Plug-and-play multifunctional mesoporous silica nanoparticles as potential platforms for cancer therapy†
Xiaofei Chen,
Xuemei Yao,
Zhe Zhang and
Li Chen*
Department of Chemistry, Northeast Normal University, Changchun 130024, P. R.China. E-mail: chenl686@nenu.edu.cn; Fax: +86 431 85099667; Tel: +86 43185099667
First published on 26th September 2014
Abstract
Multifunctional mesoporous silica nanoparticles (MMSNs) have been fabricated by the flexible supramolecular interaction between 1-adamantylamine (AD) and β-cyclodextrin (β-CD) modified by targeted molecules and fluorescein isothiocyanate. The novelty of the platform relies on its plug-and-play connection, which makes it possible to combine targeted cancer therapeutics with monitoring the delivery pathway. The in vitro drug release experiment revealed that only a small amount of the loaded DOX was released in a PBS solution at pH 7.4, while over 80% of the loaded DOX could be rapidly released in a PBS solution at pH 5.3. The experiments of MTT, CLSM and flow cytometry showed that both folate acid and lactobionic acid targeted MSNs could be greatly internalized into tumor cells compared with overexpressed free target receptors, and showed high cell inhibitions for tumor-selective therapies. These significant multifunctional characteristics will be an advantage for essential applications for cancer therapy applications.
1. Introduction
Nanotechnology, especially nanoscale materials science, has gained potential to revolutionize tumor diagnosis and therapy.1 Thus advances in nanomaterials may bring new opportunities to tumor therapeutics because of their effective cellular uptake,2 controllable release3 and significant biocompatibility.4 Various novel nanomedicine materials such as liposomes, polymers, dendrimers as well as mesoporous silica nanoparticles (MSN) are regarded as good prospective drug delivery candidates for tumor therapy due to their biocompatibility and biodegradability in vivo as well as their flexible surface chemistry, which allows efficient drug loading, functionalization and targeting.5 Over the years, various stimuli-responsive drug delivery systems (DDS) based on MSNs had been developed. Among these DDS, efficient release triggered by special external environments, such as pH,6 enzymes,7 temperature,8 photon9 and so forth was observed. Because mono-sensitive delivery cannot satisfy the practical needs of therapeutics, the multifunctional DDS has been exploited,10 which has the ability, for example, to control drug release in the exact time, at accurate location and also can monitor the process of the delivery pathway. In spite of multifunctional DDS are famous for their diverse activities, whether these materials can be used conveniently and practically is still a problem to be considered. The synthesis procedure of these multifunctional DDS should be in a simple and rapid way. Supramolecular interaction provides a successful way to briefly fabricate multifunctional materials. From the conception of peripheral devices which can be automatically detected by the computer, plug-and-play conception has been put forward to fabricate multifunctional materials. To realize this fabrication, MSN was chosen as the main board, and various functional groups were flexibly and easily modified on the surface of MSNs by the host–guest supramolecular interaction which played the important roles to accomplish the tasks such as targeting, monitoring and delivering drugs.The multifunctional mesoporous silica nanoparticles (MMSNs) composed of pH-sensitivity group, targeted molecules, and labeled fluorescein have been fabricated as shown in Fig. S1.† To verify the feasibility of using these MMSNs for intracellular monitoring and delivering drug in cancer chemotherapy, doxorubicin hydrochloride (DOX·HCl), a typical anticancer drug, was efficiently loaded into mesoporous silica nanoparticle core as illustrated in Fig. 1. To prevent drug leakage during the circulation in normal tissue or blood, movable β-cyclodextrin (β-CD), as MSNs gates' keepers, were threaded onto AD molecules via real flexible and highly activity of host–guest interaction.11 AD and MSNs were linked by the pH-sensitive benzoic–imine bond. To enhance the therapeutics efficacy to tumor cells, active-targeting folic acid (FA) or lactobionic acid (LA) was anchored on β-CD. When these DOX-loaded MMSNs arrived at tumor sites guiding by active-targeting molecules, benzoic–imine bond would be cleaved by tumor intracellular pH,12 removing parts of the capping agents β-CD, not only exposes amino group to promote tumor cells uptake the drug-loaded MSN,13 but also quickly release drugs. At the meantime, the introduction of fluorescein isothiocyanate (FITC), a kind of fluorescence molecules and it will shine strong green light under the special excitation, which make it possible to trace the pathway of β-CD capped targeted MSNs. With great biocompatibility, this MMSN has the functions of target, trace and smart drug release.
