Facile hydrothermal selective fabrication of Ni(OH)2 and Ni(HCO3)2 nanoparticulates and their electrochemical performances
Yinan Yana,
Gang Cheng*b,
Ping Wanga,
Dannong He*a and
Rong Chenb
aNational Engineering Research Center for Nanotechnology, Shanghai, 200241, P. R. China. E-mail: hdn_nercn@163.com
bSchool of Chemistry and Environmental Engineering, Wuhan Institute of Technology, Xiongchu Avenue, Wuhan, 430073, P. R. China. E-mail: gchenglab@163.com
First published on 16th September 2014
Abstract
Cone-like and flower-like nickel hydroxide and sphere-like nickel bicarbonate were synthesized via a simple hydrothermal route by varying the amount of urea without using any surfactants or additives. The surface area of the obtained materials were measured to be 17.3 m2 g−1, 45.7 m2 g−1 and 39.9 m2 g−1, and correlated electrochemical performances were briefly investigated. The maximum specific capacitance was recorded to be 1429.3 F g−1 in sphere-like nickel bicarbonate nanoparticles, suggesting their potential application in supercapacitor electrode.
Introduction
The depletion of fossil fuel and severe environmental pollution stimulate the research into developing new sustainable energy sources and rechargeable devices.1 Because of advantages, such as elevated efficiency, superior power and lengthy cycle life, supercapacitor is considered to be one of the most promising devices for bridging the gap between traditional physical capacitors and battery cells to satisfy market requirements.2 The main active ingredients in supercapacitor electrodes are divided into two categories: the electrical double-layer capacitor (EDLC) materials and the pseudo-capacitor materials. Associated with the EDLC-type carbon materials (usually less than 300 F g−1),3 transition metal oxides and hydroxides result in substantially larger specific capacitance from valence-reversible faradaic reactions.4,5Nickel compounds have been regarded as promising electrode materials among the numerous pseudo-capacitance storage materials. In the past decade, varied synthetic methods, such as chemical precipitation, hydrothermal reaction, electrochemical deposition, and sol–gel processes from different precursors, have been exploited to prepare nickel-based nanoparticles.6–10 Among the above mentioned approaches, hydrothermal method, with the ability to dissolve and recrystallize normally insoluble materials under high pressure and temperature conditions, possesses the advantages of easy-handling, energy-saving, simplicity and cost effectiveness. At the same time, the sorts of precursors utilized for precipitation of nickel-based compounds by the hydrothermal route could significantly affect the size, crystallinity and surface of nickel-based nanoparticles, which has an influence on electrochemical performance of such nanoparticles.11 Immediate precipitation by neutralizing nickel ions with hydroxide ions, also called the “direct precipitation route (DP)”, is a conventional synthetic technique. The capacity of DP produced nickel hydroxide is usually less than 576 F g−1 because of the limited surface of large particles of varied sizes.12 A modified method called the “coordination homogeneous precipitation (CHP)” was developed with the purpose of attaining granular uniformity. Affected by coordination agents, such as ammonium water, sodium succinate, ethylenediaminetetraacetic acid, sodium potassium tartrate and tri-ethanol amine, nickel ions would precipitate homogeneously in extended sedimentation time.13 Moreover, complex and versatile structures could be fabricated by involving surfactants through the CHP method via the hydrothermal route. In the presence of sodium dodecyl sulphate, PEG-400 or Tween-80, platelet-like particles from ammonium water coordination changed into cone-like, thin-flake-like or needle-like ones.14–16 Tube-like nickel oxide nanoparticles with diameters in the range of 60–80 nm were also successively fabricated with the intervention of anion surfactants. The enlarged surface area, reduced particle size and shortened diffusion path effectively improved their electrochemical performances.17
Prolonging the release of hydroxyl-ion during ureolysis at elevated temperature is helpful for yielding nanoparticles with smaller sizes, and is generally classified as the “urea homogeneous precipitation (UHP)” method. Herein, we report a facile hydrothermal approach to prepare versatile nickel nanoparticulates by simply altering the concentration of urea without involving any surfactants, in which carbonate and ammonium ions from the decomposition of urea could react with nickel ions to form Ni(OH)2, Ni(HCO3)2 or nickel mixtures. The crystal phase, structure, surface area, and correlated electrochemical properties of synthesized nanoparticulates were also investigated in this paper.
