Rh-catalyzed WO3 with anomalous humidity dependence of gas sensing characteristics†
Kwon-Il Choi,
Su-Jin Hwang,
Zhengfei Dai,
Yun Chan Kang and
Jong-Heun Lee*
Department of Materials Science and Engineering, Korea University, Seoul 136-713, Republic of Korea. E-mail: jongheun@korea.ac.kr; Fax: +82 2 928 3584; Tel: +82 2 3290 3282
First published on 23rd September 2014
Abstract
The sensing of volatile organic compounds is crucial in a variety of fields including disease diagnosis, food, and homeland security. However, the significant deterioration of gas response by water vapors often hinders the sensitive and reliable gas detection in a highly humid atmosphere. Herein, we report an Rh-loaded WO3 hollow sphere chemiresistive sensor that can be potentially used for acetone gas analysis in a highly humid atmosphere. Pure WO3 and Rh-loaded WO3 hollow spheres are synthesized via a spray pyrolysis method. The Rh-loaded WO3 sensor achieved a fast acetone response (2 s), high sensitivity, good linearity, high stability, low detection limit (40 ppb) and strong selectivity to acetone even under a highly humid (80% RH) atmosphere, compared with the unloaded WO3 sensor. Interestingly, an abnormal phenomenon occurs only with the n-type Rh-loaded WO3 sensor, where the resistance and gas response increases in humid atmospheres. The sensing mechanism by Rh loading is also addressed. The unusual improvement of gas response, selectivity, responding kinetics by Rh loading shows a good potential for the detection of acetone gas.
1. Introduction
The sensing of volatile organic compounds (VOCs) is crucial in a numerous fields, including disease diagnosis, food, and homeland security.1 Some VOCs are important for their role in disease biomarking (e.g. acetone for diabetes), toxicity, flammability or other hazardous characteristics, making the monitoring and detection of trace VOCs of prime importance.2 Recently, metal–oxide semiconducting (MOS) materials have been indicated to be good candidates for VOC gas sensors because of their irreplaceable advantages, such as low cost, simple fabrication, miniaturized size, portability and good compatibility with Si processes.3Nowadays, numerous researches have been focused on highly active nanomaterials, including nanoparticles,4 one-dimensional nanostructures,5 nanosheets6 and hollow spheres,7 as well as their building blocks,8 to develop VOC gas sensors with enhanced performance. It has been also identified that hollow and porous nanoarchitectures are more promising for large surface area to volume ratio, high gas accessibility and rapid gas responding kinetics.9 With such porous nanostructured MOS sensors, far-ranging gases and vapors can be efficiently detected and monitored with powerful sensitivity.10 However, the deterioration of sensing properties by water vapors in real-atmospheres may cause obstacles for the reliability of MOS sensors, which is an acute issue for MOS sensors. If the humidity-dependent problem could be adequately rectified, MOS hollow spheres sensors would be suitable to serve in various application areas because they possess advantages in terms of sensitivity, rapid response and miniaturization capabilities.7
Gas sensing films have been doped or surface-functionalized to achieve gas selectivity.11 To date, numerous experimental and theoretical works are reported to detect acetone gas using modified MOS films, such as Pt–In2O3,12 Pt–WO3,13 Cr–WO3,14 Si–WO3,15–17 graphene modified ZnFe2O4,18 and so forth.19 Some of them have demonstrated superior responses to acetone below 1 ppm in highly humid atmospheres (relatively humidity (RH) ≥ 80%) for the application of breath acetone monitoring.16,17,20 Nevertheless, their gas sensing behaviors at varying humidity from RH 20% to RH 80% are rarely involved. In general, the introduction of water vapor into the atmosphere deteriorates gas response, response/recovery speed, and selectivity to the reducing gas in n-type oxide semiconductor sensors,21 which hinders the reliable and rapid detection of the trace concentration of analyte gases. It should be noted that the enhancement of gas response in highly humid atmosphere, which is highly advantageous to detect VOC gases in real atmosphere, has been scarcely reported, although there was a report on the enhancement of CO response by increasing humidity in a Pd-loaded SnO2 sensor.22 The humidity-independent characteristics of gas sensors show a possibility of exhaled breath analysis because exhaled breath contains >80% humidity at 25 °C. Therefore, to materialize superior sensors, it requires not only a production of a preferable sensing material with VOCs selectivity and fast response, but also to make progressive investigations on the control of sensing properties in highly humid atmospheres.