 | ||
| Fig. 1 Plug-and-play multifunctional mesoporous silica nanoparticles and DOX loading and intracellular microenvironment triggered release from DOX-loaded MMSNs. | ||
2. Materials and methods
2.1 Reagents and materials
Tetraethylorthosilicate (TEOS), (3-aminopropyl) triethoxysilane (APTES), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS), 4-formylbenzoic acid, fluorescein isothiocyanate (FITC), folic acid (FA), 1-adamantylamine, lactobionic acid (LA), 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide (MTT) were purchased from Sigma-Aldrich. N-Cetyltrimethylammonium bromide (CTAB) was purchased from Alfa Aesar (Tianjin, China). Doxorubicin hydrochloride (DOX·HCl) was purchased from Zhejiang Hisun Pharmaceutical Co., Ltd. All the other reagents and solvents were purchased from Sinopharm Chemical Reagent Co., Ltd.2.2 Preparation of MSN-NH2
MCM-41 type mesoporous silica nanoparticles (MSN) with an average diameter of 140 nm were synthesized according to a reference procedure.14 Briefly, CTAB (1.0 g, 0.002 mol) and NaOH (0.28 g, 0.007 mol) were dissolved in 480 mL of de-ionized (DI) water and heated up to 80 °C. Then TEOS (5.0 g, 0.024 mol) was added dropwise to the solution under vigorous stirring. The reaction mixture was vigorously stirred at 80 °C for 3 h to obtain MSN. The nanoparticles were then centrifuged (9500 r per min, 10 min), washed thoroughly with water and methanol for five times and dried under vacuum overnight. Then MSN (0.1 g) was dispersed in 60 mL of methanol. 1.6 mL of (3-aminopropyl) triethoxysilane was added and the mixture was stirred for 36 h at 35 °C to obtain MSN-NH2 (with template) nanoparticles. The nanoparticles were then centrifuged (9500 rpm, 10 min), washed five times with methanol and dried under vacuum overnight. Finally, CTAB was removed by refluxing with a mixture of 80 mL of methanol and 4.5 mL of HCl (37.4%) at 65 °C for 48 h to obtain MSN-NH2 nanoparticles. The nanoparticles were then centrifuged (9500 rpm, 12 min), washed five times with methanol and dried under vacuum overnight.2.3 Preparation of MSN–NH2–benzoic–imine (MSN–BI)
MSN–NH2 (0.03 g) was dispersed in 48 mL of anhydrous DMF, and then 4-formylbenzoic acid (1.35 g, 0.01 mol) was added into the solution. The reaction mixture was stirred at 35 °C for 36 h. After the reaction, the mixture was centrifuged (9500 rpm, 10 min), and washed five times with methanol and dried under vacuum overnight.2.4 Preparation of MSN–benzoic–imine–AD (MSN–AD)
MSN–BI (0.03 g), 1-adamantylamine (4.31 g, 0.023 mol), EDC·HCl (5.73 g, 0.03 mol) and NHS (1.725 g, 0.015 mol) were dispersed in 48 mL of anhydrous DMF. The reaction mixture was stirred at 18 °C for 50 h. After the reaction, the mixture was centrifuged (9500 rpm, 10 min), and washed six times with methanol and dried under vacuum overnight.2.5 Preparation of folic acid-β-cyclodextrin (FA–CD) and lactobionic acid-β-cyclodextrin (LA–CD)
Synthesis of functionalized folic acid, lactobionic acid and β-cyclodextrin accorded to the reference procedure.10 Firstly, folic acid (0.15 g, 0.34 mmol) was dissolved into 45 mL of DMF under vigorous stirring. The un-dissolved chemicals were removed by filtration. Then, a mixture of EDC (0.13 g, 0.68 mmol) and NHS (0.0783 g, 0.68 mmol) in 5 mL of DMF was added to the above solution. The reaction was lasted for 4 h to activate the –COOH group of folic acid. After that, MSN–NH2 (0.389 g) was added to the mixture solution, which was stirred for another 48 h. Followed by evaporation under high vacuum, the mixture was extensively washed with acetone each for 3 times to remove the unreacted chemicals. The resulting product was denoted as FA–CD. Similarly, we synthesized LA–CD.2.6 Preparation of fluorescein isothiocyanate-β-cyclodextrin (FITC–CD)