Experimental section
The atomic mole ratios of nickel nitrate to urea were set as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 1
5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. In a typical procedure, 2 mmol nickel nitrate hexahydrate (Ni(NO3)2·6H2O) and urea were added into deionized water, followed by magnetic stirring for 2 h in a water bath at 60 °C to form homogeneous solutions. The solutions were transferred into 50 mL Teflon-lined autoclaves and kept at 180 °C for 12 h. The autoclaves were allowed to cool at ambient temperature, and the precipitates were collected and washed with deionized water and ethanol several times by centrifugation, then dried at 60 °C. The names of as-prepared samples were designated as S1, S2, S3, S4 and S5 respectively according to amount of urea introduced (2, 4, 6, 10 and 20 mmol).
10. In a typical procedure, 2 mmol nickel nitrate hexahydrate (Ni(NO3)2·6H2O) and urea were added into deionized water, followed by magnetic stirring for 2 h in a water bath at 60 °C to form homogeneous solutions. The solutions were transferred into 50 mL Teflon-lined autoclaves and kept at 180 °C for 12 h. The autoclaves were allowed to cool at ambient temperature, and the precipitates were collected and washed with deionized water and ethanol several times by centrifugation, then dried at 60 °C. The names of as-prepared samples were designated as S1, S2, S3, S4 and S5 respectively according to amount of urea introduced (2, 4, 6, 10 and 20 mmol).
Samples from the UHP method were characterized by various techniques. X-ray diffraction (XRD) measurements were performed using a D8 Advance (Bruker, Germany) at room temperature in specular reflection mode with Cu/Kα radiation. SEM images were taken on a field-emission electron microscope S4800 operating at an acceleration voltage of 3 or 5 kV. TEM images and SAED patterns were recorded on a JEOL 2010 electron microscope, using an accelerating voltage of 200 kV. The isotherm of nitrogen physical-adsorption was obtained using an ASAP-2020 surface analyser (Micromeritics Instruments, USA), and the specific surface area (SSA) was determined by the Brunauer–Emmett–Teller (BET) method.
Electrochemical performance was recorded by a computer-controlled CH660D electrochemical work station (CH Instruments Inc., USA) equipped with a two-compartment, three-electrode cell. Platinum gauze was used as the current collector for the work electrode, platinum foil as the auxiliary electrode, and saturated calomel electrode as the reference electrode. The prepared active material powders were mixed with acetylene black and poly(tetrafluoroethylene) in the ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. A few drops of ethanol were added to form a homogeneous slurry and the slurry was pressed and pasted on the platinum gauze with a mass of 5 mg. All electrochemical measurements were carried out in 6 M KOH solution as the electrolyte.
5. A few drops of ethanol were added to form a homogeneous slurry and the slurry was pressed and pasted on the platinum gauze with a mass of 5 mg. All electrochemical measurements were carried out in 6 M KOH solution as the electrolyte.