In this paper, we report an Rh-loaded WO3 hollow spheres (HWs) acetone sensor that can be potentially used for real-time VOCs analysis. This sensor achieves fast response (2 s), high sensitivity, low detection limit (40 ppb) and strong selectivity to trace acetone gas even under a highly humid (80% RH) atmosphere, comparing with the unloaded one. It is found that the n-type Rh-loaded WO3 sensor exhibits an abnormal phenomenon, as the resistance and gas response increase in humid atmospheres. This unusual improvement can provide a good potential in the real-time acetone gas analysis for the application in diabetic diagnosis.
2. Experimental
2.1 Sample preparation
The WO3 hollow spheres were prepared using a pre-suggested method.23 Tungsten oxide (2.3185 g, WO3, 99.995%, Sigma-Aldrich, USA) and citric acid monohydrate (2.1014 g, C6H8O7·H2O, 99.0%, Sigma-Aldrich, USA) were dissolved in 500 mL of a 2.8–3.0% diluted ammonium hydroxide aqueous solution and stirred until the solution was clear. The solution was transferred to a droplet generator for the spray pyrolysis. A large amount of droplets generated by six ultrasonic transducers (resonance frequency: 1.67 MHz) were carried to a high temperature (700 °C) quartz-tube reactor by a carrier gas (air, 40 L min−1) and then condensed. The detailed experimental setup is shown elsewhere.24 The condensed W-precursor was collected with a Teflon bag filter in the particle-collecting chamber and converted into WO3 by heat treatment at 500 °C for 1 h. To load 0.5 at% Rh on the WO3, an aqueous slurry containing rhodium trichloride hydrate (0.0113 g, RhCl3·xH2O, 99.9%, Sigma-Aldrich, USA) and WO3 (0.5 g) were stirred at 80 °C for 2 h, dried at 70 °C for 12 h, and heat treated at 500 °C for 1 h.2.2 Characterization
The morphologies and composition of the pure and Rh-loaded WO3 hollow spheres were characterized using field emission scanning electron microscopy (FE-SEM, S-4700, Hitachi Co. Ltd., Japan) and high resolution transmission electron microscopy (HRTEM, JEM-ARM-200F, JEOL, USA). The phases of the samples were analyzed by X-ray diffraction (XRD, D/MAX-2500V/PC, Rigaku, Japan) and X-ray photoelectron spectroscopy (XPS, VG Multilab ESCA 2000 system, Thermo Fisher Scientific Inc., USA). To determine the precise loading concentration of Rh in WO3 hollow spheres, an inductively coupled plasma spectrometer (ICP, iCAP 6300 SERIES, Thermo Fisher Scientific Inc., USA) was used.2.3 Gas sensing characteristics
The pure or Rh-loaded WO3 hollow spheres were dispersed in deionized water and the slurry was coated on alumina substrate (area: 1.5 × 1.5 mm2, thickness: 0.25 mm) with two Au electrodes on its top surface (electrode widths: 1 mm, separation: 0.2 mm) and a micro-heater on its bottom surface. The sensors were dried at 70 °C for 12 h and heat-treated at 500 °C for 2 h. The sensor temperatures were controlled using the micro-heater underneath the substrate and were measured using an IR temperature sensor (Metis MP25, Sensortherm GmBH, Germany). Gas sensing characteristics were measured at 300–450 °C. The concentrations of gases and relative humidity were independently controlled by mixing the gases (100 ppm CH3COCH3, CO, NH3, and 5 ppm H2S, benzene, toluene, p-xylene, NO in air balance) and dry or humid synthetic air. The Ra/Rg (Ra: resistance in air, Rg: resistance in gas) values were used as the gas responses. A constant flow rate of 500 ml min−1 was employed and a dc 2-probe resistance of the sensor was measured using a multimeter (Agilent 34970A) interfaced with a computer.3. Results and discussion
3.1 Synthesis of the microstructures of Rh-loaded WO3 HWs
The synthesis of the WO3 hollow spheres starts from the hollow W-precursor spheres (Fig. 1a) that were prepared via ultrasonic spray pyrolysis based on our previous work.23 After a heat treatment at 500 °C for 1 h, these precursors could be oxidized to crystalline WO3 hollow spheres, and the morphology and sphere size are basically unchanged (Fig. 1b). The corresponding transmission electron microscope (TEM) image (Fig. 1c) clearly shows the hollow structures. These WO3 hollow spheres are characterized by very thin shell thickness (∼25 nm in Fig. 1d), which must be beneficial to possess high gas response due to the effective electron depletion.23 Fig. 1e displays that a hollow sphere consists of highly crystalline WO3 nanoparticles. The interplanar spacing of 3.85 Å of the (001) plane and 3.69 Å of the (200) plane with an angle of 90° reveal an orthorhombic structure. In addition, the corresponding selected-area electron diffraction pattern (the inset of Fig. 1e) demonstrates that the WO3 HWs are polycrystalline.With the aim to improve the exhaled breath sensing characteristics, nanoscale rhodium oxide is functionalized on the surface of the WO3 HWs by a simple slurry mixing and after-heating process. Fig. 1f represents the TEM image of the Rh-loaded WO3 HWs with a designed 0.5 at% Rh concentration ([Rh]/[W]), showing a similar morphology, size distribution and shell thickness with prototype sample (Fig. 1c). The precise content of rhodium in Rh–loaded WO3 hollow spheres is also confirmed as 0.45 at% by inductive coupled plasma emission spectrometer (ICP) analysis. Further, elemental mapping images (Fig. 1g) suggest that Rh was uniformly dispersed on the surface of the WO3 hollow spheres.
3.2 Phase and chemical state analysis
Fig. S1† shows the X-ray diffraction (XRD) patterns for the as-synthesized WO3 HWs and Rh-loaded WO3 HWs. All the peaks for both the samples are well-matched with the standard WO3 (JCPDS no. 20-1324), indicating an orthorhombic structure with the main growth directions along the (001) and (200) plane. It also shows a good consistency with the TEM results (Fig. 1e). With respect to the Rh-loaded one, no Rh-related phase can be found in its XRD spectrum because of the small amount of Rh.Further, the X-ray photoelectron spectroscopy (XPS) measurements were carried out to detect Rh components and determine their chemical state (Fig. S2†). It demonstrates that the survey scanning energy spectra (Fig. S2a†) for both the samples are almost the same. The weak peaks located at 308.5 and 313.8 eV observed in Rh-loaded WO3 are attributed to Rh 3d5/2 and Rh 3d3/2 in Rh3+, respectively, rather than the metal phase Rh0 (Fig. S2b†).25 The binding energies of W 4f7/2 and 4f5/2 are 35.3 and 37.4 eV (Fig. S2c†) in the pure sample, respectively, indicating a 6+ state of tungsten. However, the binding energies of W 4f7/2 and 4f5/2 occur with a slight red chemical shift of 0.1 eV probably due to the increasing oxygen vacancy because of Rh loading, which is similar to a previously reported Au-loaded WO3 sample.26 Moreover, the O 1s peak at 530.2 eV of both samples is typically ascribed to W–O in WO3 (Fig. S2d†). Consequently, rhodium is obtained as Rh2O3 rather than its incorporation in the lattice of WO3, and the increased oxygen vacancy by Rh loading may bring an enhancement of gas sensing performance by promoting oxygen adsorption.27
3.3 Gas sensing characteristics
Acetone gas sensing properties of pure and Rh-loaded WO3 hollow spheres are measured in dry and various humid atmospheres. Fig. 2 shows the dynamic sensing transients of pure and Rh-loaded WO3 sensors toward 10 ppm acetone gas at 400 °C in dry, 20% RH, 50% RH, and 80% RH atmospheres, respectively. In the dry atmosphere, the acetone response (Ra/Rg) of the Rh-loaded sensor is ca. 13.34 (Fig. 2b-1), which is about two times higher than that of the pure sensor, which shows response of 6.97 (Fig. 2a-1). Moreover, the response and recovery time (τres and τrecov, time spans taken for the response to reach [in gas] and decrease by [return to air] 90% of its steady value, respectively) is decreased from 6 s and 158 s to 2 s and 129 s by Rh loading, respectively. Therefore, the sensor based on Rh-loaded WO3 HWs exhibits enhanced acetone sensing performances than those of the prototype one.The acetone sensing properties of the pure sensor in which Ra/Rg and Ra are decreased in wet ambient (20–80% RH) (Fig. 2a-2, a-3, a-4). This is a general behavior of n-type MOS gas sensor. For confirmation, pure SnO2, ZnO and In2O3 hollow spheres were prepared via spray pyrolysis and their gas sensing characteristics were measured (Fig. S3†). Indeed, all the pure sensors showed the significant decreases of Ra/Rg and Ra with increasing ambient humidity, which is a normal behavior for many MOS sensors.21 Righettoni et al.16 also reported the deterioration of the acetone sensing properties of Si-doped WO3 in highly humid atmospheres. In a stark contrast, an abnormal phenomenon occurs on Rh-loaded WO3 sensor, where the Ra/Rg and Ra increased in humid atmospheres (Fig. 2b-2, b-3, b-4). The sensing response at 80% RH increases from 5.70 to 20.68 after Rh loading. The τres of the pure WO3 sensor gradually increased from 6 s to 11 s with increasing humidity, while the τres of Rh-loaded sensor remains at 2 s throughout the humidity range. The tendency was confirmed again by the continuous operation of the sensor with increasing relative humidity (Fig. S4†). The WO3 HWs loaded with other noble metal catalysts (0.5 at% Pd or 0.5 at% Pt) showed a significant deterioration of acetone sensing characteristics (Fig. S5†), confirming that the role of the Rh catalyst to promote the gas sensing reaction under humid atmospheres is unique and unusual.
Fig. 3 presents an overview of 10 ppm acetone-sensing properties, such as Ra/Rg, Ra, τres and τrecov, as functions of the working temperature and relative humidity. The Ra/Rg values of two sensors progressively increase with increasing temperature in dry and humid atmospheres (Fig. 3a). Moreover, the Ra/Rg values of the Rh-loaded sensor are ∼2 times higher than those of the pure sensor at all temperatures in the dry atmosphere. However, the two sensors displayed different Ra/Rg vs. humidity tendencies with varying sensor temperatures. Below 325 °C, the Ra/Rg values of both sensors decrease with increasing relative humidity. Beyond 350 °C, the Rh-loaded sensor shows an inverse behavior of the increase of Ra/Rg with the relative humidity, while the Ra/Rg of the unloaded WO3 sensor still decreases with the increasing humidity. Fig. 3b shows the dependence of Ra on the working temperature at different ambient humidity. The resistances of two sensors in dry air are similar in the whole temperature range, indicating little influence on the resistances by Rh loading. In humid atmosphere, the Ra of pure WO3 sensor is decreased by water vapors, while the Ra of the Rh-loaded sensor increases (marked by arrows in Fig. 2) in all the temperature range (Fig. 3b). In Fig. 3b, it can be observed that the resistance of the pure WO3 sensor significantly increases by 27% when the operation temperature is declined from 400 °C to 300 °C in 80% RH condition. However, the increased rate of resistance of Rh-loaded WO3 sensor is only 3% when the temperature decreases from 400 °C to 300 °C (Fig. 3b), exhibiting an anti-jamming and stable characteristic. The abnormal tendencies of Ra/Rg and Ra reflect that Rh loading can probably introduce a change of humidity cross sensing mechanism at different temperatures (see the next section).
Further, we also observe the response and recovery rate of the two sensors, as shown in Fig. 3c and d, respectively. From dry to humid atmosphere, it is found that the response rate of the pure WO3 sensor becomes 1.5–2 times more sluggish, while τres values of the Rh-loaded sensor are almost invariable, in particular above 350 °C (see Fig. 3c). It should be noted that the response speeds of the Rh-loaded sensor are 2–6 and 3–12 times faster than those of the pure sensor under dry and humid condition, respectively (Fig. 3c). In addition, both the sensors have faster recovery speed under humid conditions compared to dry atmosphere, moreover the τrecov of the Rh-loaded sensor is shorter than that of the pure one in 300–400 °C range (Fig. 3d). Because all the gas sensing characteristics (Ra/Rg, τres and τrecov) are significantly enhanced by Rh loading, this Rh-loaded WO3 HWs sensor is applicable as disease diagnosis tool in an effective manner.
Fig. 4 presents the gas response of the two sensors to 0.2–20 ppm acetone in different humidities (0–80% RH) at 400 °C, indicating a good linear relation between Ra/Rg and the gas concentration. Both the sensors have stable response and recovery performances under various relative humidity conditions (Fig. S6 and S7†). The lowest acetone detection limit of the Rh-loaded sensor is calculated to be ca. 50 ppb and 40 ppb in dry air and 80% RH ambient when Ra/Rg > 1.2 was used as the criterion for gas detection, respectively, which are 4 times lower than those of the unloaded WO3 sensor.
 | ||
| Fig. 4 Gas responses of pure and Rh-loaded WO3 sensors as functions of acetone gas concentration at 400 °C in different humidity (0–80% RH) atmospheres. | ||
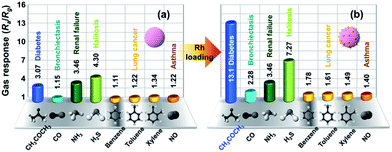
The trace acetone detection limit, fast response and negligible temperature–resistance dependence in a highly humid atmosphere of the Rh-loaded WO3 sensor can be applied for diabetes breath sensors. The key qualification for exhaled breath sensors is the selectivity of the target gas because of various interfering VOCs gases in human exhalation, such as CH3COCH3 (1.8 ppm for diabetes),28 H2S (∼0.5 ppm for halitosis),29 CO (8.6 ppm for bronchiectasis),30 NH3 (14.7 ppm for renal failure),31 benzene, toluene, xylene (10 ppb for lung cancer),32 and NO (100 ppb for asthma),33 although the precise criteria for medical diagnosis are still under investigation. Fig. 5 summarizes the sensing response for pure and Rh-loaded WO3 sensors to these gases at 400 °C in 80% RH, respectively. The concentrations of these gases are as follows: 4 ppm CH3COCH3, 20 ppm CO, 20 ppm NH3, 1 ppm H2S, 20 ppb C6H6 (benzene), 20 ppb toluene (CH3C6H5), 20 ppb xylene ((CH3)2C6H4), and 200 ppb NO. It should be noted that the responses toward ∼2 times higher concentration of biomarker gases than the detection limit for the diagnosis of diseases were measured (Table S1†) to more accurately examine the selectivity for CH3COCH3 and possible interferences from other gases. The response of the pure sensor to 4 ppm CH3COCH3 is 3.07 (see Fig. 5a), which is lower than those for 20 ppm NH3 (Renal failure, Ra/Rg = 3.46) and 1 ppm H2S (halitosis, Ra/Rg = 4.30). Therefore, if a patient is suffering from renal failure or halitosis, accurate diagnosis of diabetes is impossible. In contrast, the Rh-loaded sensor can overcome the above trouble as its Ra/Rg to 4 ppm CH3COCH3 (13.1) is 4 times higher than the prototype one (see Fig. 5b). This merit of the Rh-loaded sensor can possibly make an accurate identification of a diabetes patient even from patients suffering with either halitosis (Ra/Rg = 7.27) or renal failure (Ra/Rg = 3.46).
3.4 Gas sensing mechanism
In Fig. 2a, 3a and b, we can observe that the Ra/Rg and Ra of the pure WO3 sensor significantly decrease in highly humid atmosphere. According to the previous literature,34 decrease in the resistances of n-type semiconductor materials results from adsorbed oxygen consumption and electron generation in the presence of water vapors as described by eqn (1). The Ra/Rg is also deteriorated due to competition between reducing gas and water.| H2O(g) + O−(W) → 2OH(W) + e− | (1) |
Rh (111) has strong affinity for water compared with other metal surfaces,35 and gets easily combined with hydroxyl group and hydrogen that are obtained by the decomposition of water.36 This suppresses the direct reaction between the sensing surface (WO3 in the present study) and water, which prevents the deterioration of gas sensing characteristics. Moreover, the decomposition of water by rhodium is accelerated because the co-adsorbed oxygen is cut down to nearly half of activation energy.35 Therefore, more adsorbed oxygen can be formed through synergy between rhodium and surface oxygen adsorbed on metal oxide at high temperature, as depicted in eqn (2).
| H2O(Rh) + O−(W) → OH(Rh) + OH(W) + e− → 2H(Rh) + 2O−(W) + h+ | (2) |
Because oxygen decomposed from water is adsorbed on the sensor (WO3) surface and gets ionized, the depletion region on the surface of the sensor will be expanded, which leads to huge changes in resistance. This mechanism can well explain why the Rh-loaded sensor has higher Ra/Rg and Ra in humid atmospheres. This abnormal phenomenon suggests a new direction for the real-time developing of self-diagnosis sensor for diseases, using the direct analysis of highly humid exhaled breath.
If the hydroxyl group does not sufficiently decompose into hydrogen and oxygen due to the energy shortage (low temperature), Ra/Rg is reduced because of the competition between the target gas and water vapors reacting with surface adsorbed oxygen (O−W). It results in the differences of Ra/Rg changes at different sensing temperature (Fig. 3a). At a low temperature like 300 °C, a decrease of Ra/Rg is induced by the adsorption of hydroxyl groups formed by water vapor (eqn (1)). On the contrary, Ra/Rg is gradually raised due to the increasing adsorbed oxygen formed by the decomposition of hydroxyl group and water above 350 °C (eqn (2)).
In addition, rhodium is known to play a role of securing the selective detection of acetone gas among other VOCs,37 which is consistent with the present results. Therefore, the Rh-loaded WO3 hollow spheres possesses a high response (Ra/Rg = 20.68 to 10 ppm CH3COCH3), rapid response time (τres = 2 s), excellent acetone selectivity and negligible temperature dependence of sensor resistance in a highly humid atmosphere (RH 80%), and low detection limit (40 ppb) at 400 °C which grants a rapid and reliable detection of trace acetone (Table 1).
| Pure WO3 | Rh-loaded WO3 | |||
|---|---|---|---|---|
| Dry | RH 80% | Dry | RH 80% | |
| Ra/Rg | 6.97 | 4.57 | 13.34 | 20.68 |
| τres (s) | 6 | 11 | 2 | 2 |
| Ra (MΩ) | 14.91 | 11.43 | 14.06 | 29.53 |
| Detection limit (ppb) | 167 | 201 | 52 | 40 |
4. Conclusions
Pure WO3 and Rh-loaded WO3 hollow spheres were synthesized based on the spray pyrolysis method and their acetone gas sensing properties were measured in dry and various humid atmospheres (20–80% RH). The Rh-loaded WO3 sensor exhibited enhanced acetone sensing performances, such as fast response (ca. 2 s), four times higher response, lower detection limit (40 ppb), good linearity, high stability and strong acetone selectivity even in highly humid atmospheres (80% RH), compared with the pure WO3 sensor. Interestingly, an anomalous phenomenon occurred on the Rh-loaded sensor, where the Ra/Rg and Ra increased in humid atmospheres, compared with other sensors, which showed decreasing values of Ra/Rg and Ra. The abnormal sensing mechanism by Rh loading was also addressed. In addition, a low resistance (Ra)-temperature dependence of Rh-loaded sensor can grant a reliable detection of trace acetone in different external environments. This catalytic-Rh-induced unusual improvement of gas response, selectivity, and responding kinetics even in highly humid atmospheres can probably show a good potential for the real-acetone gas analysis containing high concentration of water vapor.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (no. 2013R1A2A1A01006545) and supported by Industrial Strategic Technology Development Program (no.10047868) funded by the Ministry of Trade, Industry & Energy (KEIT).Notes and references
- A. Modi, N. Koratkar, E. Lass, B. Q. Wei and P. M. Ajayan, Nature, 2003, 424, 171–174 CrossRef CAS PubMed; T. Hoshi and S. Lahiri, Science, 2004, 306, 2050–2051 CrossRef PubMed; J. Kong, N. R. Franklin, C. W. Zhou, M. G. Chapline, S. Peng, K. J. Cho and H. J. Dai, Science, 2000, 287, 622–625 CrossRef PubMed.
- M. Phillips, K. Gleeson, J. M. B. Hughes, J. Greenberg, R. N. Cataneo, L. Baker and W. P. McVay, Lancet, 1999, 353, 1930–1933 CrossRef CAS; H. Haick, Y. Y. Broza, P. Mochalski, V. Ruzsanyi and A. Amann, Chem. Soc. Rev., 2014, 43, 1423–1449 RSC.
- Z. Dai, L. Xu, G. Duan, T. Li, H. Zhang, Y. Li, Y. Wang, Y. Wang and W. Cai, Sci. Rep., 2013, 3, 1669 Search PubMed.
- J. Qin, M. Cao, N. Li and C. Hu, J. Mater. Chem., 2011, 21, 17167–17174 RSC.
- R. S. Devan, R. A. Patil, J. H. Lin and Y. R. Ma, Adv. Funct. Mater., 2012, 22, 3326–3370 CrossRef CAS.
- S. Some, Y. Xu, Y. Kim, Y. Yoon, H. Qin, A. Kulkarni, T. Kim and H. Lee, Sci. Rep., 2013, 3, 1868 Search PubMed.
- H.-R. Kim, K.-I. Choi, K.-M. Kim, I.-D. Kim, G. Cao and J.-H. Lee, Chem. Commun., 2010, 46, 5061–5063 RSC; K.-M. Kim, K.-I. Choi, H.-M. Jeong, H.-J. Kim, H.-R. Kim and J.-H. Lee, Sens. Actuators, B, 2012, 166, 733–738 CrossRef CAS.
- K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 6131–6144 CrossRef CAS PubMed.
- M. Tiemann, Chem.–Eur. J., 2007, 13, 8376–8388 CrossRef CAS PubMed; J.-H. Lee, Sens. Actuators, B, 2009, 140, 319–336 CrossRef.
- M. A. Lim, D. H. Kim, C.-O. Park, Y. W. Lee, S. W. Han, Z. Li, R. S. Williams and I. Park, ACS Nano, 2011, 6, 598–608 CrossRef CAS PubMed; X. Li, J. H. Cho, P. Kurup and Z. Gu, Sens. Actuators, B., 2012, 162, 251–258 CrossRef.
- M. Kimura, R. Sakai, S. Sato, T. Fukawa, T. Ikehara, R. Maeda and T. Mihara, Adv. Funct. Mater., 2012, 22, 469–476 CrossRef CAS; Z. Li, H. Zhang, W. Zheng, W. Wang, H. Huang, C. Wang, A. G. MacDiarmid and Y. Wei, J. Am. Chem. Soc., 2008, 130, 5036–5037 CrossRef PubMed.
- G. Neri, A. Bonavita, G. Micali and N. Donato, IEEE Sens. J., 2010, 10, 131–136 CrossRef CAS.
- S.-J. Choi, I. Lee, B.-H. Jang, D.-Y. Youn, W.-H. Ryu, C. O. Park and I.-D. Kim, Anal. Chem., 2013, 85, 1792–1796 CrossRef CAS PubMed.
- L. Wang, K. Kalyanasundaram, M. Stanacevic and P. Gouma, Sens. Lett., 2010, 8, 709–712 CrossRef CAS.
- M. Righettoni, A. Tricoli and S. E. Pratsinis, Anal. Chem., 2010, 82, 3581–3587 CrossRef CAS PubMed.
- M. Righettoni, A. Tricoli, S. Gass, A. Schmid, A. Amann and S. E. Pratsinis, Anal. Chim. Acta, 2012, 738, 66–75 CrossRef PubMed.
- M. Righettoni and A. Tricoli, J. Breath Res., 2011, 5, 037109 CrossRef PubMed.
