallization” was applied to treat the thin-film samples prepared by spin casting. Unique morphologies of crystallization which would be different with the original one of neat PLLA or that of PCL were expected to acquire. Detail discussions on the crystallization morphologies of PLLA/PCL blends were also performed. By analyzing the results of POM and AFM in depth, suggested mechanism for the crystal growth in PLLA/PCL blends under two-step crystallization was proposed. Understanding the crystallization behaviors and morphologies of fully biodegradable PLLA/PCL blends would be very useful for their future end-use relating to advanced applications of biochemistry, bioscience and biomaterial.1–9
Experimental
Materials and blend preparation
Poly(L-lactic acid) (PLLA) supplied by Polysciences Inc. was used in this study. The molecular weight and the PDI value of PLLA (indicated by GPC) are 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and 1.1, respectively. Poly(ε-caprolactone) (PCL) was purchased from Aldrich. It is with a molecular weight of 20
000 g mol−1 and 1.1, respectively. Poly(ε-caprolactone) (PCL) was purchased from Aldrich. It is with a molecular weight of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a PDI value of 2.5.
000 g mol−1 and a PDI value of 2.5.
PLLA/PCL blends with compositions of 100/0, 90/10, 80/20, 20/80, 10/90, and 0/100 were prepared by spin casting using chloroform (CHCl3) as the common solvent with a concentration of 2 wt%. A drop of the polymer solution was deposited on a micro glass slide and spun at 3500 rpm for 40 s to obtain an ultrathin film (thickness: 1 μm). The blends were then treated with two-step isothermal crystallization. First, the samples were heated on a hot stage to the maximum melt temperature (Tmax = 190 °C) and held for 3 min to remove prior crystals or thermal histories. The samples were then rapidly moved to another hot stage that was pre-set at the designated isothermal temperature of Tc1 = 108 to 127 °C until PLLA fully crystallized (around 2–6 h), and then the temperature was reduced to Tc2 = room temperature to 47 °C until PCL fully crystallized.
Apparatus and procedures
Results and discussion
Crystal morphology of PLLA/PCL blends
Before the discussion of the crystallization morphology and the structure, the miscibility between PLLA and PCL were also preliminary investigated by DSC and OM. Immiscible state rather than miscible state could be suggested for the blends of PLLA and PCL. The data of non-isothermal crystallization also presents two non-isothermal crystallization peaks for the PLLA/PCL blends. This result could also suggest the state of immiscibility for the PLLA/PCL blends. Relative information and discussions for preliminary understanding the miscibility and the crystallization behavior by DSC and OM have been included as the “ESI†” of the present work. To explore the crystal morphology, PLLA/PCL blend samples were prepared with spin coating at 3500 rpm for 40 s prior to thermal treatment with two-step isothermal crystallization as shown in Fig. 1 (left) for sample compositions of 100/0, 90/10, 80/20, 20/80, 10/90, and 0/100. The neat PLLA and neat PCL samples were treated with single-step melt and isothermal crystallization, as shown in Fig. 1 (right), with 90 °C (for neat PCL) and 190 °C (for neat PLLA) pre-set as the maximum temperature, to remove the thermal history or prior crystals, held for 3 min. The samples were then rapidly cooled to the crystallization temperature (Tc = 117 °C for neat PLLA; Tc = 30 °C for neat PCL). | ||
| Fig. 1 Scheme for crystallization step. Two-step crystallization for PLLA/PCL blends (left) and single-step crystallization for neat PLLA and PCL (right). | ||
Fig. 2 shows POM micrographs of melt-crystallized PLLA/PCL blends (with various compositions) under two-step or single-step crystallization. It should note that for two-step crystallization, first crystallization temperature (Tc1) was set as 117 °C and second crystallization temperature (Tc2) was set as 30 °C. In general, spherulites with periodic or rhythmic ring-band structures could be obtained in neat polymer or in polymer blends under crystallization.23,24 In this study, neat PLLA (PLLA/PCL = 100/0) exhibits concentric ring-band spherulites with a hexagonal shape in their center. It also has a non-birefringence form as shown in Fig. 2(a). Nurkhamidah et al.25 also found similar morphology for neat PLLA in their study. They reported that neat PLLA could present non-birefringence spherulite in thinner film prepared by spin casting. In addition, the morphology of concentric ring band was also revealed for neat PLLA under melt crystallization.25
