Template confined synthesis of Cu- or Cu2O-doped SiO2 aerogels from Cu(II)-containing composites by in situ alcohothermal reduction†
Weiwei Xu,
Ai Du*,
Jun Tang,
Peng Yan,
Xiaoguang Li,
Zhihua Zhang,
Jun Shen and
Bin Zhou*
Shanghai Key Laboratory of Special Microstructure Materials and Technology, School of Physics Science and Engineering, Tongji University, Shanghai 200092, P. R. China. E-mail: zhoubin863@.tongji.edu.cn; duai@tongji.edu.cn; Fax: +86-21-65986071; Tel: +86-21-65982762
First published on 10th September 2014
Abstract
Uniform and highly dispersed Cu- or Cu2O-doped SiO2 aerogels were synthesized via an in situ alcohothermal reduction strategy. The initial templates containing an accurately controllable doping fraction were prepared through a co-gelation method. After alcohothermal reduction and CO2 supercritical drying, a similar morphology of the resulting samples to the initial templates was retained. The entire reducing process was systematically studied through comparative experiments, separately using ethanol, ethylene glycol and glycerol as reducing agents, with the corresponding converted products obtained being cubic Cu2O- and Cu-contained silica composites. The specific surface area of the resulting products ranged from 500 m2 g−1 to 850 m2 g−1. The related microstructure evolution mechanism was comprehensively studied through analysis of the pore-size distribution. The high specific surface area and controlled doping amount make it suitable for possible use in specific applications, such as high efficiency photocatalysis and backlight targets.
1. Introduction
Aerogel material assembled by nanoscale colloidal particles or polymers could be recognized as a state of matter, rather than as a functional material.1,2 Among various aerogels, metal and metal-oxide porous materials have attracted increasing attention in special applications, such as gas sensors, electrochemistry, catalysis, thermal insulation, and inertial confinement fusion (ICF) targets.3–9 However, due to the high reactivity and weak cross-linking nature of nanoscale metal particles, it is difficult to prepare a freestanding aerogel composed of pure metal.10 Thus, most studies have focused on the preparation of metal-doped silica aerogels, thus adopting the functionality of the metal and the stability of silica networks.11–16 The general idea is to directly glue the metal or metal oxide particles using silica sol.17,18 However, due to the influence of gravity, sedimentation could lead to longitudinal heterogeneity of the final aerogels during gelation and the drying process.19–21 The concentration inhomogeneity results in inhomogeneity properties, which may significantly affect their application. For example, in inertial-confinement fusion experiments (ICF), the distribution of metal particles plays an important role in the conversion efficiency of converting laser energy into multi-keV X-rays.22,23 Thus, it is still a problem how to prepare homogeneous metal- or metal oxide-doped silica aerogels.In this work, the in situ alcohothermal reduction method was employed to synthesize homogeneous Cu/SiO2 composite aerogels with an accurately controllable doping fraction. The Cu(II)-contained silica gel templates were prepared through a propylene oxide pre-reaction method, with the Cu(II) compound and silica colloidal particles simultaneously cross-linked into the homogeneous binary gel frameworks. Significantly, the mole ratio of Cu to Si could be accurately controlled and could reach as high as 20% via this method. Afterward, the Cu(II)-contained silica gels were directly reduced by the alcohothermal method with a different alcohol as a reducing agent. Finally, Cu- or Cu2O-doped SiO2 aerogels were obtained after supercritical fluid drying. According to our knowledge, to date, there has been no related report about the in situ alcohothermal reduction of metal-based gels in composite networks. In addition, the uniform nanoparticles size and high dispersion of the resulting products not only exhibit a better performance, but also provide a quantitative media to study the mechanism in photocatalysis or ICF backlights.
2. Experiment
Tetramethoxysilane (TMOS), copper chloride (CuCl2·2H2O), 1,2-epoxy propane (PO, 99.0%), acetonitrile, ethanol, ethylene glycol, and glycerol were purchased from Sinopharm Chemical Reagent Co. Ltd., China. All chemicals used in the research are used without further purification.The propylene oxide pre-reaction method was used to synthesize the Cu(II)-contained silica composite templates according to the following procedure. First, tetramethoxysilane, acetonitrile, deionized water, and propylene oxide with the volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.018
0.018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.63 were mixed together for pre-reaction. The solution was then poured into another mixture of CuCl2·2H2O (2.264 g), acetonitrile (20 ml), and water (1.972 ml). Following mixing, propylene oxide (3 ml) was added into the mixed solution, and stirred for several minutes. Finally, homogeneous dark-green gels were obtained after sufficient hydrolysis and condensation of the precursors within 30 min at 35 °C. The wet gels were aged for at least 48 h and then sealed together with 35 ml of reducing agent in a Teflon-lined stainless-steel autoclave (50 ml). The autoclave was heated to 200 °C, at a rate of 1 °C min−1, dwelling there for 10 h and undergoing natural cooling. The resulting products were washed with ethanol several times, and then dried with CO2 supercritical drying. In order to systematically study the whole reducing process, ethanol, ethylene glycol, and glycerol were separately used as reducing agents. In this paper, the initial aerogel templates and corresponding products reduced by ethanol, ethylene glycol, and glycerol were marked as AG-1, AG-2, AG-3, and AG-3, respectively.
0.63 were mixed together for pre-reaction. The solution was then poured into another mixture of CuCl2·2H2O (2.264 g), acetonitrile (20 ml), and water (1.972 ml). Following mixing, propylene oxide (3 ml) was added into the mixed solution, and stirred for several minutes. Finally, homogeneous dark-green gels were obtained after sufficient hydrolysis and condensation of the precursors within 30 min at 35 °C. The wet gels were aged for at least 48 h and then sealed together with 35 ml of reducing agent in a Teflon-lined stainless-steel autoclave (50 ml). The autoclave was heated to 200 °C, at a rate of 1 °C min−1, dwelling there for 10 h and undergoing natural cooling. The resulting products were washed with ethanol several times, and then dried with CO2 supercritical drying. In order to systematically study the whole reducing process, ethanol, ethylene glycol, and glycerol were separately used as reducing agents. In this paper, the initial aerogel templates and corresponding products reduced by ethanol, ethylene glycol, and glycerol were marked as AG-1, AG-2, AG-3, and AG-3, respectively.
The surface morphologies of the samples were examined by a scanning electron microscope (SEM, Philips-XL30), which was additionally equipped with an energy dispersive X-ray spectrometer (EDS). A high-resolution transmission electron microscope (TEM, JEOL JEM-2010) was used to characterize the microstructure. The components and crystal structure of the resulting products were detected by X-ray diffraction (XRD, Rigaku D/Max-RB). The specific surface area and pore structure parameters of the aerogels were obtained from nitrogen sorption isotherms tested at 77 K, using a Quantachrome Autosorb-1 analyzer. The Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods were used, respectively, to calculate the specific surface area and pore size distribution.
3. Results and discussions
3.1 The formation mechanisms of Cu(II)-contained SiO2 gels
The whole experiment process is shown in Scheme 1. Acetonitrile, rather than ethanol, was selected as the solvent, because the ethanol system exhibits not only a mismatch of gelation time between the two precursors, but also a much larger shrinkage during the ageing and drying processes. This could be explained by the discrepancy between the different solvents in polarity and coordination effects.24–26 As a strong polar solvent, acetonitrile exhibited more significant solvent effects than ethanol. That is to say the nitrile group partly replaced the coordinated H2O on [Cu(H2O)n(OH)x−n]2+ (x ≤ 6), which stabilized the copper component reactants and, as a result, slowed down the condensation rate to some extent. Due to the fact that acetonitrile is a highly polar aprotic solvent that does not form hydrogen bonds with the silicate nucleophile, the hydrolysis rate of TMOS can be significantly accelerated compared to the ethanol solvent system.27,28 In addition, a propylene oxide pre-reaction method was employed during the TMOS solution reaction, which also partly improved the extent of the hydrolysis. Therefore, the reaction rate was balanced between two different precursors, which was beneficial for the formation of homogeneous Cu(II)/SiO2 binary networks.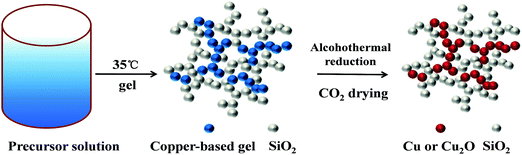 | ||
| Scheme 1 Schematic of the formation of Cu(II)-contained silica gels and the subsequent alcohothermal reduction process. | ||
As shown in the following equation, a similar ring-opening addition reaction of the propylene oxide was conducted in different precursor solutions. In this reaction, PO acts as an irreversible proton capture agent, and two products are generated with similar chemistry structural formulas.
