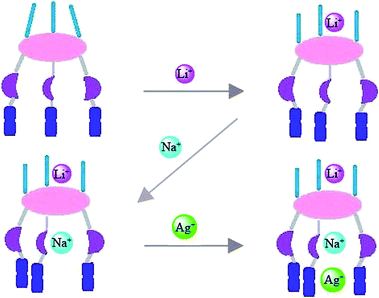
Synthesis and inclusion behavior of a heterotritopic receptor based on hexahomotrioxacalix[3]arene†
Cheng-Cheng Jina,
Hang Conga,
Xin-Long Nib,
Xi Zengb,
Carl Redshawc and
Takehiko Yamato*a
aDepartment of Applied Chemistry, Faculty of Science and Engineering, Saga University, Honjo-machi 1, Saga-shi, Saga 840-8502, Japan. E-mail: yamatot@cc.saga-u.ac.jp
bKey Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang, Guizhou 550025, China
cDepartment of Chemistry, The University of Hull, Cottingham Road, Hull, Yorkshire, HU6 7RX, UK
First published on 4th July 2014
Abstract
A heterotritopic hexahomotrioxacalix[3]arene receptor with the capability of binding two alkali metals and a transition metal in a cooperative fashion was synthesized. The binding model was investigated by using 1H NMR titration experiments in CDCl3–CD3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the results revealed that the transition metal was bound at the upper rim and the alkali metals at the lower and upper rims. Interestingly, the alkali metal ions Li+ and Na+ bind at the lower and upper rim respectively depending on the dimensions of the alkali metal ions versus the size of the cavities formed by the calix[3]arene derivative. The hexahomotrioxacalix[3]arene receptor acts as a heterotritopic receptor, binding with the transition metal ion Ag+ and the alkali metals ions Li+ and Na+. These findings were not applicable to other different sized alkali metals, such as K+ and Cs+.
1, v/v), and the results revealed that the transition metal was bound at the upper rim and the alkali metals at the lower and upper rims. Interestingly, the alkali metal ions Li+ and Na+ bind at the lower and upper rim respectively depending on the dimensions of the alkali metal ions versus the size of the cavities formed by the calix[3]arene derivative. The hexahomotrioxacalix[3]arene receptor acts as a heterotritopic receptor, binding with the transition metal ion Ag+ and the alkali metals ions Li+ and Na+. These findings were not applicable to other different sized alkali metals, such as K+ and Cs+.
Introduction
Calixarenes and their derivatives are attractive compounds for use in host–guest and supramolecular chemistry. In particular, hexahomotrioxacalix[3]arene derivatives with C3-symmetry can selectively bind ammonium ions which play important roles in both chemistry and biology.1,2 Furthermore, the incorporation of two types of recognition sites via the introduction of different ionophores on the homotrioxacalix[3]arene will create potential heteroditopic receptors with the capability of binding cations and anions, e.g. ammonium ions and halides.Recently, we reported a novel ditopic receptor possessing two complexation sites and bearing a thiacalix[4]arene in the 1,3-alternate conformation. The binding behaviour with Na+, K+ and Ag+ ions was examined by 1H NMR titration experiments. Although the formation of a heterogeneous di-nuclear complex was not clearly observed, the exclusive formation of mononuclear complexes of the 1,3-alternate-derivative with metal cations is of particular interest with respect to the observation of positive/negative allosteric effects within the thiacalix[4]arene family.3
On the other hand, Nabeshima et al. reported a novel calix[4]arene derivative bearing two 2,2′-bipyridine moieties and two ester groups at the lower rim in the cone conformation to construct sophisticated molecular devices and systems.4 Indeed, bipyridyl containing calixarenes have been extensively used to complex various metal ions.5–12 Di- or polytopic receptors are those constructed with two or more binding subunits within the same macrocyclic structure.13–15 It is well known that these kinds of systems are suitable candidates for the allosteric regulation5–7 of host–guest interactions with metal cations which play a major role in biological systems.
Moving from our interest in the synthesis of heteroditopic or heteropolytopic receptors that function as multiple types of cation binder, we introduced a 2,2′-bipyridyl group linked via a carbonyl group at the upper rim and diethylacetamide group at the lower rim of the hexahomotrioxacalix[3]arene. Herein, we report the synthesis and complexation studies of these cone-hexahomotrioxacalix[3]arene triamide derivatives that serve as tritopic receptors for Ag+, Li+ and Na+ ions in a cooperative fashion. The recognition behaviour towards multiple types of cation was investigated by 1H NMR experiments in CDCl3–CD3CN solution.
Results and discussion
Synthesis
The preparation of cone-7,15,23-triethoxycarbonyl-25,26,27-tris-(N,N-diethylaminocarbonylmethoxy)-2,4,10,12,18,20-hexahomo-3,11,19-trioxacalix[3]arene (cone-4) is shown in Scheme 1. Thus, bis(hydroxymethylation) of ethyl 4-hydroxybenzoate (1) with formaldehyde in aqueous NaOH for one week afforded ethyl 3,5-bis(hydroxymethyl)-4-hydroxybenzoate (2)16 in 41% yield. Heating compound (2) to reflux in p-xylene for 24 h afforded hexahomotrioxacalix[3]arene (3).17 The O-alkylation of compound (3) with N,N-diethylchloroacetamide in the presence of NaI/NaH in refluxing THF/DMF(v/v = 5/1) gave cone-tris(N,N-diethylamino-carbonylmethoxy)hexahomotrioxacalix[3]arene cone-4 (ref. 17) in 45% yield. Hydrolysis of the O-alkylated compound, cone-4, was carried out with NaOH in a mixture of ethanol–water (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 50 °C for 2 h to yield the cone-hexahomotrioxacalix[3]arene tricarboxylic acid cone-5.17
1) at 50 °C for 2 h to yield the cone-hexahomotrioxacalix[3]arene tricarboxylic acid cone-5.17
Cone-hexahomotrioxacalix[3]arene triamide derivative (cone-7) was prepared by a condensation reaction of cone-5 with 6 in the presence of dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopryidine (DMAP) at room temperature for 3 days in dichloromethane (Scheme 2).
Cone-7 immobilised in a ‘flattened cone’ conformation (in which the phenolic rings are tilted to open up the calixarene cavity), was obtained in moderate yield. Conformational assignments for cone-7 were firmly established by the presence of the bridging methylene protons with a ΔδH separation between Hax and Heq of 0.41 ppm in the 1H NMR spectra (CDCl3). For the calix[4]arenes, the ΔδH value of the ArCH2Ar protons has been correlated with the orientation of adjacent aromatic rings.2d,e,18,19 The same findings were observed for homotrioxacalix[3]arenes.20
UV-vis spectroscopy studies
Cone-7 as a tritopic hexahomotrioxacalix[3]arene ligand was synthesized, which possessed N,N-diethylacetamide groups at the lower rim and 2,2′-bipyridyl groups at the upper rim linked by a carbonyl group. Consequently, the binding behaviour of cone-7 towards different metal cations can be investigated by UV-vis absorption spectroscopy. As shown in Fig. 1 and S4,† the UV-vis spectra of cone-7 displayed a typical absorption at around 290 nm in CH2Cl2–CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The effects of the addition of various metal ions such as Li+, Na+, K+, Cs+, Ag+, Cu2+, Zn2+, Pb2+ and Hg2+ as their perchlorate salts in CH2Cl2–CH3CN solution have been studied. As can be seen, an obvious absorption change in the UV-vis spectrum occurred upon addition of Li+, Na+ and transition metal ions. The electronic absorption spectrum of cone-7 exhibited a red shift in the presence of transition metals, whereas only an intensity change was observed for alkali metals. For the metal Zn2+, it was noticed that the absorption band was split into two absorption bands at around 310 nm and 320 nm, respectively. No significant UV-vis absorption changes were observed upon the addition of K+ and Cs+ ions. Thus, it can be explained that the 2,2′-bipyridyl group acted as a chromophore displaying a red-shift absorption after binding with transition metals. According to this observation, we can demonstrate the transition metals bound with 2,2′-bipyridyl group at the upper rim and alkali metals bound with other sites respectively. This finding also can be proved by the 1H NMR titration experiments.
1, v/v). The effects of the addition of various metal ions such as Li+, Na+, K+, Cs+, Ag+, Cu2+, Zn2+, Pb2+ and Hg2+ as their perchlorate salts in CH2Cl2–CH3CN solution have been studied. As can be seen, an obvious absorption change in the UV-vis spectrum occurred upon addition of Li+, Na+ and transition metal ions. The electronic absorption spectrum of cone-7 exhibited a red shift in the presence of transition metals, whereas only an intensity change was observed for alkali metals. For the metal Zn2+, it was noticed that the absorption band was split into two absorption bands at around 310 nm and 320 nm, respectively. No significant UV-vis absorption changes were observed upon the addition of K+ and Cs+ ions. Thus, it can be explained that the 2,2′-bipyridyl group acted as a chromophore displaying a red-shift absorption after binding with transition metals. According to this observation, we can demonstrate the transition metals bound with 2,2′-bipyridyl group at the upper rim and alkali metals bound with other sites respectively. This finding also can be proved by the 1H NMR titration experiments.
 | ||
Fig. 1 UV-vis absorption spectra response of cone-7 (1 × 10−6 M) in CH2Cl2–CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to 1 × 10−5 M various tested metal ions. λmax = 290 nm, ε = 1.89 × 105 cm−1 M−1. 1, v/v) to 1 × 10−5 M various tested metal ions. λmax = 290 nm, ε = 1.89 × 105 cm−1 M−1. | ||
1H NMR titration studies
To investigate the binding behaviour between cone-7 with Li+, Na+ and Ag+ ions, 1H NMR spectroscopic studies were carried out in CDCl3/CD3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The spectral differences are shown in Fig. 2. In the presence of an equivalent of Li+, for example, the ΔδH value for Hax and Heq for the ArCH2O methylene protons changed from δ 0.39 ppm to δ 0.27 ppm, The ΔδH′ value for the –NCH2CH3 methylene proton changed from δ 0.11 ppm to δ 0.30 ppm. In comparison with the complex cone-7 ⊃ Li+, in the spectra of cone-7 ⊃ Na+ complex, the ΔδH value for the ArCH2O methylene protons barely changed but the signals for the ArCH2O methylene protons both were both shifted upfield, i.e. δ 0.19 ppm. The ΔδH′ value for the –NCH2CH3 methylene proton changed from δ 0.11 ppm to δ 0.25 ppm. In addition, obvious downfield chemical shifts for Ar-H (δ 0.33 ppm) and bipy-CH2 (δ 0.11 ppm) were observed for the complex cone-7 ⊃ Na+.
1, v/v). The spectral differences are shown in Fig. 2. In the presence of an equivalent of Li+, for example, the ΔδH value for Hax and Heq for the ArCH2O methylene protons changed from δ 0.39 ppm to δ 0.27 ppm, The ΔδH′ value for the –NCH2CH3 methylene proton changed from δ 0.11 ppm to δ 0.30 ppm. In comparison with the complex cone-7 ⊃ Li+, in the spectra of cone-7 ⊃ Na+ complex, the ΔδH value for the ArCH2O methylene protons barely changed but the signals for the ArCH2O methylene protons both were both shifted upfield, i.e. δ 0.19 ppm. The ΔδH′ value for the –NCH2CH3 methylene proton changed from δ 0.11 ppm to δ 0.25 ppm. In addition, obvious downfield chemical shifts for Ar-H (δ 0.33 ppm) and bipy-CH2 (δ 0.11 ppm) were observed for the complex cone-7 ⊃ Na+.
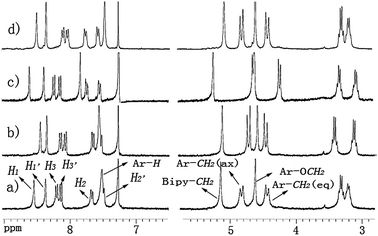 | ||
Fig. 2 Partial 1H NMR titration of cone-7/guest complex (H/G = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); (a) free cone-7; (b) cone-7 ⊃ Li+; (c) cone-7 ⊃ Na+; (d) cone-7 ⊃ Ag+; solvent: CDCl3/CD3CN (10 1); (a) free cone-7; (b) cone-7 ⊃ Li+; (c) cone-7 ⊃ Na+; (d) cone-7 ⊃ Ag+; solvent: CDCl3/CD3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v). | ||
The addition of an equiv. of AgClO4 to cone-7 caused instant complexation at the upper rim as demonstrated by the downfield shifts of the 2,2′-bipyridyl protons (H2′, Δδ = −0.08 ppm, H2, Δδ = −0.10 ppm), and the upfield shifts of the 2,2′-bipyridyl protons (H3′, Δδ = +0.10 ppm, H3, Δδ = +0.10 ppm) for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of cone-7 ⊃ Ag+ (Ka = 2.24 × 105 M−1) as shown in Fig. 2d, whereas the lower rim protons were scarely affected in the presence of Ag+. This results strongly suggested that Ag+ can be selectively bound by the nitrogen atoms of the 2,2′-bipyridyl group.
1 complex of cone-7 ⊃ Ag+ (Ka = 2.24 × 105 M−1) as shown in Fig. 2d, whereas the lower rim protons were scarely affected in the presence of Ag+. This results strongly suggested that Ag+ can be selectively bound by the nitrogen atoms of the 2,2′-bipyridyl group.
The Li+ formed a complex with the N,N-diethylmethoxy-carbonylmethoxy group of cone-7 and adopted the more-upright C3-symmetric form. It is known that the introduction of bulky substituents onto the OH groups forces the phenol units to stand upright from the calixarene ring plane.1 This inclination was reflected by the chemical-shift difference (ΔδH) between the axial and equatorial ArCH2 protons, the small ΔδH value for Hax and Heq indicated that the phenol groups in the complex are positioned in a more upright orientation. We have already reported that when a Li+ ions was bound to the ionophoric group at the lower rim, the calix cavity changed from a “flattened cone” to a more-upright form.21 The Na+ ion was bound in the cavity formed by the three phenoxy rings, as evidenced by the upfield chemical shift of the axial and equatorial ArCH2 protons (i.e. δ 0.19 ppm), the downfield chemical shifts for the Ar-H (δ 0.33 ppm) and bipy–CH2 (δ 0.11 ppm).
We also carried out 1H NMR titration experiments for cone-7 with K+ and Cs+ ions (Fig. S5 and S6†). An equivalent of KClO4 and CsClO4 were added to the solution of cone-7, and no obvious chemical shift change was observed. Because of the size of K+ and Cs+ ions, they are not suitable for binding with the lower rim or upper rim cavities.
The complexation modes of receptor cone-7 with Ag+ and Li+ were investigated by 1H NMR spectroscopy. The addition of an equiv. of AgClO4 to cone-7 caused instant complexation at the upper rim as demonstrated in Fig. 3b. Fig. 3c showed the 1H NMR spectrum after the addition of Li+ ion to the cone-7 ⊃ Ag+ complex. When an equivalent of LiClO4 was added, the ΔδH value for Hax and Heq for the ArCH2O methylene protons changed, the ΔδH value (from peaks around δ 4.42–4.69 ppm) for the LiClO4 ⊂ [cone-7 ⊃ Ag+] (δ 0.27 ppm) was smaller than that of the cone-7 ⊃ Ag+ (from peaks around δ 4.42–4.80 ppm) (δ 0.38 ppm). The ΔδH′ value for the –NCH2CH3 methylene protons (δ 0.29 ppm) of LiClO4 ⊂ [cone-7 ⊃ Ag+] was larger than that of the cone-7 ⊃ Ag+ (δ 0.12 ppm). This result implied that Li+ formed a complex with the N,N-diethylmethoxy-carbonylmethoxy group after cone-7 complexed with Ag+ and adopted the more upright C3-symmetric form. This result was also observed after changing the binding sequence of metal ions, first to form the complex cone-7 ⊃ Li+ and then to form the complex AgClO4 ⊂ [cone-7 ⊃ Li+] (Fig. S7†). Thus, the cone-hexahomotrioxacalix[3]arene triamide derivative cone-7 can serve as a receptor for Ag+ and Li+ in a cooperative fashion. Similar findings were observed for the NaClO4 ⊂ [cone-7 ⊃ Ag+] complex.
 | ||
Fig. 3 Partial 1H NMR titration of cone-7/guest complex (H/G = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); (a) free cone-7; (b) cone-7 ⊃ AgClO4; (c) LiClO4 ⊂ [cone-7 ⊃ Ag+]; solvent: CDCl3/CD3CN (10 1); (a) free cone-7; (b) cone-7 ⊃ AgClO4; (c) LiClO4 ⊂ [cone-7 ⊃ Ag+]; solvent: CDCl3/CD3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v). | ||
1H NMR titration experiments were also carried out with the Na+ ion and solutions of cone-7 ⊃ Ag+ as shown in Fig. 4c and d. When 0.4 equivalents of NaClO4 was added, the complex NaClO4 ⊂ [cone-7 ⊃ Ag+] and the free species [cone-7 ⊃ Ag+] both existed in the system. However, when 1 equivalent of NaClO4 was added to the solution of cone-7 ⊃ Ag+, the free species [cone-7 ⊃ Ag+] gradually disappeared and only the complex Na+ ⊂ [cone-7 ⊃ Ag+], as shown in Fig. 4d, was observed. The corresponding protons shifts were given by 1H NMR complexation experiments. Thus, cone-7 first bound with Ag+ at the upper rim, then bound with Na+ ion in the cavity formed by the three phenoxy rings of the oxacalix[3]arene. ΔδH value for Hax and Heq for ArCH2O methylene protons mostly did not change, however the signals for the ArCH2O methylene protons were both shifted upfield, i.e. δ 0.20 ppm (Heq, δ 4.45 ppm to δ 4.23 ppm and Hax, δ 4.84 ppm to δ 4.64 ppm, respectively). The ΔδH′ value for the –NCH2CH3 methylene protons (δ 0.24 ppm) for NaClO4 ⊂ [cone-7 ⊃ Ag+] was larger than that of the cone-7 ⊃ Ag+ (δ 0.11 ppm). The Ar-H proton was shifted downfield (δ 0.32 ppm) and the bipy–CH2 proton was shifted downfield (δ 0.20 ppm). When 0.4 equivalents of NaClO4 was added to the complex cone-7 ⊃ Ag+, the complex NaClO4 ⊂ [cone-7 ⊃ Ag+] and the free species [cone-7 ⊃ Ag+] both existed in the system. It was necessary to consider whether the negative allosteric effect caused by the binding of Ag+ existed or not, so the sequence of metal ions addition was changed, viz. initially bind with Na+ ion, then to the Ag+ ion as shown in Fig. 5. However, when 0.4 equivalents of NaClO4 was added to cone-7, the complex cone-7 ⊃ Na+ and the free species cone-7 were both observed as shown in Fig. 5c. On further addition of the metal ion Na+ (1 equiv.), the free species disappeared and only the complex cone-7 ⊃ Na+ existed. In most other work, a passive/negative allosteric effect was caused by the binding with Ag+, but here, there was no observation of the allosteric effect.
Until now, the ability of the cone-7 to serve as a heteroditopic receptor has been demonstrated, but now to illustrate that cone-7 can serve as a heterotritopic receptor, cone-7 was allowed to complex with Li+, Na+ and Ag+ metal ions in a cooperative fashion. 1H NMR spectroscopic titration experiments were carried out by addition of Li+ ions to the solution of cone-7, by Na+ ions to the solution of cone-7 ⊃ Li+ and by Ag+ ions to the solution of Na+ ⊂ [cone-7 ⊃ Li+] as shown in Fig. 6. In the presence of an equivalent of Li+, the ΔδH values for Hax and Heq for the ArCH2O methylene protons changed from δ 0.40 ppm to δ 0.24 ppm, and the ΔδH′ value for the –NCH2CH3 methylene protons changed from δ 0.11 ppm to δ 0.28 ppm. When 1 equiv. of NaClO4 was added to the solution of cone-7 ⊃ Li+, the ΔδH value for Hax and Heq of the ArCH2O methylene protons changed from δ 0.24 ppm to δ 0.34 ppm, and the signals for the ArCH2O methylene protons were both shifted upfield, i.e. δ 0.18 ppm (Heq, δ 4.48 ppm to δ 4.30 ppm and Hax, δ 4.72 ppm to δ 4.64 ppm, respectively), indicating that binding was occurring between the cone-7 ⊃ Li+ and Na+; the corresponding chemical shift changes were attributable to the cooperative effects by the Li+ and Na+ ions. The Ar-H proton was shifted downfield (δ 0.15 ppm) and the bipy–CH2 proton was shifted downfield (δ 0.06 ppm). After addition of Ag+ ion to the solution of Na+ ⊂ [cone-7 ⊃ Li+], we also observed the same downfield shifts for the 2,2′-bipyridyl protons (H2′, Δδ = −0.08 ppm, H2, Δδ = −0.10 ppm). Thus, cone-7 can serve as a heterotritopic receptor. This result was also observed after changing the binding sequence of the metal ions. Firstly, the complex of cone-7 ⊃ Ag+ was formed, then the complex LiClO4 ⊂ [cone-7 ⊃ Ag+], Na+ ⊂ {Li+ ⊂ [cone-7 ⊃ Ag+]} (Fig. S8†) was formed. We observed the same1H NMR spectrum as shown in Fig. 6d and S8d,† and thus it was proved that cone-7 can serve as a heterotritopic receptor for the Ag+, Li+ and Na+ ions in a cooperative fashion (Fig. 7).
As shown in Table 1, the nitrogen atom N1 in the bipyridine ring pointed away from the calix cavity in free cone-7 because of the electron repulsion between the nitrogens. After complexation, the nitrogen turned inwards towards the cavity to complex with the Ag+ and thus affected the 2,2′-bipyridyl protons with downfield shifts for H2′ (Δδ = −0.08 ppm) and H2 (Δδ = −0.10 ppm), upfield shifts for H3′ (Δδ = +0.10 ppm), H3 (Δδ = +0.10 ppm) and H1 (Δδ = +0.04 ppm) (Table 1) due to the tetrahedral interaction of the N–Ag+ motif.
| Compd. | Chemical shift,δppma,b | |||||
|---|---|---|---|---|---|---|
| H1 | H2 | H3 | H′1 | H′2 | H′3 | |
| a Δδ values are the difference of the chemical shift between cone-7 in CDCl3–CD3CN at 27 °C.b A minus sign (−) denotes a shift to lower magnetic field, a plus sign (+) denotes a shift to higher magnetic.c The midpoint values of multiplet are indicated. | ||||||
| cone-7 | 8.55 | 7.67c | 8.21c | 8.37 | 7.50c | 8.14c |
| cone-7⊃Ag+ | 8.51 | 7.77 | 8.11 | 8.36 | 7.58 | 8.04 |
| Δδ | +0.04 | −0.10 | +0.10 | +0.01 | −0.08 | +0.10 |
Furthermore, after complexation, H3′ and H3, H2′ and H2 have similar magnetic environments, and therefore the downfield/upfield shifts were similar.
Complexation studies
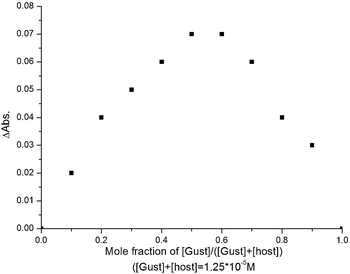
The stoichiometries of the cone-7 complexes with Ag+ and Li+ were determined by UV-vis absorption spectra [CH2Cl2/CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)], using the continuous variation method, the absorption reached a maximum at around 0.5 mol fraction for this cation (Fig. 8), which clearly indicated that the Ag+ formed a 1
1, v/v)], using the continuous variation method, the absorption reached a maximum at around 0.5 mol fraction for this cation (Fig. 8), which clearly indicated that the Ag+ formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with cone-7. Thus, Ag+ was completely bound by the soft bipyridine cavity of cone-7 and the homotrioxacalix[3]arene cavity did not participate in the complexation. The stoichiometry of the cone-7 complexes with Li+ was also determined by UV-vis absorption spectra [CH2Cl2/CH3CN (10
1 complex with cone-7. Thus, Ag+ was completely bound by the soft bipyridine cavity of cone-7 and the homotrioxacalix[3]arene cavity did not participate in the complexation. The stoichiometry of the cone-7 complexes with Li+ was also determined by UV-vis absorption spectra [CH2Cl2/CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)] (Fig. S9†), using the continuous variation method. The absorption also reached maximum at 0.5 mol fraction for this cation, indicating that the Li+ ion formed a 1
1, v/v)] (Fig. S9†), using the continuous variation method. The absorption also reached maximum at 0.5 mol fraction for this cation, indicating that the Li+ ion formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with cone-7, and the Li+ ion was completely bound by the N,N-diethylaminocarbonyl-methoxy groups. The molar ratio method was used to determine the stoichiometry of cone-7 complexed with Na+ by UV-vis absorption spectra [CH2Cl2/CH3CN (10
1 complex with cone-7, and the Li+ ion was completely bound by the N,N-diethylaminocarbonyl-methoxy groups. The molar ratio method was used to determine the stoichiometry of cone-7 complexed with Na+ by UV-vis absorption spectra [CH2Cl2/CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)] (Fig. S10†), which also indicated that the Na+ ion formed a 1
1, v/v)] (Fig. S10†), which also indicated that the Na+ ion formed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with cone-7.
1 complex with cone-7.
UV-vis spectrophotometric analysis was employed to detemine the association constant of the inclusion complex of cone-7 and Ag+. The decrease in absorbance at 290 nm versus the increase in concentration of the Ag+ was fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model to determine the association constant, which was found to be 2.24 × 105 M−1. The association constant for cone-7 and Li+ was 2.58 × 105 M−1 and for cone-7 and Na+, which was found to be 1.55 × 105 M−1 (Fig. S11–13†).
1 binding model to determine the association constant, which was found to be 2.24 × 105 M−1. The association constant for cone-7 and Li+ was 2.58 × 105 M−1 and for cone-7 and Na+, which was found to be 1.55 × 105 M−1 (Fig. S11–13†).
Conclusions
A cone-hexahomotrioxacalix[3]arene receptor cone-7 bearing 2,2′-bipyridyl linked via a carbonyl group at the its upper rim and N,N-diethylacetamide chains at the lower rim, respectively, has been synthesized. The receptor cone-7 can serve as a heterotritopic hexahomotrioxacalix[3]arene receptor with capability for binding two types of cations in a cooperative fashion. The binding of the alkali metal ion Li+ took place at the lower rim, and the alkali metal ion Na+ and transition metal ion Ag+ at the upper rim, respectively. In addition, given the Na+ ion is larger than the Li+ ion, the Li+ ion bound with the lower rim cavity through the oxygens whereas the Na+ ion chose to bind with the larger cavity formed by the three phenoxy rings of the oxacalix[3]arene, which was verified by 1H NMR titration experiments.The nitrogen atom in the bipyridine ring pointed away from the calix cavity in the cone-7 because of the electronic repulsion between the nitrogens. After complexation, the nitrogen atom in the bipyridine ring turned inwards towards the cavity to complex with Ag+ to allow for the tetrahedral disposition of the N⋯Ag+ motif.
Further studies on the synthesis of tritopic receptors based on the hexahomotrioxacalix[3]arene are also underway in our laboratory.
Experimental
General
All melting points (Yanagimoto MP-S1) are uncorrected.1H NMR and 13C NMR spectra were recorded on a Nippon Denshi JEOL FT-300 NMR spectrometer and Varian-400MR-vnmrs400 with SiMe4 as an internal reference: J-values are given in Hz. IR spectra were measured for samples as KBr pellets on a Nippon Denshi JIR-AQ2OM spectrophotometer. Mass spectra were obtained with a Nippon Denshi JMS-HX110A Ultrahigh Performance mass spectrometer at 75 eV by using a direct-inlet system. UV-vis spectra were recorded using a Shimadzu UV-3150UV-vis-NIR spectrophotometer. Elemental analyses were performed by a Yanaco MT-5.Materials
cone-7,15,23-Tris(hydroxycarbonyl)-25,26,27-tris(N,N-diethyl-aminocarbonylmethoxy)-2,4,10,12,18,20-hexahomo-3,11,19-trioxacalix[3]arene triacid (cone-5) was synthesized from cone-7,15,23-tris(ethoxycarbonyl)-25,26,27-trihydroxy-2,4,10,12,18,20-hexahomo-3,11,19-trioxacalix[3]arene cone-3 as following the reported procedure.21 5′-Methyl-2,2′-bipyridyl-5-ylmethanol 6 was prepared according to the reported procedure.22Synthesis of 7,15,23-tris(5′-methyl-2,2′-bipyridyl-5-yl-methyl-oxycarbonyl)-25,26,27-tris(N,N-diethylaminocarbonyl-methoxy)-2,4,10,12,18,20-hexahomo-3,11,19-trioxacalix[3]arene (cone-7)
To a solution of cone-5 (100 mg, 0.11 mmol), 5′-methyl-2,2′-bipyridyl-5-ylmethanol 6 (110 mg, 0.55 mmol) and 1-hydroxybenzotriazole (DMAP) (67.2 mg, 0.55 mmol) in CH2Cl2 (10 mL), was added dropwise a solution of dicyclohexylcarbodiimide (DCC) (190 mg, 0.92 mmol) in CH2Cl2 (10 mL) at 0 °C. The reaction mixture was stirred for 3 days at room temperature then condensed under reduced pressure. The residue was extracted with ethyl acetate (2 × 30 mL). The combined extracts were washed with 10% citric acid (2 × 20 mL), 5% sodium bicarbonate (20 mL), water (20 mL) and saturated brine (20 mL); the solution was dried (MgSO4) and condensed under reduced pressure. The cone-7 was obtained from column chromatography [(CHCl3–MeOH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v)) (88 mg, 56%) as colorless prisms. M.p. 84.5–85 °C. IR: υmax(KBr)/cm−1 = 1723 (COOR) and 1650 (CONRR′).1H NMR (300 MHz, CDCl3): δ = 1.11–1.12 (18H, m, –CH2CH3), 2.40 (9H, s, Bipy–CH3), 3.30–3.41 (12H, m, –NCH2), 4.50 (6H, d, J = 13.2 Hz, Ar-CH2), 4.67 (6H, s, Ar-OCH2), 4.92 (6H, d, J = 12.6 Hz, Ar-CH2), 5.21 (6H, s, Bipy–CH2), 7.57 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.58 (6H, s, Ar-H), 7.74 (3H, dd, J = 10.2, J = 2.0 Hz, Bipy-H), 8.21 (3H, d, J = 8.1 Hz, Bipy-H), 8.28 (3H, d, J = 8.1 Hz, Bipy-H), 8.45 (3H, s, Bipy-H) and 8.62 (3H, s, Bipy-H) ppm. 13C NMR (100 MHz, CDCl3): δ = 13.5 (CH3), 18.5 (CH3), 40.5 (CH2), 63.5 (CH2), 67.0 (CH2), 72.5 (CH2), 120.7–160.1 (Ar-C, Bipy-C), 165.0 (C
1, v/v)) (88 mg, 56%) as colorless prisms. M.p. 84.5–85 °C. IR: υmax(KBr)/cm−1 = 1723 (COOR) and 1650 (CONRR′).1H NMR (300 MHz, CDCl3): δ = 1.11–1.12 (18H, m, –CH2CH3), 2.40 (9H, s, Bipy–CH3), 3.30–3.41 (12H, m, –NCH2), 4.50 (6H, d, J = 13.2 Hz, Ar-CH2), 4.67 (6H, s, Ar-OCH2), 4.92 (6H, d, J = 12.6 Hz, Ar-CH2), 5.21 (6H, s, Bipy–CH2), 7.57 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.58 (6H, s, Ar-H), 7.74 (3H, dd, J = 10.2, J = 2.0 Hz, Bipy-H), 8.21 (3H, d, J = 8.1 Hz, Bipy-H), 8.28 (3H, d, J = 8.1 Hz, Bipy-H), 8.45 (3H, s, Bipy-H) and 8.62 (3H, s, Bipy-H) ppm. 13C NMR (100 MHz, CDCl3): δ = 13.5 (CH3), 18.5 (CH3), 40.5 (CH2), 63.5 (CH2), 67.0 (CH2), 72.5 (CH2), 120.7–160.1 (Ar-C, Bipy-C), 165.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 167.0 (C
O) and 167.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) ppm. FABMS: m/z: 1426.78 (M+). C81H87O15N9 (1426.61): calcd C 68.19, H 6.15; N 8.84. Found: C 68.31, H 6.24, N 8.93.
O) ppm. FABMS: m/z: 1426.78 (M+). C81H87O15N9 (1426.61): calcd C 68.19, H 6.15; N 8.84. Found: C 68.31, H 6.24, N 8.93.
1H NMR complexation experiments
To a CDCl3 solution (500 μL, 5 × 10−3 M) of cone-7 in an NMR tube was added a CD3CN solution (50 μL, 5 × 10−3 M) of LiClO4, NaClO4, KClO4, CsClO4 and AgClO4. The spectrum for each was recorded after the addition metal ions. The temperature of the 1H NMR probe was kept constant at 27 °C. The1H NMR data of the most representative complexes are given below.The 1H NMR data of the most representative complexes was given below:
cone-7 ⊃ Li+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): δH (CDCl3/CD3CN, 10
1): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.12–3.42 (12H, m, –NCH2), 4.46 (6H, d, J = 13.2 Hz, Ar-CH2), 4.59 (6H, s, Ar-OCH2), 4.73 (6H, d, J = 12.6 Hz, Ar-CH2), 5.12 (6H, s, Bipy–CH2), 7.54 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.56 (6H, s, Ar-H), 7.66 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.08 (3H, d, J = 8.1 Hz, Bipy-H), 8.15 (3H, d, J = 8.1 Hz, Bipy-H), 8.35 (3H, s, Bipy-H) and 8.45 (3H, s, Bipy-H) ppm.
1, v/v): 3.12–3.42 (12H, m, –NCH2), 4.46 (6H, d, J = 13.2 Hz, Ar-CH2), 4.59 (6H, s, Ar-OCH2), 4.73 (6H, d, J = 12.6 Hz, Ar-CH2), 5.12 (6H, s, Bipy–CH2), 7.54 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.56 (6H, s, Ar-H), 7.66 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.08 (3H, d, J = 8.1 Hz, Bipy-H), 8.15 (3H, d, J = 8.1 Hz, Bipy-H), 8.35 (3H, s, Bipy-H) and 8.45 (3H, s, Bipy-H) ppm.
cone-7 ⊃ Na+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): δH (CDCl3/CD3CN, 10
1): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.10–3.35 (12H, m, –NCH2), 4.26 (6H, d, J = 13.2 Hz, Ar-CH2), 4.64 (6H, s, Ar-OCH2), 4.66 (6H, d, J = 12.6 Hz, Ar-CH2), 5.26 (6H, s, Bipy–CH2), 7.56 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.85 (6H, s, Ar-H), 7.75 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.16 (3H, d, J = 8.1 Hz, Bipy-H), 8.25 (3H, d, J = 8.1 Hz, Bipy-H), 8.40 (3H, s, Bipy-H) and 8.63 (3H, s, Bipy-H) ppm.
1, v/v): 3.10–3.35 (12H, m, –NCH2), 4.26 (6H, d, J = 13.2 Hz, Ar-CH2), 4.64 (6H, s, Ar-OCH2), 4.66 (6H, d, J = 12.6 Hz, Ar-CH2), 5.26 (6H, s, Bipy–CH2), 7.56 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.85 (6H, s, Ar-H), 7.75 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.16 (3H, d, J = 8.1 Hz, Bipy-H), 8.25 (3H, d, J = 8.1 Hz, Bipy-H), 8.40 (3H, s, Bipy-H) and 8.63 (3H, s, Bipy-H) ppm.
cone-7 ⊃ Ag+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): δH (CDCl3/CD3CN, 10
1): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.21–3.32 (12H, m, –NCH2), 4.45 (6H, d, J = 13.2 Hz, Ar-CH2), 4.62 (6H, s, Ar-OCH2), 4.84 (6H, d, J = 12.6 Hz, Ar-CH2), 5.10 (6H, s, Bipy–CH2), 7.58 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.47 (6H, s, Ar-H), 7.77 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.04 (3H, d, J = 8.1 Hz, Bipy-H), 8.11 (3H, d, J = 8.1 Hz, Bipy-H), 8.36 (3H, s, Bipy-H) and 8.51 (3H, s, Bipy-H) ppm.
1, v/v): 3.21–3.32 (12H, m, –NCH2), 4.45 (6H, d, J = 13.2 Hz, Ar-CH2), 4.62 (6H, s, Ar-OCH2), 4.84 (6H, d, J = 12.6 Hz, Ar-CH2), 5.10 (6H, s, Bipy–CH2), 7.58 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.47 (6H, s, Ar-H), 7.77 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.04 (3H, d, J = 8.1 Hz, Bipy-H), 8.11 (3H, d, J = 8.1 Hz, Bipy-H), 8.36 (3H, s, Bipy-H) and 8.51 (3H, s, Bipy-H) ppm.
[cone-7 ⊃ Ag+] ⊃ Li+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): δH (CDCl3/CD3CN, 10
1): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.08–3.37 (12H, m, –NCH2), 4.42 (6H, d, J = 13.2 Hz, Ar-CH2), 4.54 (6H, s, Ar-OCH2), 4.69 (6H, d, J = 12.6 Hz, Ar-CH2), 5.08 (6H, s, Bipy–CH2), 7.62 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.51 (6H, s, Ar-H), 7.78 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.01 (3H, d, J = 8.1 Hz, Bipy-H), 8.08 (3H, d, J = 8.1 Hz, Bipy-H), 8.33 (3H, s, Bipy-H) and 8.43 (3H, s, Bipy-H) ppm.
1, v/v): 3.08–3.37 (12H, m, –NCH2), 4.42 (6H, d, J = 13.2 Hz, Ar-CH2), 4.54 (6H, s, Ar-OCH2), 4.69 (6H, d, J = 12.6 Hz, Ar-CH2), 5.08 (6H, s, Bipy–CH2), 7.62 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.51 (6H, s, Ar-H), 7.78 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.01 (3H, d, J = 8.1 Hz, Bipy-H), 8.08 (3H, d, J = 8.1 Hz, Bipy-H), 8.33 (3H, s, Bipy-H) and 8.43 (3H, s, Bipy-H) ppm.
[cone-7 ⊃ Ag+] ⊃ Na+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4): 3.13–3.35 (12H, m, –NCH2), 4.23 (6H, d, J = 13.2 Hz, Ar-CH2) complex, 4.45 (6H, d, J = 13.2 Hz, Ar-CH2) free, 4.64 (6H, s, Ar-OCH2), 4.64 (6H, d, J = 12.6 Hz, Ar-CH2) complex, 4.84 (6H, d, J = 12.6 Hz, Ar-CH2) free, 5.29 (6H, s, Bipy–CH2) complex, 5.09 (6H, s, Bipy–CH2) free, 7.64 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H) free, 7.72 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H) complex, 7.81 (6H, s, Ar-H) complex, 7.48 (6H, s, Ar-H) free, 7.99 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.15 (3H, d, J = 8.1 Hz, Bipy-H), 8.23 (3H, d, J = 8.1 Hz, Bipy-H) complex, 8.09 (3H, d, J = 8.1 Hz, Bipy-H) free, 8.37 (3H, s, Bipy-H) and 8.59 (3H, s, Bipy-H) complex and 8.53 (3H, s, Bipy-H) free ppm.
0.4): 3.13–3.35 (12H, m, –NCH2), 4.23 (6H, d, J = 13.2 Hz, Ar-CH2) complex, 4.45 (6H, d, J = 13.2 Hz, Ar-CH2) free, 4.64 (6H, s, Ar-OCH2), 4.64 (6H, d, J = 12.6 Hz, Ar-CH2) complex, 4.84 (6H, d, J = 12.6 Hz, Ar-CH2) free, 5.29 (6H, s, Bipy–CH2) complex, 5.09 (6H, s, Bipy–CH2) free, 7.64 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H) free, 7.72 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H) complex, 7.81 (6H, s, Ar-H) complex, 7.48 (6H, s, Ar-H) free, 7.99 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.15 (3H, d, J = 8.1 Hz, Bipy-H), 8.23 (3H, d, J = 8.1 Hz, Bipy-H) complex, 8.09 (3H, d, J = 8.1 Hz, Bipy-H) free, 8.37 (3H, s, Bipy-H) and 8.59 (3H, s, Bipy-H) complex and 8.53 (3H, s, Bipy-H) free ppm.
[cone-7 ⊃ Ag+] ⊃ Na+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): δH (CDCl3/CD3CN, 10
1): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.11–3.35 (12H, m, –NCH2), 4.23 (6H, d, J = 13.2 Hz, Ar-CH2), 4.65 (6H, s, Ar-OCH2), 4.64 (6H, d, J = 12.6 Hz, Ar-CH2), 5.29 (6H, s, Bipy–CH2), 7.72 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.80 (6H, s, Ar-H), 7.97 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.15 (3H, d, J = 8.1 Hz, Bipy-H), 8.23 (3H, d, J = 8.1 Hz, Bipy-H), 8.35 (3H, s, Bipy-H) and 8.59 (3H, s, Bipy-H) ppm.
1, v/v): 3.11–3.35 (12H, m, –NCH2), 4.23 (6H, d, J = 13.2 Hz, Ar-CH2), 4.65 (6H, s, Ar-OCH2), 4.64 (6H, d, J = 12.6 Hz, Ar-CH2), 5.29 (6H, s, Bipy–CH2), 7.72 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.80 (6H, s, Ar-H), 7.97 (3H, dd, J = 10.2 Hz, J = 2.0 Hz, Bipy-H), 8.15 (3H, d, J = 8.1 Hz, Bipy-H), 8.23 (3H, d, J = 8.1 Hz, Bipy-H), 8.35 (3H, s, Bipy-H) and 8.59 (3H, s, Bipy-H) ppm.
cone-7 ⊃ Na+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4): δH (CDCl3/CD3CN, 10
0.4): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.15–3.31 (12H, m, –NCH2), 4.25 (6H, d, J = 13.2 Hz, Ar-CH2) complex, 4.40 (6H, d, J = 13.2 Hz, Ar-CH2) free, 4.62 (6H, s, Ar-OCH2), 4.62 (6H, d, J = 12.6 Hz, Ar-CH2) complex, 4.79 (6H, d, J = 12.6 Hz, Ar-CH2) free, 5.24 (6H, s, Bipy–CH2) complex, 5.14 (6H, s, Bipy–CH2) free, 7.50 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.83 (6H, s, Ar-H) complex, 7.50 (6H, s, Ar-H) free, 7.68 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.14 (3H, d, J = 8.1 Hz, Bipy-H), 8.20 (3H, d, J = 8.1 Hz, Bipy-H), 8.39 (3H, s, Bipy-H) and 8.56 (3H, s, Bipy-H) ppm.
1, v/v): 3.15–3.31 (12H, m, –NCH2), 4.25 (6H, d, J = 13.2 Hz, Ar-CH2) complex, 4.40 (6H, d, J = 13.2 Hz, Ar-CH2) free, 4.62 (6H, s, Ar-OCH2), 4.62 (6H, d, J = 12.6 Hz, Ar-CH2) complex, 4.79 (6H, d, J = 12.6 Hz, Ar-CH2) free, 5.24 (6H, s, Bipy–CH2) complex, 5.14 (6H, s, Bipy–CH2) free, 7.50 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.83 (6H, s, Ar-H) complex, 7.50 (6H, s, Ar-H) free, 7.68 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.14 (3H, d, J = 8.1 Hz, Bipy-H), 8.20 (3H, d, J = 8.1 Hz, Bipy-H), 8.39 (3H, s, Bipy-H) and 8.56 (3H, s, Bipy-H) ppm.
[cone-7 ⊃ Na+] ⊃ Ag+ (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): δH (CDCl3/CD3CN, 10
1): δH (CDCl3/CD3CN, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v): 3.06–3.31 (12H, m, –NCH2), 4.19 (6H, d, J = 13.2 Hz, Ar-CH2), 4.62 (6H, s, Ar-OCH2), 4.60 (6H, d, J = 12.6 Hz, Ar-CH2), 5.26 (6H, s, Bipy–CH2), 7.65 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.78 (6H, s, Ar-H), 7.90 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.13 (3H, d, J = 8.1 Hz, Bipy-H), 8.21 (3H, d, J = 8.1 Hz, Bipy-H), 8.32 (3H, s, Bipy-H) and 8.56 (3H, s, Bipy-H) ppm.
1, v/v): 3.06–3.31 (12H, m, –NCH2), 4.19 (6H, d, J = 13.2 Hz, Ar-CH2), 4.62 (6H, s, Ar-OCH2), 4.60 (6H, d, J = 12.6 Hz, Ar-CH2), 5.26 (6H, s, Bipy–CH2), 7.65 (3H, dd, J = 6.7 Hz, J = 1.2 Hz, Bipy-H), 7.78 (6H, s, Ar-H), 7.90 (3H, dd, J = 10.2 Hz, J = 1.2 Hz, Bipy-H), 8.13 (3H, d, J = 8.1 Hz, Bipy-H), 8.21 (3H, d, J = 8.1 Hz, Bipy-H), 8.32 (3H, s, Bipy-H) and 8.56 (3H, s, Bipy-H) ppm.
Stoichiometry of metal complexation and determination of association constants
Job's plot experiment was carried out using the absorption spectrum, make the volume fixed and the concentration of [Host]+[Guest] = 1.25 × 10−5 M, [Guest]/([Host]+[Guest]) changed from 0.1 to 0.9, and the association constants also determined by the absorption spectrum in a varying guest concentration of 0–1.25 μM and a constant concentration of host receptors with 1 μM. As a probe the absorption intensity signal was used. The association constant values were calculated by the intensity changes in the complex and the free host molecules.Acknowledgements
This work was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices (Institute for Materials Chemistry and Engineering, Kyushu University)”. We would like to thank the OTEC at Saga University and the International Collaborative Project Fund of Guizhou province at Guizhou University for financial support. CR thanks the EPSRC for a travel grant.Notes and references
- (a) C. D. Gutsche, Calixarenes, An Introduction, Royal Society of Chemistry, Cambridge, U.K., 2008 Search PubMed; (b) A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713–1734 CrossRef CAS; (c) J. S. Kim and D. T. Quang, Chem. Rev., 2007, 107, 3780–3799 CrossRef CAS PubMed; (d) D. Coquière, S. Le Gac, U. Darbost, O. Sénèque, I. Jabin and O. Reinaud, Org. Biomol. Chem., 2009, 7, 2485–2500 RSC.
- (a) A. Casnati, P. Minari, A. Pochini and R. Ungaro, J. Chem. Soc., Chem. Commun., 1991, 1413–1414 RSC; (b) S. Chang, M. Jang, S. Han, J. Lee, M. Kang and K. No, Chem. Lett., 1992, 1937–1940 CrossRef CAS; (c) M. Takeshita, F. Inokuchi and S. Shinkai, Tetrahedron Lett., 1995, 36, 3341–3344 CrossRef CAS; (d) H. Matsumoto, S. Nishio, M. Takeshita and S. Shinkai, Tetrahedron, 1995, 51, 4647–4654 CrossRef CAS; (e) M. Takeshita and S. Shinkai, Chem. Lett., 1994, 125–128 CrossRef CAS.
- X.-L. Ni, H. Cong, A. Yoshizawa, S. Rahman, H. Tomiyasu, U. Rayhan, X. Zeng and T. Yamato, J. Mol. Struct., 2013, 1046, 110–115 CrossRef CAS.
- T. Nabeshima, T. Saiki and K. Sumitomo, Org. Lett., 2002, 4, 3201–3209 CrossRef.
- T. Nabeshima, T. Saiki, K. Sumitomo and S. Akine, Tetrahedron Lett., 2004, 45, 4719–4722 CrossRef CAS.
- T. Nabeshima, Y. Yoshihira, T. Saiki, S. Akine and E. Horn, J. Am. Chem. Soc., 2003, 125, 28–29 CrossRef CAS PubMed.
- T. Saiki, J. Iwabuchi, S. Akine and T. Nabeshima, Tetrahedron Lett., 2004, 45, 7007–7710 CrossRef CAS.
- P. D. Beer, J. P. Martin and M. G. B. Drew, Tetrahedron, 1992, 48, 9917–9928 CrossRef CAS.
- V. J. B. Regnouf, J. O. Dalbavie, R. Lamartine and B. Fenet, Tetrahedron Lett., 2001, 42, 2681–2684 CrossRef.
- S. Pellet-Rostaing, V. J. B. Regnouf, R. Lamartine and B. Fenet, Inorg. Chem. Commun., 1999, 2, 44–47 CrossRef CAS.
- N. Psychogios and V. J. B. Regnouf, Tetrahedron Lett., 2001, 42, 2799–2800 CrossRef CAS.
- N. Psychogios and V. J. B. Regnouf, Tetrahedron Lett., 2002, 43, 77–80 CrossRef CAS.
- T. Nabeshima, Coord. Chem. Rev., 1996, 148, 151–169 CrossRef CAS.
- B. Linton and A. D. Hamilton, Chem. Rev., 1997, 97, 1669–1680 CrossRef CAS PubMed.
- T. Nabeshima, S. Akine and T. Saiki, Rev. Heteroatom Chem., 2000, 22, 219–239 CAS.
- A. Zinke, R. Ott, E. Leggeewie, A. Hassanein and G. Zankl, Monatsh. Chem., 1956, 87, 552–559 CrossRef CAS.
- (a) Z. Zhong, A. Ikeda and S. Shinkai, J. Am. Chem. Soc., 1999, 121, 11906–11907 CrossRef CAS; (b) A. Ikeda, Y. Suzuki, M. Yoshimura and S. Shinkai, Tetrahedron, 1998, 54, 2497–2508 CrossRef CAS; (c) A. Ikeda, M. Yoshimura, H. Udzu, C. Fukuhara and S. Shinkai, J. Am. Chem. Soc., 1999, 121, 4296–4297 CrossRef CAS; (d) A. Ikeda, H. Udzu, M. Yoshimura and S. Shinkai, Tetrahedron, 2000, 56, 1825–1832 CrossRef CAS.
- F. P. Gabbaï, A. Schier, J. Riede and M. J. Hynes, J. Chem. Soc., Chem. Commun., 1998, 76, 989–992 Search PubMed.
- (a) G. Bifulco, L. Gomez-paloma, R. Riccio, C. Gaeta, F. Troisi and P. Neri, Org. Lett., 2005, 7, 5757–5760 CrossRef CAS PubMed; (b) G. Bifulco, R. Riccio, C. Gaeta and P. Neri, Chem.–Eur. J., 2007, 13, 7185–7194 CrossRef CAS PubMed.
- (a) T. Yamato, C. Pérez-Casas, S. Rahman, Z. Xi, M. R. J. Elsegood and C. Redshaw, J. Inclusion Phenom. Macrocyclic Chem., 2007, 58, 193–197 CrossRef CAS; (b) C. Pérez-Casas, S. Rahman, N. Begum, Z. Xi and T. Yamato, J. Chem. Res., 2007, 2, 76–78 Search PubMed; (c) T. Yamato, S. Rahman, Z. Xi, F. Kitajima and J. T. Gil, Can. J. Chem., 2006, 84, 58–64 CrossRef CAS; (d) T. Yamato, F. Kitajima and J. T. Gil, J. Inclusion Phenom. Macrocyclic Chem., 2005, 53, 257–262 CrossRef CAS.
- X.-L. Ni, J. Tahara, S. Rahman, X. Zeng, D. L. Hughes, C. Redshaw and T. Yamato, Chem.–Asian J., 2012, 7, 519–527 CrossRef CAS PubMed.
- M. Takimoto, X. L. Ni, S. Rahman, X. Zeng and T. Yamato, J. Inclusion Phenom. Macrocyclic Chem., 2011, 70, 69–80 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H/13C NMR spectra of cone-7 and the detailed 1H NMR titration spectra data. See DOI: 10.1039/c4ra04566a |
| This journal is © The Royal Society of Chemistry 2014 |