Fig. 2(b)–(e) shows POM micrographs of melt-crystallized PLLA/PCL blends (with various compositions) under two-step crystallization. Non-birefringence with a round shape in the center appears for PLLA/PCL = 90/10. Negative-sign spherulites can be seen for PLLA/PCL = 80/20. Theoretically, negative-sign spherulites are assigned when the first and third quadrants are blue and the second and fourth quadrants are orange. These two samples have small dark voids or domains that are well-distributed over the surface of the sample. This indicates that the two polymers are phase-separated. The voids or domains are increased in size with increasing PCL content. Similar results were reported by Can et al.26 in the literature. It suggested that PLLA and PCL have different solubility parameters and thus they tend to demix from each other during solvent evaporation and coalesce in separate domains to reduce the interfacial area. Dell'Erba et al.27 also showed that the blends of PLLA and PCL are immiscible. Furthermore, they suggested that the presence of PCL would enhance the nucleation rate of crystallization for PLLA in the blends. Although PLLA and PCL are phase-separated, the homogenous dispersion of the minor PCL phase as shown in the image of PLLA/PCL = 90/10 blend could imply that there is a certain extent of interaction between two polymers.
Fig. 2(d) and (e), as represented for a major PCL content, exhibited a wrinkle band pattern in samples of PLLA/PCL = 20/80 and 10/90. The term “wrinkle band” is named for an irregular pattern of ring band with highly twisted peripherals of the rings. They have disordered pattern for alternating ridge and valley. The wrinkle band formed at the crystallization temperature of PLLA (Tc = 117 °C) and negative-sign spherulites formed at the PCL crystallization temperature (Tc = 30 °C). The wrinkle band separately forms in different places on the sample. Both the crystallization temperature and blend composition affect the morphology of wrinkle band patterns. Phase separation clearly appeared with increasing PCL content, reflecting the increasing size of voids or domains with increasing PCL content. Thus, PCL-rich compositions having a small proportion of PLLA linked with PCL. The wrinkle band formation mechanism is explained below. Fig. 2(f) shows that neat PCL has negative-sign ringless spherulites.
Fig. 3 shows POM micrographs of PLLA/PCL = 20/80 blend under step-crystallization with various first crystallization temperatures (from 108 °C to 125 °C) and a fixed second crystallization temperature of 30 °C. The first step crystallization encountered disordered pattern for wrinkle band with non-birefringence spherulites. It shows various forms of morphology for generated pattern by crystallizing the sample at different first crystallization temperatures. That is, wrinkle band generated at different first crystallization temperatures does not have any fixed form as observed by POM.
Fig. 4 shows POM micrographs for PLLA/PCL = 20/80 blend under step-crystallization with a fixed first crystallization temperature of 117 °C and second crystallization temperatures of 30 °C, 42 °C, and 47 °C. As can be seen, the crystal morphology changed with second crystallization temperature. Ringless negative-sign spherulites appeared for lower second crystallization temperatures along with wrinkle bands, which formed at the first crystallization temperature. The morphology of wrinkle band does not have any fixed pattern which is indicated from first crystallization temperature images. They all have a different pattern under same condition and composition blends. Further explanations are given below to elaborate the formation of wrinkle band as shown by POM images. In addition, ringless positive-sign spherulites appeared for higher second crystallization temperature with branch-like bands replacing wrinkle bands as formed at lower second crystallization temperature.
Wrinkle pattern with small concentric ring-band spherulites in PCL-rich PLLA/PCL blends
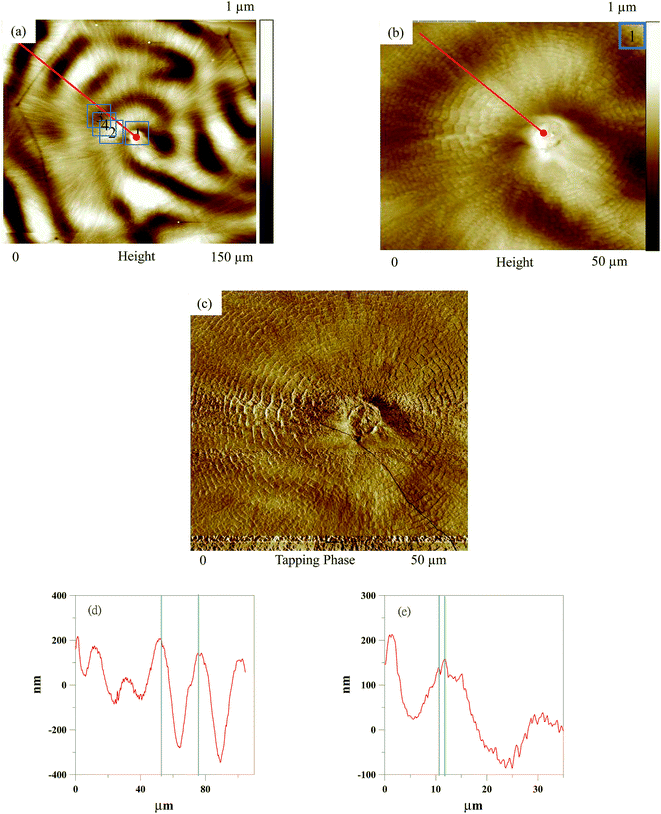
Fig. 5 presents the overall view of high-resolution crystal morphology of the PLLA/PCL = 20/80 blend. Fig. 5(a) shows a height image with a 150 μm magnitude. Large wrinkle bands with an irregular alternating ridge and valley bands appear. The average relative height of the wrinkle bands is about 170 nm and the band spacing is about 20 μm. Fig. 5(b) and (c) show a height and phase image for a 50 μm magnitude, respectively. In Fig. 5(b) and (c) with high resolution images, small concentric ring band are also resolved. Fig. 5(d) shows the height profile of Fig. 5(a) with the magnitude as 150 μm. Fig. 5(e) shows the height profile of Fig. 5(b) with the magnitude as 50 μm. The band spacing of small concentric ring bands is about 1.2 μm, which is higher than that of neat PLLA (6–7 nm).22 Average value estimated for the relative height of the small concentric ring bands is about 23 nm. In previous studies,28–30 concentric ring-banded spherulites were also obtained from ultrathin films (100–300 nm) either by melt crystallization or solvent-induced crystallization. The formation of wrinkle bands in PLLA/PCL blends might be associated with the difference in surface tension or total surface free energy (γS). Hirotsu et al.31 and Yasin et al.32 have characterized the γS of PLLA and that of PCL by the polar component of the surface free energy (γpS) and the dispersive component of the surface free energy (γDS). Estimated value of γS is close to 34 mJ m−2 for PLLA and 48 mJ m−2 for PCL. In general, it would suggest that the low-surface-tension component would migrate to the surface of the film.33 PLA has a lower surface tension than that of PCL and thus migrated to the surface of the film. In other words, PCL was being rejected in the tangential direction by PLA. Fig. 5 clearly shows a wrinkle pattern along with small concentric ring-banded spherulites in AFM images.The detailed lamellar pattern on the surface of small concentric ring-band spherulites was also observed using AFM. Images of spot regions including ridges, valleys, and the interface between ridge and valley regions of wrinkle bands are displayed in Fig. 6–8, respectively.
Fig. 6 shows AFM images and a scheme of lamellar arrangement and growth direction for the ridge of wrinkle bands (the area indicated as “2” in Fig. 5) in PLLA/PCL = 20/80 blend as crystallized at Tc1 = 117 °C and Tc2 = 30 °C. Fig. 6(a) shows an AFM height image for the ridge of wrinkle bands. Detail information of height profile is shown in Fig. 6(b). Average value estimated for the relative height of the ridge of wrinkle bands is about 48 nm, and the band spacing is about 1.8 μm. Fig. 6(c) shows a phase image associated with the lamellar ridge arrangement of concentric ring bands on the ridge of wrinkle bands. There is a flat-on lamellar orientation with an irregular radial growth direction. Schematic presentation for the lamellar arrangement and growth direction of concentric ring bands on the ridge of wrinkle bands is displayed in Fig. 6(d).
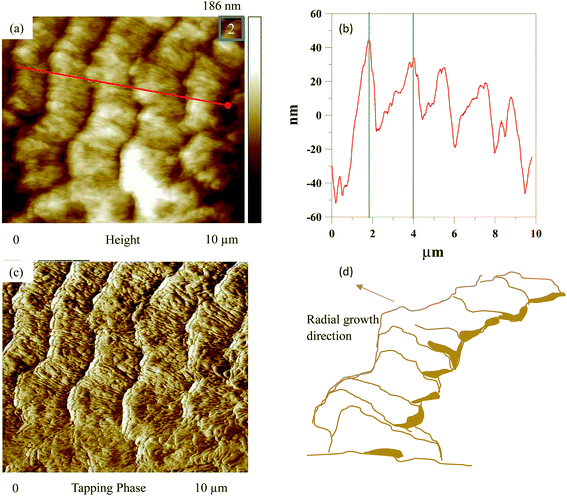 | ||
| Fig. 6 AFM images and a scheme of lamellar arrangement and growth direction for the ridge of wrinkle bands (the area indicated as “2” in Fig. 5) in PLLA/PCL = 20/80 blend as crystallized at Tc1 = 117 °C and Tc2 = 30 °C: (a) height image, (b) height profile, (c) phase image, and (d) scheme of lamellar arrangement and growth direction. | ||
Fig. 7 shows AFM images and a scheme of lamellar arrangement and growth direction for the valley of wrinkle bands (the area indicated as “3” in Fig. 5) in PLLA/PCL = 20/80 blend as crystallized at Tc1 = 117 °C and Tc2 = 30 °C. Fig. 7(a) presents an AFM height image for the valley of wrinkle bands. In addition, Fig. 7(b) displays the height profile of Fig. 7(a). Average value estimated for the relative height of the valley of wrinkle bands is about 22 nm, and the band spacing is about 0.8 μm. A phase image associated with the lamellar valley arrangement of wrinkle bands is shown in Fig. 7(c). There is a flat-on lamellar orientation with an irregular radial growth direction, as shown in Fig. 7(d), with schematic illustration.
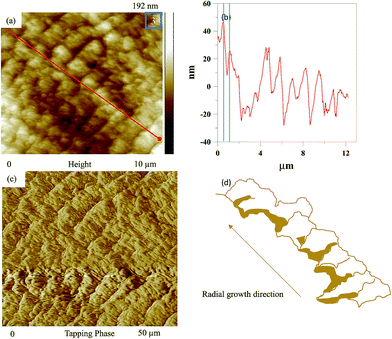 | ||
| Fig. 7 AFM images and a scheme of lamellar arrangement and growth direction for the valley of wrinkle bands (the area indicated as “3” in Fig. 5) in PLLA/PCL = 20/80 blend as crystallized at Tc1 = 117 °C and Tc2 = 30 °C: (a) height image, (b) height profile, (c) phase image, and (d) scheme of lamellar arrangement and growth direction. | ||
Fig. 8 shows AFM images and a scheme of lamellar arrangement and growth direction of the interface between ridges and valleys on wrinkle bands (the area indicated as “4” in Fig. 5) in PLLA/PCL = 20/80 blend as crystallized at Tc1 = 117 °C and Tc2 = 30 °C. Fig. 8(a) shows an AFM height image for the interface between ridges and valleys of wrinkle bands. Relative height profile is presented in Fig. 8(b). From the results of Fig. 8(b), the average height is about 24 nm and the band spacing is about 0.9 μm. The lamellar arrangement and growth direction could be discussed by the results of Fig. 8(c) and (d). Fig. 8(c) shows a phase image associated with the lamellar arrangement of the interface between ridges and valleys on wrinkle bands. There is a flat-on lamellar pattern with various relative height between the ridges and valleys of wrinkle bands (as shown in Fig. 8(c), red line). The lamellar growth direction of PLLA could also be indicated along with the presence of concentric ring bands which might be induced by rhythmic growth. Similar characterization has also been performed in pervious literature.25 The illustration is displayed in Fig. 8(d) for clarifying the lamellar arrangement and growth direction.
 | ||
| Fig. 8 AFM images and a scheme of lamellar arrangement and growth direction of the interface between ridges and valleys on wrinkle bands (the area indicated as “4” in Fig. 5) in PLLA/PCL = 20/80 blend as crystallized at Tc1 = 117 °C and Tc2 = 30 °C: (a) height image, (b) height profile, (c) phase image, and (d) scheme of lamellar arrangement and growth direction. | ||
Huang et al.34 have proposed the concept of rhythmic growth to study the crystallization morphology in neat PLLA. The concept is also adopted herein to further discuss the peculiar morphology as large wrinkle band along with concentric ring-band in PLLA/PCL blend. Fig. 9 illustrates the formation of large wrinkle band along with small concentric ring-band spherulites melt-crystallized at Tc1 = 117 °C and Tc2 = 30 °C. Initially, the PLLA/PCL blend is melted under the maximum temperature (Tmax = 190 °C) for 3 min to form amorphous chains of polymers, as shown in Fig. 9(a). Fig. 9(b) shows the subsequent rejection of PLLA to the surface of the film due to its surface tension being lower than that of PCL. PLLA in the blends could mainly diffuse up to the sample surface rather than be hid at the sample bottom theoretically owing to its lower surface tension. In addition, the results of AFM characterization could also support the suggestion for the diffusion of PLLA up to the sample surface. As shown in Fig. 5, concentric ring bands which can be identified as the structural feature of PLLA are revealed in the images of AFM topography. Therefore, the diffusion of PLLA up to the sample surface can also be proved experimentally. Further descriptions for the formation of wrinkle bands along with concentric ring bands are depicted as following. Fig. 9(c) shows how the concentric ring bands and a ridge band of wrinkle bands form simultaneously. Generally, the different thermal properties between the components lead to surface tension. The amorphous PCL is like dilute agent. It helps PLLA to diffuse to the growth front. The concentration gradient G (G = Co − CG/l), where Co is concentration of the whole solution and CG is concentration of polymer chains at growth front, rapidly approaches the maximum value and the diffusion length, l, reaches its minimum value. Consequently, according to the Fick's first law of diffusion, the number of polymer chains per unit volume that diffuse to the growth front also reaches a maximum. Ultimately, ridge bands of PLLA crystals form as the lamellae growing upward and forward, as shown in Fig. 9(d)–(f). However, the lack of PLLA's polymer chains at the growth front due to the consumption of crystal growth makes a decrease of concentration gradient. The diffusion length suddenly increases due to diffusion of neighboring polymer chains to the growth front for covering lack of polymer chains, as shown in Fig. 9(d). At this step, the ridge cannot further grow and a valley band of concentric ring-banded spherulites is thus obtained. The processes of ridge and valley formation occur rhythmically. Therefore, concentric ring-banded spherulites induced by rhythmic growth is suggested and are signed with red line in Fig. 9(e) and (f). The molten PCL (as amorphous chains) under small concentric ring bands could further form either ridges or valleys of wrinkle bands. With higher local concentration of molten PCL under small concentric ring bands, a ridge of wrinkle band would be generated. On the other hand, a valley of wrinkle band would be formed with lower local concentration of molten PCL. The wrinkle band/pattern is signed with blue line in Fig. 9(e) and (f) for clarifying the structure in details. The formation of distorted ring-band spherulites in PLLA/PCL blend under two-step crystallization with lower Tc2 is displayed in Fig. 9 schematically. The cross-section of large wrinkle bands along with small concentric ring-band spherulites is illustrated in Fig. 9(f).
 | ||
| Fig. 9 Scheme of the formation of large wrinkle band along with small concentric ring-band spherulites melt-crystallized at Tc1 = 117 °C and Tc2 = 30 °C. | ||
Branched band at high second crystallization temperature in PLLA/PCL blends
In previous discussions, we have studied about the morphology of wrinkle bands along with small concentric ring band formed by two-step crystallization at lower second step crystallization temperature (Tc2 = 30 °C). In the following investigations, we would also discuss the crystallization morphology at the higher Tc2 as 47 °C, as shown in Fig. 10.Fig. 10 shows the AFM images of PLLA/PCL = 20/80 blend which is prepared via two-step crystallization at higher Tc2 as 47 °C. The wrinkle bands with rhythmically waving ridge and valley structure cannot be identified for the PLLA/PCL = 20/80 blend by AFM height image of Fig. 10(a). That is, wrinkle band which can be generated at lower Tc2 is vanished while treating the sample at higher Tc2 as 47 °C. The POM images of Fig. 4(e) and (f) might also evidence the structural transformation. They show the transformation from wrinkle pattern to branch-like pattern while crystallizing the blends from the lower Tc2 to the higher Tc2. The wrinkle bands with significant ridge and valley structure were vanished by changing the Tc2 to the higher one. To have more detail investigations, the white and dark region in Fig. 10(a) (the regions of “1” and “2”), which are indicated as the higher and lower region respectively, were also observed and discussed. Fig. 10(b) shows AFM phase image of the higher region in Fig. 10(a); in addition, Fig. 10(c) shows AFM phase image of the lower region in Fig. 10(a). A flat-on lamellae orientation is displayed in either the image of Fig. 10(b) or (c).
Fig. 11 suggests a scheme to illustrate the crystal formation for PLLA/PCL blend crystallized at Tc1 = 117 °C and Tc2 = 47 °C. The schematic illustration of the formation of wrinkle bands along with small concentric ring bands is shown in Fig. 9. The initial steps of crystallization mechanism for PLLA/PCL blend under higher Tc2 is suggested to be similar to that under lower Tc2 as shown from Fig. 9(a)–(e). It proposes that the PLLA crystals should be formed by the mechanism of rhythmic growth as demonstrated from Fig. 10(a)–(e). However, as illustrated in Fig. 11(f), no significant wrinkle pattern along with rhythmically and regularly waved ridge or valley is shown. This result might be resulted from the sufficient diffusion and reorganization of PCL chains during their crystallization at higher Tc2 as 47 °C. PCL chains with high mobility could be diffused sufficiently and reorganized finely under the pre-formed crystals of PLLA as crystallized at Tc1. It suggests more thermodynamic stable and perfect crystals of PCL could be developed with higher Tc2 rather than those imperfect ones formed at lower Tc2 with fast crystallization and less reorganization. Consequently, at higher Tc2, it would generate more perfect crystals of PCL which could be inserted uniformly under the pre-formed crystals of PLLA by sufficient diffusion and reorganization. The final structure of PLLA/PCL blend treated by two-step crystallization with higher Tc2 is illustrated as Fig. 11(f). According to the AFM results with high-resolution images, it should be noted that the final structure of PLLA/PCL blend under higher Tc2 is different with that under lower Tc2, showing significant pattern of rhythmically and regularly waving ridge or valley structure.
 | ||
| Fig. 11 Scheme illustration of the crystal formation for PLLA/PCL blend crystallized at Tc1 = 117 °C and Tc2 = 47 °C. | ||
Conclusion
In this study, distorted ring-band spherulites formed in the PLLA/PCL blends treated with two-step crystallization were investigated in detail. Wrinkle patterns or bands along with small concentric ring bands were observed. At the first crystallization temperature (117 °C), wrinkle patterns or bands were induced by the phase separation of component due to a difference in surface tension. PLLA has a lower surface tension than that of PCL. The lower-surface-tension component as PLLA in the blend tended to migrate to the surface of the film. Meanwhile, the top of surface encountered a small concentric ring band pre-formed by PLLA crystals which were induced by rhythmic growth mechanism. This mechanism could initiate the formation of wrinkle bands. The amorphous PCL diffused with PLLA to the growth front and inserted into the concentric ring-banded spherulites of PLLA. The alternating ridges and valleys of wrinkle bands formed due to the different amounts of amorphous PCL inserted into the concentric ring bands of PLLA. When the sample was quenched to the second-crystallization temperature (30 °C), the amorphous PCL is transformed into crystals quickly. Thus, unique morphology of wrinkle pattern along with small concentric ring band was induced in PLLA/PCL blend upon crystallizing the sample by two-step crystallization. However, by comparing with the results of lower second-crystallization temperature, the wrinkle bands formed at lower second-crystallization temperature were replaced by the branch-like band upon crystallizing the samples at higher second-crystallization temperature. At higher second-crystallization temperature as 47 °C, highly mobile PCL chains could be sufficiently diffused and uniformly inserted into the pre-formed crystals of PLLA, and more perfect PCL crystals could also be generated subsequently via the second-step crystallization. Wrinkle bands with regularly waving ridge and valley patterns gradually diminished upon crystallizing the samples at higher second-crystallization temperature of two-step crystallization in PLLA/PCL blends.Acknowledgements
This work has been financially supported by basic research grants of NSC-102-E-035-085- and NSC-102-2221-E-006-268-MY3 from Taiwan's Ministry of Science and Technology (MOST), to which the authors express their gratitude.References
- D. J. Sawyer, Macromol. Symp., 2003, 201, 271–281 CrossRef CAS.
- R. E. Drumright, P. R. Gruber and D. E. Henton, Adv. Mater., 2000, 12, 1841–1846 CrossRef CAS.
- J. R. Dorgan, H. J. Lehermeier, L. I. Palade and J. Cicero, Macromol. Symp., 2001, 175, 55–66 CrossRef CAS.
- J. W. Park, S. S. Im, S. H. Kim and Y. H. Kim, Polym. Eng. Sci., 2000, 40, 2539–2550 CAS.
- M. A. Abdelwahab, A. Flynn, B. S. Chiou, S. Imam, W. Orts and E. Chiellini, Polym. Degrad. Stab., 2012, 97, 1822–1828 CrossRef CAS PubMed.
- C. C. Chen, J. Y. Chueh, H. Tseng, H. M. Huang and S. Y. Lee, Biomaterials, 2003, 24, 1167–1173 CrossRef CAS.
- J. H. Zhang, J. Xu, H. Y. Wang, W. Q. Jin and J. F. Li, Mater. Sci. Eng., C, 2009, 29, 889–893 CrossRef CAS PubMed.
- P. Sangwan, C. Way and D. Y. Wu, Macromol. Biosci., 2009, 9, 677–686 CrossRef CAS PubMed.
- A. A. Kumar, K. Karthick and K. P. Arumugam, Int. J. Biosci., Biochem. Bioinf., 2011, 1, 173–176 Search PubMed.
- C. C. Rusa and A. E. Tonelli, Macromolecules, 2000, 33, 5321–5324 CrossRef CAS.
- Y. H. Na, Y. He, X. T. Shuai, Y. Kikkawa, Y. Doi and Y. Inoue, Biomacromolecules, 2002, 3, 1179–1186 CrossRef CAS PubMed.
- M. Todo, S. D. Park, T. Takayama and K. Arakawa, Eng. Fract. Mech., 2007, 74, 1872–1883 CrossRef PubMed.
- G. Maglio, M. Malinconico, A. Migliozzi and G. Groeninckx, Macromol. Chem. Phys., 2004, 205, 946–950 CrossRef CAS.
- Y. Q. Zhang, Z. K. Wang, F. Jiang, J. Bai and Z. G. Wang, Soft Matter, 2013, 9, 5771–5778 RSC.
- Z. Bartczak, A. Galeski and N. P. Krasnikova, Polymer, 1987, 28, 1627–1634 CrossRef CAS.
- W. Wenig and M. Asresahegn, Polym. Eng. Sci., 1993, 33, 877–888 CAS.
- W. Shao, Y. Q. Zhang, Z. G. Wang, Y. H. Niu, R. J. Yue and W. P. Hu, Ind. Eng. Chem. Res., 2012, 51, 15953–15961 CrossRef CAS.
- M. Tsuburaya and H. Saito, Polymer, 2004, 45, 1027–1032 CrossRef CAS PubMed.
- X. H. Zhang, Z. G. Wang, X. Dong, D. J. Wang and C. C. Han, J. Chem. Phys., 2006, 125, 024907 CrossRef PubMed.
- X. H. Zhang, Z. G. Wang, M. Muthukumar and C. C. Han, Macromol. Rapid Commun., 2005, 26, 1285–1288 CrossRef CAS.
- H. Wang, K. Shimizu, E. K. Hobbie, Z. G. Wang, J. C. Meredith, A. Karim, E. J. Amis, B. S. Hsiao, E. T. Hsieh and C. C. Han, Macromolecules, 2002, 35, 1072–1078 CrossRef CAS.
- F. Sakai, K. Nishikawa, Y. Inoue and K. Yazawa, Macromolecules, 2009, 42, 8335–8342 CrossRef CAS.
- Z. G. Wang, L. J. An, B. Z. Jiang and X. H. Wang, Macromol. Rapid Commun., 1998, 19, 131–133 CAS.
- Z. G. Wang, L. J. An, W. Jiang, B. Z. Jiang and X. H. Wang, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 2682–2691 CrossRef CAS.
- S. Nurkhamidah and E. M. Woo, Macromol. Chem. Phys., 2013, 214, 673–680 CrossRef CAS.
- E. Can, G. Udenir, A. I. Kanneci, G. Kose and S. Bucak, AAPS PharmSciTech, 2011, 12, 1442–1453 CrossRef CAS PubMed.
- R. Dell'Erba, G. Groeninckx, G. Maglio, M. Malinconico and A. Migliozzi, Polymer, 2001, 42, 7831–7840 CrossRef.
- J. Xu, B. H. Guo, Z. M. Zhang, J. J. Zhuo, Y. Jiang, S. K. Yan, L. Li, Q. Wu, G. Q. Chen and J. M. Schultz, Macromolecules, 2004, 37, 4118–4123 CrossRef CAS.
- T. Kyu, H. W. Chiu, A. J. Guenthner, Y. Okabe, H. Saito and T. Inoue, Phys. Rev. Lett., 1999, 83, 2749–2752 CrossRef CAS.
- Y. X. Duan, Y. Zhang, S. K. Yan and J. M. Schultz, Polymer, 2005, 46, 9015–9021 CrossRef CAS PubMed.
- T. Hirotsu, K. Nakayama, T. Tsujisaka, A. Mas and F. Schue, Polym. Eng. Sci., 2002, 42, 299–306 CAS.
- M. Yasin and B. J. Tighe, Biomaterials, 1992, 13, 9–16 CrossRef CAS.
- G. X. Sun and C. M. Chan, Colloid Polym. Sci., 2013, 291, 1495–1501 CAS.
- S. Y. Huang, H. F. Li, H. Y. Wen, D. H. Yu, S. C. Jiang, G. Li, X. S. Chen and L. J. An, CrystEngComm, 2014, 16, 94–101 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08658a |
| This journal is © The Royal Society of Chemistry 2014 |