Result and discussion
The purity and crystallinity of as-synthesized products were examined by powder X-ray diffraction (XRD). The XRD-patterns of S1 and S2 are shown in Fig. 1a and b. Both diffraction patterns consist of prominent peaks at 19.5° (001), 32.55° (100), 38.95° (101), 51.5° (102) and 58.5° (110), readily indexed to pure hexagonal phase β-nickel hydroxide (P-3m1(164), JCPDS Card no. 74-2075; a = 3.13, c = 4.63), indicating the crystalline formation of pure Ni(OH)2 materials. The morphologies of nickel hydroxide were investigated by FESEM and TEM. The FESEM images of S1 are shown in Fig. 1c and e. Geometrically cone-like particles are seen scattered all over the spotting field. Each particle was determined to be about 100 nm in diameter and 200 nm in length. From the SEM image of S2 shown in Fig. 1d and f, numerous sheets were observed to be stacked together and constituted into large flower-like structures in the range of 400–800 nm. The edges of nanosheets can be clearly discerned along the macropores on the rough particle surfaces. TEM images of S1 and S2 are presented in Fig. 1g and h, from which it can be seen that the particle size and the shape in TEM images agree with the SEM images. The HRTEM image of nickel hydroxide nanoparticles (S1) is shown in Fig. 1i, and the lattice spacing is discerned to be 0.155 nm, corresponding to the (110) planes. The corresponding selected area electron diffraction pattern characterizations (SAED) of nickel hydroxide nanospheres are shown in Fig. 1j. The disperse rings were indexed to the crystal planes of (110), (111), (210), and (221), confirming the well-established crystalline feature. | ||
| Fig. 1 (a and b) XRD patterns; (c–f) SEM images; (g–i) TEM images; and (j) SAED pattern of the as-synthesized nickel hydroxide products: (a, c, e, g and i) from S1; (b, d, f, h and j) from S2. | ||
XRD pattern of S5 was compared with JCPDS card no. 15-782 (shown in Fig. 2a), and it was revealed to compose of nickel carbonate. The peak at 14.95°, corresponding to (110) plane is more intense than others, implying its dominant orientation. The size of nickel carbonate particles was determined to be in the range of 40–80 nm from SEM and TEM images (shown in Fig. 2b and c). The clear dots in SAED pattern (the inset of Fig. 2d) suggest that the nickel bicarbonate from hydrothermal reaction has a high order of crystallinity.18 In HRTEM image shown in Fig. 2d, the fringe of S5 marked in white letters was measured to be 0.59 nm.
 | ||
| Fig. 2 (a) XRD pattern, (b) SEM, (c) TEM and (d) HRTEM images of the as-synthesized nickel bicarbonate (S5). | ||
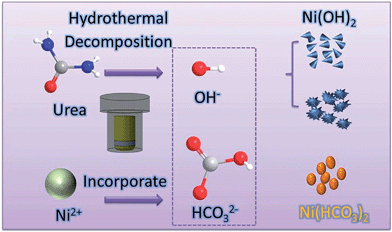
It was found that urea plays an important role in the fabrication of Ni(OH)2 and Ni(HCO3)2. As shown in Fig. 3, the XRD patterns of S3 and S4 indicate that both of them are composed of Ni(OH)2 (JCPDS no. 74-2075) and Ni(HCO3)2 (JCPDS no. 15-782) when the amount of urea is 6 or 10 mmol. Based on the above results, the formation mechanism of nickel-based nanoparticles can be deducted as follows: urea decomposition results in the release of CO32− and NH4+, and their hydration process generate HCO3− and OH−. At lower urea amounts of 2 and 4 mmol, the generation of OH− is predominant and tends to precipitate Ni(OH)2 particles (cone-like and flower-like forms). When the amount of urea is raised to the range of 6 to 10 mmol, HCO3− and OH− coexist and precipitate mixtures of Ni(OH)2 and Ni(HCO3)2. While the amount of urea exceeds 20 mmol, the generation of HCO3− predominantly occurs during the ureolysis, resulting in the formation of Ni(HCO3)2 phase. It is well-known that the formation of anisotropic nanostructures with shape control in the crystallization process includes nucleation and growth.19 In the present reaction system, lower amounts of urea might be advantageous for the formation of Ni(OH)2 nanoparticles. However, the involvement of higher amounts of urea could enhance the nucleation rate of Ni(OH)2 nanocrystals, resulting in the formation of spherical nanoparticle aggregates. Compared with Ni(OH)2, Ni(HCO3)2 has a unique crystal structure and different crystal growth habit, which might be beneficial for the formation of nanoparticles by such a hydrothermal route. Further investigation on the influence of urea's concentration and other experimental parameters on the formation of Ni-based nanostructures with different morphologies is still in progress. On the basis of our observations, Ni(OH)2 and Ni(HCO3)2 with different crystal structures and morphologies could be selectively synthesized by varying the initial urea amount used in this method. The illustration of formation process is shown in Scheme 1.