- F. Liu, X. Chu, Y. Dong, W. Zhang, W. Sun and L. Shen, Sens. Actuators, B, 2013, 188, 469–474 CrossRef CAS.
- S. Ryabtsev, A. Shaposhnick, A. Lukin and E. Domashevskaya, Sens. Actuators, B, 1999, 59, 26–29 CrossRef CAS.
- J. Shin, S.-J. Choi, I. Lee, D.-Y. Youn, C. O. Park, J.-H. Lee, H. L. Tuller and I.-D. Kim, Adv. Funct. Mater., 2013, 23, 2357–2367 CrossRef CAS.
- K.-I. Choi, M. Hübner, A. Haensch, H.-J. Kim, U. Weimar, N. Barsan and J.-H. Lee, Sens. Actuators, B, 2013, 183, 401–410 CrossRef CAS; K.-I. Choi, H.-J. Kim, Y. C. Kang and J.-H. Lee, Sens. Actuators, B, 2014, 194, 371–376 CrossRef.
- N. Barsan and U. Weimar, J. Phys.: Condens. Matter, 2003, 15, R813 CrossRef CAS.
- Y. H. Cho, Y. C. Kang and J.-H. Lee, Sens. Actuators, B, 2013, 176, 971–977 CrossRef CAS.
- J. R. Sohn, Y. C. Kang and H. D. Park, Jpn. J. Appl. Phys., Part 1, 2002, 41, 3006–3009 CrossRef CAS.
- A. A. Tolia, R. Smiley, W. Delgass, C. G. Takoudis and M. J. Weaver, J. Catal., 1994, 150, 56–70 CrossRef CAS.
- C. Navío, S. Vallejos, T. Stoycheva, E. Llobet, X. Correig, R. Snyders, C. Blackman, P. Umek, X. Ke, G. Van Tendeloo and C. Bittencourt, Mater. Chem. Phys., 2012, 134, 809–813 CrossRef.
- N. Yamazoe and K. Shimanoe, Sens. Actuators, B, 2008, 128, 566–573 CrossRef CAS.
- C. Deng, J. Zhang, X. Yu, W. Zhang and X. Zhang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 810, 269–275 CrossRef CAS.
- F. L. Suarez, J. K. Furne, J. Springfield and M. D. Levitt, J. Dent. Res., 2000, 79, 1773–1777 CrossRef CAS PubMed.
- I. Horvath, S. Loukides, T. Wodehouse, S. Kharitonov, P. Cole and P. Barnes, Thorax, 1998, 53, 867–870 CrossRef CAS PubMed.
- S. Davis, P. Spanel and D. Smith, Kidney Int., 1997, 52, 223–228 CrossRef.
- D. Poli, P. Carbognani, M. Corradi, M. Goldoni, O. Acampa, B. Balbi, L. Bianchi, M. Rusca and A. Mutti, Respir. Res., 2005, 6, 71 CrossRef PubMed.
- S. A. Kharitonov and P. J. Barnes, Biomarkers, 2002, 7, 1–32 CrossRef CAS PubMed.
- K. Grossmann, R. G. Pavelko, N. Barsan and U. Weimar, Sens. Actuators, B, 2012, 166–167, 787–793 CrossRef CAS.
- P. J. Feibelman, Phys. Rev. Lett., 2003, 90, 186103 CrossRef PubMed.
- M. Pozzo, G. Carlini, R. Rosei and D. Alfè, J. Chem. Phys., 2007, 126, 164706 CrossRef CAS PubMed; A. Shavorskiy, T. Eralp, E. Ataman, C. Isvoranu, J. Schnadt, J. N. Andersen and G. Held, J. Chem. Phys., 2009, 131, 214707 CrossRef PubMed.
- C. Houtman and M. A. Barteau, J. Phys. Chem., 1991, 95, 3755–3764 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray diffraction patterns, X-ray photoelectron spectroscopy, gas sensing behaviors of pure and Rh-loaded WO3 hollow spheres and other reference materials. See DOI: 10.1039/c4ra06654e |
| This journal is © The Royal Society of Chemistry 2014 |