Fluorescein isothiocyanate (0.194 g, 0.5 mmol) and β-cyclodextrin (0.57 g, 0.5 mmol) were dispersed in 35 mL of anhydrous DMF. The reaction mixture was stirred at 25 °C for 30 h. The solution was evaporated under high vacuum and the remains dialyzed in de-ionized water for 24 h. Finally, the solution was lyophilized to give the product FITC–CD.2.7 In vitro drug loading and release
Doxorubicin (DOX) was used as a model drug for in vitro drug loading and release. DOX loaded in MMSNs were prepared by a simple dialysis technique.15 Typically, MSN (160.0 mg), DOX (35.0 mg) and β-CD (50.0 mg) were mixed in 4.0 mL of DMF. The mixture was stirred at room temperature for 6 h and then added dropwise into 20.0 mL of PBS at pH 7.4. Then DMF was removed by dialysis against deionized water at pH 7.4 for 36 h. The dialysis medium was refreshed four times and the whole procedure was performed in the dark. After removing the blank particles by centrifugation, the supernatant was measured by UV-Vis spectrophotometer. According to a standard curve obtained from DOX/DMF solutions at a series of DOX concentrations, we obtained drug loading content.In vitro drug release profiles of drug-loaded capped MMSNs were investigated in PBS (at pH 5.3, 6.8 or 7.4). The pre-weighed freeze-dried DOX loaded capped MSN were suspended in 3 mL of release medium and transferred into a dialysis bag (MWCO 1000 Da). The release experiment was initiated by placing the end-sealed dialysis bag into 50 mL of release medium at 37 °C with continuous shaking at 75 rpm. At predetermined intervals, 2 mL of external release medium was taken out and an equal volume of fresh release medium was replenished. The amount of released DOX was determined by using fluorescence measurement. The release experiments were conducted in triplicate.
2.8 Intracellular drug release
The cellular uptake and intracellular release behaviors of DOX-loaded capped MSN were assessed by confocal laser scanning microscopy (CLSM) and flow cytometric analyses.2.9 Cell viability assays
The relative cytotoxicities of MMSN against HeLa and HepG2 cells were evaluated in vitro by a standard MTT assay. The cells were seeded in 96-well plates at 1 × 104 cells per well in 200.0 μL of complete RPMI 1640 and incubated at 37 °C in 5% CO2 atmosphere for 24 h. The culture medium was then removed and solutions in complete RPMI 1640 at different concentrations (0–50 mg L−1) were added. The cells were subjected to MTT assay after being incubated for additional 48 h. The absorbance of the solution was measured on a Bio-Rad 680 microplate reader at 490 nm. Cell viability (%) was calculated based on eqn (1):| Cell viability (%) = Asample /Acontrol × 100 | (1) |
The cytotoxicities of DOX-loaded MMSN against HepG2 and HeLa cells were also evaluated in vitro by a MTT assay. Similarly, cells were seeded into 96-well plates at 1 × 104 cells per well in 200.0 μL of complete RPMI 1640 and further incubated for 24 h. As for group of target competition, having added the target molecules 1 μg mL−1 for 1 h, removed medium and washed once again, then added fresh RPMI 1640. After washing cells with PBS, 180.0 μL of complete RPMI 1640 and 20.0 μL of DOX-loaded MMSN-FA, MMSN with free FA (MMSN-COM), MMSN-LA and MMSN solutions in PBS were added to form culture media with different DOX concentrations (0–10.0 mg L−1 DOX). The cells were subjected to MTT assay after being incubated for 24, 48 and 72 h. The absorbance of the solution was measured on a Bio-Rad 680 microplate reader at 490 nm. Cell viability (%) was also calculated based on eqn (1).