Surface area analysis of S1, S2 and S5 were conducted by nitrogen adsorption–desorption tests (the adsorption–desorption curves of S1, S2 and S5 are shown in Fig. 4a). The surface area of flower-like large Ni(OH)2 particles in S2 is about 45.7 m2 g−1, which is much larger than others. The minimum value was measured in the cone-like Ni(OH)2 particles in S1 at 17.3 m2 g−1. Besides, the sphere-like Ni(HCO3)2 particles showed an intermediate surface area value at 39.9 m2 g−1. The majority of adsorption and desorption curves overlapped, and small hysteresis loops were observed above the value of P/P0 in the range of 0.8–1, suggesting no mesopore was formed in the aggregation of nanoparticles.
The electrochemical performances were investigated in 6 M KOH with standard calomel reference electrodes. Cyclic voltammetry (CV) curves scanned at 10 mV s−1 are shown in Fig. 4b. All the CV curves comprise a pair of strong symmetry redox peaks, reflecting faradaic pseudo-activity induced capacitance. The anodic peaks can be observed in the range of 0.25–0.4 V, which can be attributed to the oxidation of Ni(OH)2 or Ni(HCO3)2 into NiOOH, while the catholic peaks at 0.0–0.1 V is related to the conversion of NiOOH back to Ni(OH)2 or Ni(HCO3)2. The mechanism20 is described by the following equations:
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e− |
| Ni(HCO3)2 + 3OH− ↔ NiOOH + 2HCO3− + H2O + e− |
The galvanic charge–discharge curves at the current density of 2 A g−1 within the voltage range of −0.2–0.5 V is shown in Fig. 4c. Distinguishable from the EDLC-type isosceles, the shape of charge–discharge curves is non-linear, while the flat stage in the curves clearly demonstrates the pseudo-capacitance induced by faradaic charges. The specific capacitance can be calculated from CV curves and galvanic charge–discharge curves using the following equation:
| C = S/2vmΔV, C = ∫IΔt/mΔV |
We have also tried assembling supercapacitors according to the literature.22–24 The active materials (active carbon as anode and nickel bicarbonate as cathode) were casted on the nickel foam with a density of 5 mg cm−2 (1 cm × 1 cm). The asymmetric supercapacitor was assembled by pressing the two electrodes into a cell, which was separated by a nylon paper. The electrolyte used was 6 M KOH aqueous solution. Fig. 5 shows the cyclic voltammogram and galvanic charge–discharge curves of the asymmetric cell. The rudimentary measured capacitance of as-made asymmetric cell is about 64.2 F g−1 in the scan range of 0–1.5 V at the scan rate of 1 mV s−1, which is higher than the reported value of 37 F g−1 in NiO/active carbon asymmetric cell.25 The above results suggest that the as-synthesized Ni(HCO3)2 nanoparticles is a promising electrode material.
 | ||
| Fig. 5 (a) Cyclic voltammogram of asymmetric cell at 1 mV s−1; (b) galvanic charge–discharge curves of the assembled asymmetric cell at 1 A g−1. | ||
Conclusions
In this communication, cone-like, flower-like nickel hydroxide and sphere-like nickel bicarbonate were facilely synthesized without surfactants. The crystal phase, structure, surface area, and correlated electrochemical properties of the as-synthesized products were systematically investigated. The surface areas of obtained materials are measured to be 17.3 m2 g−1, 45.7 m2 g−1 and 39.9 m2 g−1, respectively, and the correlated electrochemical performance was systematically compared. The maximum specific capacitance was 1429.3 F g−1 in sphere-like nickel bicarbonate, suggesting their potential application as supercapacitor electrode materials.Notes
The authors declare no competing financial interest.References
- B. E. Conway, Electrochemical supercapacitors: scientific fundamentals and technological applications, Plenum Publishers, New York, 1st edn, 1999 Search PubMed.