 | (1) |
In the TMOS system, H+ was the hydrolysis product of TMOS, and was then combined with the oxygen atom on the epoxy group. Due to the instability of the hydroxylating carbon–oxygen ring, the ring-opening reaction and the subsequent nucleophilic addition process of the methoxyl group easily occurred, which partly accelerated formation of the silica gel network.29 It is notable that the hydrolysis product, –OCH3, exhibited weak electronegativity and that as the solution was in an acidic environment, the ring-opening addition reaction tended to take place first, with the alkoxyalcohol product mainly being the second item.30,31 When the two solutions were mixed together, there was a similar reaction on PO and hydrogen halide, which were produced by the hydrolysis of [Cu(H2O)n(OH)x−n]2+. However, the strong electronegativity of the chloride ion made the major reaction tend to generate the chloroalcohol product with the first form of the structural formula, as shown in eqn (1).32 A homogeneous and coherently supported binary gel with randomly interconnected networks was obtained through the co-gelation process previously mentioned.
3.2 Conversion of Cu(II)-contained silica gels into Cu2O- or Cu-doped SiO2 aerogels
The main oxidation–reduction reaction is shown in eqn (2) (x = 1, 2). During the alcohothermal process, the Cu(II)-contained gel skeletons were gradually reduced by the hydroxyl groups in the alcohols, which were then converted into the aldehyde or carbonyl derivatives.33,34
 | (2) |
As shown in Fig. 1, a macroscopic difference in the resulting products is apparent. From the left to right, the samples with separate densities of 230 mg cm−3, 340 mg cm−3, 263.6 mg cm−3, and 237.7 mg cm−3 are, respectively, the initial aerogels (blue, AG-1) and the corresponding reducing products of ethanol (yellow, AG-2), ethylene glycol (dark red, AG-3), and glycerol (brick red, AG-4), based on the solvothermal method. Distinctly, the gels had different shrinkages during the whole process, which could be explained as follows. On the one hand, the Cu(II) component can recombine under high-temperature and high-pressure conditions. On the other hand, damage by the capillary forces to the porous structure was unavoidable during the drying process. In addition, the reaction between the Cu(II) particles and the alcohol reducing agent is a complex process. Whether the monolithic structure could be reserved is mainly determined by the tolerance of the silica frameworks to the alcohothermal process.
 | ||
| Fig. 1 Photographs of the samples: (a) Cu(II)-contained silica composite gels (left image) and different reduction products; (b) the corresponding aerogels after CO2 drying. | ||
In order to analyze the crystal phase composition of Cu(II)-contained silica composites and the converted products, X-ray diffraction (XRD) patterns were obtained, as shown in Fig. 2. The initial composite aerogels (Fig. 2a) exhibited a broad diffraction peak around 22°, which was associated with amorphous silica and which appeared in subsequent diffraction curves (Fig. 2b–d). After ethanol-thermal reduction, obvious diffraction peaks (Fig. 2b) at 2θ values of 29.6°, 36.5°, 42.3°, 61.5°, and 73.4° were present as the cubic Cu2O phase (PDF#075-4299). When ethylene glycol and glycerol were used as reducing agents, similar diffraction peaks appeared at 43.3°, 50.4°, and 74.1° and were indexed as the (111), (200), and (220) diffraction of cubic Cu (PDF#04-0836). By contrast, the peak intensity in Fig. 2d is stronger than that in Fig. 2c, which reveals that the copper nanoparticles in the glycerol system exhibit a higher crystalline structure than that of ethylene glycol under the same conditions. In addition, the average crystal grain size was estimated from the XRD patterns by using Jade software with the Scherrer equation,35 and the related values were 9.9 nm (Fig. 2c) and 22.4 nm (Fig. 2d) respectively, which are consistent with the TEM results.
 | ||
| Fig. 2 XRD patterns of composite aerogels: (a) Cu(II)-contained silica composites, and related products with different reducing agents: (b) ethanol, (c) ethylene glycol, (d) glycerol. | ||
3.3 Morphology and microstructure analysis
SEM analyses of the resulting composites were characterized using scanning electron microscopy (SEM). As shown in Fig. 3a, the initial Cu(II)-contained silica composite aerogels exhibited a typical three-dimensional disordered porous framework, which was assembled by uniform primary spherical particles with diameters of about 10–50 nm. The porous structure was preserved after the alcohothermal reduction, while partial aggregation of the nanoparticles was observed in Fig. 3b–d. Meanwhile, the pore structure was slightly collapsed, due to the recombination of the Cu(II) gel network, in which the Cu(II) colloidal particles were converted to Cu or Cu2O nanocrystals under reducing conditions. As shown in Fig. S2 to S5,† the molar ratio (nCu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nSi) of the initial templates was about 19.91 ± 2.42%, obtained through statistics at different points using the EDS method, indicating that the Cu(II) component was homogeneously distributed in the whole framework. During the subsequent in situ alcohothermal reduction process, the loss of copper atoms was inevitable under high temperature conditions. The doping fractions of the resulting aerogels reduced by ethanol, ethylene glycol, and glycerol were around 18.6%, 17.8%, and 13.8%, respectively (Table 1). In addition, there was only a slight ratio discrepancy of the reduced sample at different points, while the doping fraction was relatively high, compared with other doping methods.
nSi) of the initial templates was about 19.91 ± 2.42%, obtained through statistics at different points using the EDS method, indicating that the Cu(II) component was homogeneously distributed in the whole framework. During the subsequent in situ alcohothermal reduction process, the loss of copper atoms was inevitable under high temperature conditions. The doping fractions of the resulting aerogels reduced by ethanol, ethylene glycol, and glycerol were around 18.6%, 17.8%, and 13.8%, respectively (Table 1). In addition, there was only a slight ratio discrepancy of the reduced sample at different points, while the doping fraction was relatively high, compared with other doping methods.
 | ||
| Fig. 3 The SEM images of the resulting aerogels: (a) Cu(II)-contained silica composites; (b) after ethanol reduction; (c) after ethylene glycol reduction; and (d) after glycerol reduction. | ||
| Specimen | Bulk density (mg cm−3) | BET (m2 g−1) | Average pore size (nm) | Grain size (nm) | Doping fraction |
|---|---|---|---|---|---|
| AG-1 | 230 | 631 | 17.5 | — | 19.91% |
| AG-2 | 340 | 846.4 | 10.4 | 8.6 | 18.6% |
| AG-3 | 263.6 | 722.1 | 23.09 | 9.9 | 17.8% |
| AG-4 | 237.7 | 531.2 | 19.42 | 22.4 | 13.8% |
The microstructures of various aerogels were characterized using transmission electron microscopy (TEM). These nanocomposites clearly displayed the typical microstructure of common aerogels, i.e., that of nanoparticles clustered together to form a mesoporous structure. As shown in Fig. 4a, the initial aerogels are mainly composed of small spherical particles with a diameter of about 1–10 nm, and both the components are uniformly dispersed without any obvious aggregation of colloidal particles. However, after ethanol-thermal reduction, the shape of the skeleton is similar to that of the randomly distributed root, and some Cu2O submicron particles are detected in the networks, as shown in Fig. 4b. This could be explained by the nucleation and growth of Cu2O nanocrystal particles being easily realized under high-temperature and high-pressure conditions. In contrast, Fig. 4c and d demonstrate that the Cu nanocrystal particles are uniformly dispersed in the networks, and that there is no clear aggregation in the primary structure. During the reaction process, the framework of the Cu(II) component was gradually consumed under reducing conditions, and the corresponding copper nanoparticles with a weak interaction were simultaneously obtained. Interestingly, the copper colloidal particles reduced by glycerol have a larger grain size than those reduced by ethylene glycol, which reveals that glycerol has a stronger reducibility compared with ethylene glycol.36,37
 | ||
| Fig. 4 TEM images of (a) Cu(II)-contained silica composite aerogels and the corresponding products with different reducing agents: (b) ethanol, (c) ethylene glycol, and (d) glycerol. | ||
Nitrogen adsorption/desorption isotherms of the resulting samples were obtained by the Brunauer–Emmett–Teller (BET) method. As described in Fig. S1,† the initial aerogels and corresponding samples reduced by ethanol, ethylene glycol, and glycerol exhibited similar typical IV isotherms and narrow H1-type hysteresis loops, which are consistent with the mesoporous structure. The specific surface areas were 631.1 m2 g−1, 846.4 m2 g−1, 722.1 m2 g−1, and 531.2 m2 g−1, respectively. In addition, Fig. 5a shows the corresponding cumulative pore volumes and size distribution as that calculated from the desorption branch of the isotherm applying the BJH theory. The initial Cu(II)-contained silica aerogels exhibited a broad pore size distribution, mainly in the mesopore range (10–50 nm), which represents the typical characterization of most mesoporous aerogel materials. After the ethanol-thermal process, some micropores (≤2 nm) that give rise to the surface area appeared.38 Meanwhile, the main-peak value of the mesopore transformed from 35.82 nm down to 17.75 nm, compared with the AG-1 sample. The change of pore size distribution could be reasonably explained by two factor: (1) the generated micropores were mostly attributed to the in situ pore-fabrication during the conversion process from the Cu(II) to the Cu2O component; (2) the shift of the main-peak position was mainly caused by the obvious shrinkage of the obtained wet gels during the supercritical drying caused by the unavoidable capillary forces, which could be visibly certified from the related photographs of the ethanol-reduced wet gels and the final aerogels in Fig. 1. As for sample AG-3, a similar pore-size distribution to the initial ones is shown in Fig. 5a. The main peak was still at about 35 nm, while an additional sub-peak at 24 nm appeared after the ethylene glycol-thermal reduction. Interestingly, we found that the pore-size distribution of the AG-4 sample exhibited a narrower distribution, and the peak value dropped down to 24.6 nm, which was approximately the same as the sub-peak of AG-3. In regards the as-described change of pore-size distribution, this could be explained by the reaction mildly taking place when ethylene glycol was used as a reducing agent, and the colloidal frameworks then essentially underwent internal stress, so that the reduced sample exhibited a similar pore-size distribution as that of the initial templates. As for the generation of some small mesopores, except for the inevitable bulk shrinkage during CO2 supercritical drying, this is mostly attributed to the filling and further fusion effects of the converted copper nanoparticles, whereby the initial pores were partly filled with the primary copper nanoparticles, which then gradually fused into larger sized ones. In contrast, the glycol provided stronger reducing conditions for the initial templates, and the reduced copper nanocrystal particles in the existing mesopores were then easily aggregated into larger ones. More importantly, the colloidal skeletons could barely tolerate the internal stress caused by the reaction between the Cu(II) compound and glycerol, with the consequence that the composite frameworks partly shrunk, and even collapsed to varying degrees, following the mechanism described in Fig. 5b. Consequently, this reaction condition might not only result in macroscopic shrinkage of the bulk, but could also lead to a decrease in the specific surface area.
 | ||
| Fig. 5 The distribution and formation mechanism of the hierarchical pores of the resulting composite aerogels. | ||
4. Conclusion
An in situ alcohothermal reduction strategy was successfully used to synthesize highly dispersed Cu- or Cu2O-doped SiO2 composite aerogels. The rapid preparation of the initial Cu(II)-contained silica templates was attributed to the employment of both PO pre-reaction and acetonitrile solvent, but in different ways. After the alcohothermal reaction with ethanol, ethylene glycol, and glycerol separately as reducing agents, the resulting Cu2O/SiO2 and Cu/SiO2 composite aerogels retained some unique properties of the initial templates, such as monolithic appearance, high surface area, and nanoscale porous framework. By analysis of the crystal phase composition and pore-size distribution, we could conclude that the reducibility was enhanced with the increasing number of –OH on the alcohol molecules under the same conditions. Because of the uniform nanoparticle size and high dispersion, the resulting products could find use in specific applications, such as in high efficiency photocatalysis, and backlight targets.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51102184, 51172163), National High Technology Research and Development Program of China (2013AA031801), National Key Technology Research and Development Program of China (2013BAJ01B01), Shanghai Municipal Science and Technology Commission Nano Special Project, China (12nm0503001, 11nm0501600), and Science and Technology Innovation Fund of Shanghai Aerospace, China (SAST201254, SAST201321).References
- A. Du, B. Zhou, Z. H. Zhang and J. Shen, Materials, 2013, 6, 941–968 CrossRef CAS PubMed.
- A. E. Gash, T. M. Tillotson, J. H. Satcher Jr, J. F. Poco, L. W. Hrubesh and R. L. Simpson, J. Non-Cryst. Solids, 2001, 285, 22–28 CrossRef CAS.
- J. S. Lee, T. J. Ha, M. H. Hong and H. H. Park, Thin Solid Films, 2013, 529, 98–102 CrossRef CAS PubMed.
- Y. P. Liu, K. Huang, H. Luo, H. X. Li, X. Qi and J. X. Zhong, RSC Adv., 2014, 4, 17653–17659 RSC.
- X. Z. Guo, L. Q. Yan, H. Yang, J. Li, C. Y. Li and X. B. Cai, Acta Phys.–Chim. Sin., 2011, 27, 2478–2484 CAS.
- H. B. Ren, L. Zhang, C. W. Shang, X. Wang and Y. T. Bi, J. Sol-Gel Sci. Technol., 2010, 53, 307–311 CrossRef CAS PubMed.
- G. Q. Zu, J. Shen, L. P. Zou, W. Q. Wang, Y. Lian, Z. H. Zhang and A. Du, Chem. Mater., 2013, 25, 4757–4764 CrossRef CAS.
- Z. H. Zhou, X. X. Zhang, C. H. Lu, L. Lan and G. P. Yuan, RSC Adv., 2014, 4, 8966–8972 RSC.
- Y. Tokudome, K. Nakanishi, K. Kanamori, K. Fujita, H. Akamatsu and T. Hanada, J. Colloid Interface Sci., 2009, 338, 506–513 CrossRef CAS PubMed.
- A. K. Herrmann, P. Formanek, L. Borchardt, M. Klose, L. Giebeler, J. Eckert, S. Kaskel, N. Gaponik and A. Eychmuller, Chem. Mater., 2014, 26, 1074–1083 CrossRef CAS.
- Y. Q. Zhao, H. L. Zhao, Y. H. Liang, Q. Y. Jia and B. B. Zhang, Trans. Nonferrous Met. Soc. China, 2010, 20, 1463–1469 CrossRef CAS.
- Z. Ulker, I. Erucar, S. Keskin and C. Erkey, Microporous Mesoporous Mater., 2013, 170, 353–358 CrossRef PubMed.
- T. Kristiansen, J. A. Stoeneng, M. A. Einarsrud, D. G. Nicholson and K. Mathisen, J. Phys. Chem. C, 2012, 116, 20368–20379 CAS.
- J. X. Liu, F. Shi, L. N. Bai, X. Feng, X. K. Wang and L. Bao, J. Sol-Gel Sci. Technol., 2014, 69, 93–101 CrossRef CAS PubMed.
- H. Z. Guo, X. Liu, Q. S. Xie, L. Wang, D. L. Peng, P. S. Branco and M. B. Gawande, RSC Adv., 2013, 3, 19812–19815 RSC.
- J. Tang, A. Du, W. W. Xu, G. W. Liu, Z. H. Zhang, J. Shen and B. Zhou, J. Sol-Gel Sci. Technol., 2013, 68, 102–109 CrossRef CAS PubMed.
- W. N. Zhang, J. L. Lu, J. W. Zhu and W. B. Cui, Asian J. Chem., 2013, 25, 877–879 CAS.
- C. A. Morris, M. L. Anderson, R. M. Stroud, C. I. Merzbacher and D. R. Rolison, Science, 1999, 284, 621–624 CrossRef.
- P. R. Aravind, P. Mukundan, P. K. Pillai and K. G. K. Warrier, Microporous Mesoporous Mater., 2006, 96, 14–20 CrossRef CAS PubMed.
- K. Brodzik, J. Walendziewski, M. Stolarski, L. V. Ginneken, K. Elst and V. Meynen, J. Porous Mater., 2008, 15, 541–549 CrossRef CAS.
- K. Grosse, L. Ratke and B. Feuerbacher, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 2897–2902 Search PubMed.
- K. B. Fournier, J. H. Satcher, M. J. May, J. F. Poco, C. M. Sorce, J. D. Colvin, S. B. Hansen, S. A. MacLaren, S. J. Moon, J. F. Davis, F. Girard, B. Villette, M. Primout, D. Babonneau, C. A. Coverdale and D. E. Beutler, Phys. Plasmas, 2009, 16, 052703/1–052703/13 CrossRef CAS PubMed.
- K. B. Fournier, C. Constantin, J. Poco, M. C. Miller, C. A. Back, L. J. Suter, J. Satcher, J. Davis and J. Grun, Phys. Rev. Lett., 2004, 5196, 194–204 CAS.
- P. D. Brown, S. K. Gill and L. J. Hope-weeks, J. Mater. Chem., 2011, 21, 4204–4208 RSC.
- P. Brown, D. U. Cearnaigh, E. K. Fung and L. J. Hope-weeks, J. Sol-Gel Sci. Technol., 2012, 61, 104–111 CrossRef CAS PubMed.
- O. Malay, I. Yilgor and Y. Z. Menceloglu, J. Sol-Gel Sci. Technol., 2013, 67, 351–361 CrossRef CAS.
- I. Artaki, T. W. Zerda and J. Jonas, Mater. Lett., 1985, 3, 493–496 CrossRef CAS.
- I. Artaki, T. W. Zerda and J. Jonas, J. Non-Cryst. Solids, 1986, 81, 381–395 CrossRef CAS.
- W. Y. Zhang, H. Wang, Q. B. Li, Q. N. Dong, N. Zhao, W. Wei and Y. H. Sun, Appl. Catal., A, 2005, 294, 188–196 CrossRef CAS PubMed.
- M. N. Timofeeva, V. N. Panchenko, A. Gil, Y. A. Chesalov, T. P. Sorokina and V. A. Likholobov, Appl. Catal., B, 2011, 102, 433–440 CrossRef CAS PubMed.
- R. E. Parker and N. S. Isaacs, Chem. Rev., 1959, 59, 737–799 CrossRef CAS.
- A. Du, B. Zhou, W. W. Xu, Q. J. Yu, Y. Shen, Z. H. Zhang, J. Shen and G. M. Wu, Langmuir, 2013, 29, 11208–11216 CrossRef CAS PubMed.
- B. Zhou, H. X. Wang, Z. G. Liu, Y. Q. Yang, X. Q. Huang, Z. Lu, Y. Sui and W. H. Su, Mater. Chem. Phys., 2011, 126, 847–852 CrossRef CAS PubMed.
- J. M. Du, B. X. Han, Z. M. Liu and Y. Q. Liu, Cryst. Growth Des., 2007, 7, 900–904 CAS.
- A. L. Patterson, Phys. Rev., 1939, 56, 978–982 CrossRef CAS.
- X. Y. Chen, X. Wang, Z. H. Wang, J. X. Wan, J. L. Liu and Y. T. Qian, Nanotechnology, 2004, 15, 1685–1687 CrossRef CAS.
- H. Tuysuz, Y. Liu, C. Weidenthaler and F. Schuth, J. Am. Chem. Soc., 2008, 130, 14108–14110 CrossRef PubMed.
- D. J. Suh and T. J. Park, Chem. Mater., 1996, 8, 509–513 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06633b |
| This journal is © The Royal Society of Chemistry 2014 |