3. Results and discussion
3.1 Synthesis of MSN–AD
The FT-IR spectra of MSN–CTAB, MSN–NH2, MSN–BI and MSN–AD were shown in Fig. 2. It could be observed that MSN–CTAB gave the characteristic C–H deformation vibration at 2923 and 2858 cm−1 and C–H deformation vibration about 1472 cm−1 due to a large amount of CTAB existing in the channels. While these peaks attributed to CTAB all disappeared after CTAB had been removed. The peak appeared at 1631 cm−1 assigned to N–H vibration further confirmed the successful preparation of MSN–NH2. There appeared the peak at 1665 cm−1 belonged to t the absorbance of the imine bond16 confirmed that the successful graft of 4-formylbenzoic acid on MSN–NH2. The adsorption peaks at 1552, 1651, 1717 cm−1, which assigned to the N–H bending vibration, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the amide group, and the C
O stretching vibration in the amide group, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the carboxyl group, respectively all demonstrated the successful synthesis of MSN–AD. Thus MSN–AD could be treated as a kind of main body, some functional groups could easily modify MSN through host–guest interaction, such as, between AD and β-CD.
O stretching vibration in the carboxyl group, respectively all demonstrated the successful synthesis of MSN–AD. Thus MSN–AD could be treated as a kind of main body, some functional groups could easily modify MSN through host–guest interaction, such as, between AD and β-CD.
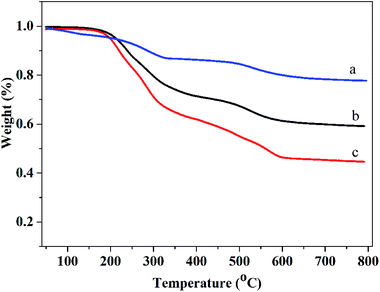
According to the TGA curves of these nanoparticles, shown in Fig. 3, it could be concluded that the mass loss of ratio of 4-formylbenzoic acid and 1-adamantylamine was nearly 18% and 16%, so their molar ratio was nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 just as expected.
1 just as expected.
There were three diffraction peaks associated with the 100, 110, and 200 reflections of hexagonal symmetry in XRD pattern of MSN–NH2 (Fig. 4A), suggesting a similar ordered hexagonal mesostructure.17 The prepared MSN–NH2 particles were uniform spherical with a mean diameter of approximately 140 nm according to the TEM images shown in Fig. 4B. An array of ordered mesoporous network could be clearly observed.
 | ||
| Fig. 4 Low-angle X-ray diffraction (XRD) pattern of MSN–NH2 powders (A) and TEM image of MSN–NH2 (B). | ||
The surface area and pore size of different nanoparticles were determined byTThanks for your Brunauer−Emmett−Teller (BET) and Barrett−Joyner−Halenda (BJH) analyses. The BET surface areas of nanoparticles decreased from 1010 m2 g−1 to 511 m2 g−1 after MSN–NH2 were anchored with β-CD (Fig. 5). The BJH pore sizes of nanoparticles also decreased from 2.48 nm to 2.07 nm after graft (Fig. S2†). These results suggest that the mesopores were successfully capped by β-CD.
3.2 synthesis of FA–CD, LA–CD and FITC–CD
The molecules structure of folic acid-β-cyclodextrin (FA–CD), lactobionic acid-β-cyclodextrin (LA–CD) and isothiocyanate-β-cyclodextrin (FITC–CD) were confirmed by 1H NMR spectrum. Obviously, as shown in Fig. S3,† the shift of g and f reflected the successfully synthesis of FA–CD. And according to the previous report of Z. Zhong et al.,18 the chemical shifts of a and b clearly reflected the structure of LA–CD had been successful synthesis shown by Fig. S4.† Even though there exists isomer of isothiocyanate, the 1H NMR spectrum of Fig. S5† distinctly demonstrated the resonances at 7.3–8.1 ppm belonged to benzene and 4.4–4.9 belonged to the double bonds, which indicated we succeeded in synthesis of FITC–CD.3.3 In vitro DOX loading and triggered release
In general, MSNs has great DOX loading efficiency, and we have calculated the DOX loading content of MMSN@DOX was as high as to be 12.26 wt%. Then in vitro release behaviours of these DOX-loaded MMSNs were investigated at pH 5.3, 6.8 and 7.4, mimicking the physiological pH in late endosome, tumor extracellular microenvironment, and blood and normal tissue, respectively. Over 80% of DOX was released from DOX-loaded MMSNs during the first 12 h in PBS at pH 5.3 indicated in Fig. 6. When the pH value increases from 5.3 to 7.4, the DOX release rate accordingly decreased. These different release behaviours were likely to result from the acid-triggered cleavage of benzoic–imine bond which leading to the removal of the capped β-CD from the nanoparticle surface. Further, the release behaviours of MSN@DOX at different pH values were performed. As shown in Fig. S6,† at pH 5.3 the release ratio of DOX was nearly the same as that at pH 6.8. Noticeably, there was nearly 60% DOX released from MSN even at pH 7.4, indicating that the release of DOX depended on the solubility and diffusion of DOX under different pH conditions without the intelligent cap. However, the release ratio of DOX from MMSN@DOX was only nearly 30% and 15%, respectively. This difference between them could be attributed to the gate keepers (β-cyclodextrin) anchored benzoic–imine linker. In addition, DOX loading content of MSN@DOX was 6.92 wt%, which was lower than that of MMSN@DOX. So this intermediate pH-sensitive linker has the ability to enhance DOX loading content and to effectively control drug holding or rapidly release at different pH environment. Therefore, this pH-sensitive MMSN@DOX can be regarded as effective drug delivery system for further use.3.4 Intracellular DOX release and cellular proliferation inhibition
As we known, FA has specific interaction with HeLa cells and LA with HepG2 cells. The aim to introduce these specific and unspecific target molecules is to prove the specific targeted DOX-loaded MMSNs has a higher tumor inhibition than others. Since MMSNs possess flexible and convenience plug-and-play way of synthesis, we could easily choose different target molecules to specifically treat different tumors. Then antitumor activity of DOX-loaded MMSN-FA, DOX-loaded MMSN-LA, DOX-loaded MMSN-FA + free FA and DOX-loaded MMSN towards HeLa cells was investigated using MTT assays. Compared with DOX-loaded MMSN, DOX-loaded MMSN-FA induced greater antitumor effects toward HeLa cells (Fig. 7). There are two reasons for this result: one is because that FA is the receptor to positive tumor cells,19 which leads to a higher endocytic activity of HeLa cells; the other is because the benzoic–imine bond would cleave triggered by the endosomal acid environment. The cell viabilities of DOX-loaded MMSN-LA were lower than that of DOX-loaded MMSN-FA towards HeLa cells, which indicated that the targeting FA molecules could seduced the specific interaction with HeLa cells while LA molecules could not. On the other hand, compared with DOX-loaded MMSN-FA + free FA, DOX-loaded MMSN-FA had a higher inhibition towards HeLa cells, because the cell inhibition was mainly caused by the cell internalization of the nanoparticles. The targeting is suppressed due to 1 μg mL−1 of free folic acid presenting and binding.20 Similar result has been exhibited towards HepG2 cells. From MTT assays, it indicated that DOX-loaded MMSN-LA had the highest efficient inhibition of cell proliferation than any other DOX-loaded MMSNs. Because of the presence of competition free target molecules LA, DOX-loaded MMSN–LA + free LA showed weakest antitumor efficiency of all (Fig. S7†). Based on the above data, the target molecules could accurately guide pH-sensitive DOX-loaded MMSNs to the tumor site, and rapidly release drugs. Therefore the MMSNs have clear functions of target and pH-sensitivity, which showed an efficient drug delivery platform for inhibition of cancer cells. And DOX-loaded MMSN-FA exhibited the lowest half maximal inhibitory concentration (IC50) of 1.08 μg mL−1, compared with that of DOX-loaded MMSNs by 4.56 μg mL−1 in HeLa cells. This result quantitatively confirmed the differences in cellular proliferation inhibitions and revealed the target responsive ability of DOX-loaded MMSNs in cells.Confocal laser scanning microscopy (CLSM) of DOX-loaded MMSNs capped by FA-CD and FITC-CD toward HeLa cells assays have been carried out to prove the possibility of assembled interactions between MMSNs and two different groups in vitro and each group could maintain their specific functions. As expected, the strongest intracellular DOX fluorescence was observed in the HeLa cells after incubation with DOX-loaded MMSN-FA for 3 h. We also observed that the internalized nanoparticles with high level of green fluorescence were mainly located in the HeLa cells (Fig. 8). The weakest fluorescence intensity of DOX-loaded MMSN-FA + free FA than other DOX-loaded MMSNs in the nuclei was observed. After cells were treated with free FA, as a kind of competing molecule, inhibited DOX-loaded MMSN-FA to bind the tumor cells and leaded to prevent DOX accumulation in cells as well. And the fluorescence intensities were in the following order: DOX-loaded MMSN-FA > DOX-loaded MMSN-LA > DOX-loaded MMSN > DOX-loaded MMSN-FA with free FA, respectively. Through anchored the fluorescent labels, we could easily know the exact pathway of DOX-loaded MMSNs and also know target molecules increasing effect of therapeutics. Such tumor inhibition results also observed towards HepG2 cells showed in Fig. S8.† The fluorescence intensities ordered: DOX-loaded MMSN-LA > DOX-loaded MMSN-FA > DOX-loaded MMSN > DOX-loaded MMSN-LA with free LA, respectively. Thus it is convenience to learn the therapeutic efficiency because of function provided by FITC.
Drug triggered release in intracellular environment was also observed by flow cytometric analyses. As shown in Fig. 9, the flow cytometric histogram for the HeLa cells incubated with DOX-loaded MMSN-FA shifted to the obviously the highest fluorescence intensity region in contrast to that for the cells incubated with other DOX-loaded MMSNs. Then the ordering of them from the higher to the lower were DOX-loaded MMSN-FA, DOX-loaded MMSN-LA, DOX-loaded MMSN and DOX-loaded MMSN-FA + free FA. The quantity mean of them were 3915, 2982, 2164, 1874 and 164, respectively. Similarly, the flow cytometric profiles towards HepG2 cells also have been performed. As showed in Fig. S9,† DOX-loaded MMSN-LA showed the highest fluorescence intensity while the group of DOX-loaded MMSN-LA + free LA performed the lowest fluorescence intensity. The quantity mean of intensity were 4004, 1885 respectively. And the quantity of DOX-loaded MMSN-FA, DOX-loaded MMSN were 2879 and 2161. These results demonstrated that DOX-loaded MMSN with targeted molecules owned significant bindings with tumor cells, abruptly drug release and an efficient inhibition to targeted cells. And all of these positive results indicated that MMSN has taken advantage of target, pH-sensitivity and fluorescence.
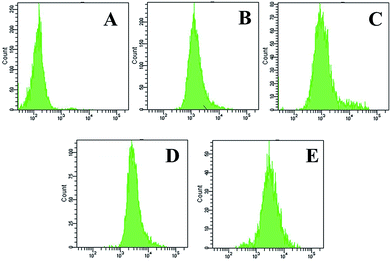 | ||
| Fig. 9 Flow cytometric profiles of HeLa cells blank (A) and incubated with DOX-loaded MMSN-FA + free FA (B), DOX-loaded MMSN (C), DOX-loaded MMSN-LA (D) and DOX-loaded MMSN-FA (E) for 3 h. | ||
It also should be noted that empty MMSNs were nontoxic to HeLa and HepG2 cells for 48 hours up to a tested concentration (Fig. S10†). These data showed that the low cytotoxic and great biocompatible MMSNs can be safely employed as efficient nanocarriers for drug delivery.
4. Conclusions
We have developed a biocompatible and intracellular pH-responsive drug delivery system. Different functional β-CDs were easily, even simultaneously mixed used, modified onto MSNs through the conception of plug-and-play. The DOX-loaded MMSNs could be efficiently uptaken via endocytosis by cells and specifically released DOX, resulting in comparable antitumor activity towards HeLa and HepG2 cells in a time-dependent behaviors. Marked by fluorescence FITC, we could clearly trace the pathway of DOX-loaded MMSNs. Thus, the excellent biocompatibility and efficient intracellular drug release manners of the MMSN outline the significant potential for future biomedical applications.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Projects 21474012, 51273037, 50903012), Jilin Science and Technology Bureau (International Cooperation Project 20120729, 20130206074GX), Jilin Human Resources and Social Security Bureau (201125020).Notes and references
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- X. Liu, Y. Chen, H. Li, N. Huang, Q. Jin, K. Ren and J. Ji, ACS Nano, 2013, 7, 6244–6257 CrossRef CAS PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- S. Zhang, Z. Chu, C. Yin, C. Zhang, G. Lin and Q. Li, J. Am. Chem. Soc., 2013, 135, 5709–5716 CrossRef CAS PubMed.
- M. Cassidy, H. Chan, B. Ross, P. Bhattacharya and C. Marcus, Nat. Nanotechnol., 2013, 8, 363–368 CrossRef CAS PubMed.
- R. Guillet-Nicolas, A. Popat, J. L. Bridot, G. Monteith, S. Z. Qiao and F. Kleitz, Angew. Chem., Int. Ed., 2013, 125, 2374–2378 CrossRef.
- C. Coll, L. Mondragón, R. Martínez-Máñez, F. Sancenón, M. D. Marcos, J. Soto, P. Amorós and E. Pérez-Payá, Angew. Chem., Int. Ed., 2011, 50, 2138–2140 CrossRef CAS PubMed.
- X. Kang, Z. Cheng, D. Yang, P. A. Ma, M. Shang, C. Peng, Y. Dai and J. Lin, Adv. Funct. Mater., 2012, 22, 1470–1481 CrossRef CAS.
- J. Croissant and J. I. Zink, J. Am. Chem. Soc., 2012, 134, 7628–7631 CrossRef CAS PubMed.
- Z. Luo, X. Ding, Y. Hu, S. Wu, Y. Xiang, Y. Zeng, B. Zhang, H. Yan, H. Zhang and L. Zhu, ACS Nano, 2013, 7, 10271–10284 CrossRef CAS PubMed.
- J. Chiba, A. Sakai, S. Yamada, K. Fujimoto and M. Inouye, Chem. Commun., 2013, 49, 6454–6456 RSC.
- E. S. Lee, Z. Gao and Y. H. Bae, J. Controlled Release, 2008, 132, 164–170 CrossRef CAS PubMed.
- S. E. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613–11618 CrossRef CAS PubMed.
- J. Zhang, Z.-F. Yuan, Y. Wang, W.-H. Chen, G.-F. Luo, S.-X. Cheng, R.-X. Zhuo and X.-Z. Zhang, J. Am. Chem. Soc., 2013, 135, 5068–5073 CrossRef CAS PubMed.
- H. He, L. Yan, F. Meng, Z. Xie, X. Jing and Y. Huang, Phys. Chem. Chem. Phys., 2013, 15, 14210–14218 RSC.
- C. Ding, L. Zhao, F. Liu, J. Cheng, J. Gu, S. Dan, C. Liu, X. Qu and Z. Yang, Biomacromolecules, 2010, 11, 1043–1051 CrossRef CAS PubMed.
- C. Kresge, M. Leonowicz, W. Roth, J. Vartuli and J. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- R. Yang, F. Meng, S. Ma, F. Huang, H. Liu and Z. Zhong, Biomacromolecules, 2011, 12, 3047–3055 CrossRef CAS PubMed.
- W. Cao, J. Zhou, A. Mann, Y. Wang and L. Zhu, Biomacromolecules, 2011, 12, 2697–2707 CrossRef CAS PubMed.
- S. Chen, X.-Z. Zhang, S.-X. Cheng, R.-X. Zhuo and Z.-W. Gu, Biomacromolecules, 2008, 9, 2578–2585 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08552c |
| This journal is © The Royal Society of Chemistry 2014 |