- X. Wang, X. Lu, B. Liu, D. Chen, Y. Tong and G. Shen, Adv. Mater., 2014, 26, 4763–4782 CrossRef CAS PubMed.
- Y. Yan, T. Kuila, N. H. Kim and J. H. Lee, Carbon, 2014, 74, 195 CrossRef CAS PubMed.
- L. Mai, F. Yang, Y. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381 CrossRef PubMed.
- K. M. Hercule, Q. Wei, A. M. Khan, Y. Zhao, X. Tian and L. Mai, Nano Lett., 2013, 13, 5685–5691 CrossRef CAS PubMed.
- D. S. Kong, J. M. Wang, H. B. Shao, J. Q. Zhang and C. N. Cao, J. Alloys Compd., 2011, 509, 5611 CrossRef CAS PubMed.
- W.-K. Hu, X.-P. Gao, D. Noréus, T. Burchardt and N. K. Nakstad, J. Power Sources, 2006, 160, 704 CrossRef CAS PubMed.
- H. Li, S. Liu, C. Huang, Z. Zhou, Y. Li and D. Fang, Electrochim. Acta, 2011, 58, 89 CrossRef CAS PubMed.
- Y. Kido, K. Nakanishi, N. Okumura and K. Kanamori, Microporous Mesoporous Mater., 2013, 176, 64 CrossRef CAS PubMed.
- M. S. Wu and K. C. Huang, Int. J. Hydrogen Energy, 2011, 36, 13407 CrossRef CAS PubMed.
- Y. Miao, L. Ouyang, S. Zhou, L. Xu, Z. Yang, M. Xiao and R. Ouyang, Biosens. Bioelectron., 2014, 53, 428 CrossRef CAS PubMed.
- R. Acharya, T. Subbaiah, S. Anand and R. P. Das, J. Power Sources, 2002, 109, 494 CrossRef CAS.
- T. Subbaiah, R. Mohapatra, S. Mallick, K. G. Misra, P. Singh and R. P. Das, Hydrometallurgy, 2003, 68, 151 CrossRef CAS.
- X. C. Song, X. Wang, Z. A. Yang and Y. F. Zheng, Mater. Lett., 2011, 65, 2348 CrossRef CAS PubMed.
- Q. Li, H. Ni, Y. Cai, X. Cai, Y. Liu, G. Chen, L.-Z. Fan and Y. Wang, Mater. Res. Bull., 2013, 48, 3518 CrossRef CAS PubMed.
- C. Coudun, F. Grillon and J.-F. Hochepied, Colloids Surf., A, 2006, 280, 23 CrossRef CAS PubMed.
- X. Liu and L. Yu, J. Power Sources, 2004, 128, 326 CrossRef CAS PubMed.
- H. J. Liu, T. Y. Peng, D. Zhao, K. Dai and Z. H. Peng, Mater. Chem. Phys., 2004, 87, 81 CrossRef CAS PubMed.
- Y. C. Cao, J. Am. Chem. Soc., 2004, 126, 7456 CrossRef CAS PubMed.
- J. Sun, Z. Li, J. Wang, Z. Wang, L. Niu, P. Gong, X. Liu, H. Wang and S. Yang, J. Alloys Compd., 2013, 581, 217 CrossRef CAS PubMed.
- T. Kuila, P. Khanra, N. H. Kim, J. K. Lim and J. H. Lee, J. Mater. Chem. A, 2013, 1, 9294 CAS.
- J. Huang, P. Xu, D. Cao, X. Zhou, S. Yang, Y. Li and G. Wang, J. Power Sources, 2014, 246, 371 CrossRef CAS PubMed.
- J. Yan, Z. Fan, W. Sun, G. Ning, T. Wei, Q. Zhang, R. Zhang, L. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632 CrossRef CAS.
- Y. Tang, Y. Liu, S. Yu, Y. Zhao, S. Mu and F. Gao, Electrochim. Acta, 2014, 123, 158–166 CrossRef CAS PubMed.
- D. Wang, F. Li and H. Cheng, J. Power Sources, 2008, 185, 1563–1568 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |